Shutterstock.com
NHS England has begun working with the government’s Antivirals Taskforce to scope out the deployment of oral antivirals to treat COVID-19 in the community, The Pharmaceutical Journal has learnt.
A spokesperson for NHS England told The Pharmaceutical Journal that Keith Ridge, chief pharmaceutical officer for England, has taken on the role of senior responsible officer for oral antiviral deployment and is currently working with the taskforce to decide on the next steps.
The deployment will be informed by evidence from clinical trials of antiviral treatment in the UK and from overseas, some of which have shown promising results on shortening COVID-19 infection time.
The Antivirals Taskforce was established by the Department of Health and Social Care (DHSC) in April 2021, with the aim of identifying at least two effective oral antiviral treatments in 2021 that can be taken at home by individuals with a positive COVID-19 test, or who have been exposed to someone with the virus.
The taskforce sits alongside the government’s existing Therapeutics Taskforce, which aims to roll out treatments quickly for COVID-19 — such as the corticosteroid dexamethasone — and prevent hospital admissions.
In July 2021, the National Institute for Health Research issued a research call for applications to support a two-year community-based clinical trial to test antivirals for COVID-19 and start recruitment of patients by early October 2021.
As well as preventing hospitalisations, the research call said that antivirals may also be useful in managing outbreaks — alongside public health interventions — to prevent infection in known contacts of positive cases, and to offer protection to those who are not vaccinated or do not respond to vaccination.
The Pharmaceutical Journal asked the DHSC for an update on the outcome of the research call but a spokesperson said that it was not able to announce the results yet.
Ultan Power, professor of molecular virology at Queen’s University Belfast, said the main challenge with antiviral drugs is getting them in early enough in the infectious disease process.
“For acute infections like SARS-CoV-2, a lot of the disease is caused by the inflammatory response in response to the infection — so getting rid of the virus is not going to turn this off,” he explained.
“If they are given at a much earlier stage in the infection process then they work well. But, a lot of the time, people are only going to seek medication if they’re sick. And if they’re very sick it may already be too late.”
Power said that he expected that, for the best outcome for patients, both antivirals and targeted anti-inflammatory drugs would need to be used in tandem.
“[We would need to administer the] antivirals early and [then] dampen down, at the right time, the inflammatory response so we can stop the damage that is done by the virus during its replication cycle.
“Most of the damage caused [by respiratory viruses] is caused by the immune response — we have to know the details of what is being turned on by these viruses and target those [processes] specifically.”
Which antivirals may reduce hospitalisation in patients with COVID-19?
Several antivirals are being tested for their efficacy in reducing the risk of hospitalisation or death in non-hospitalised patients with COVID-19.
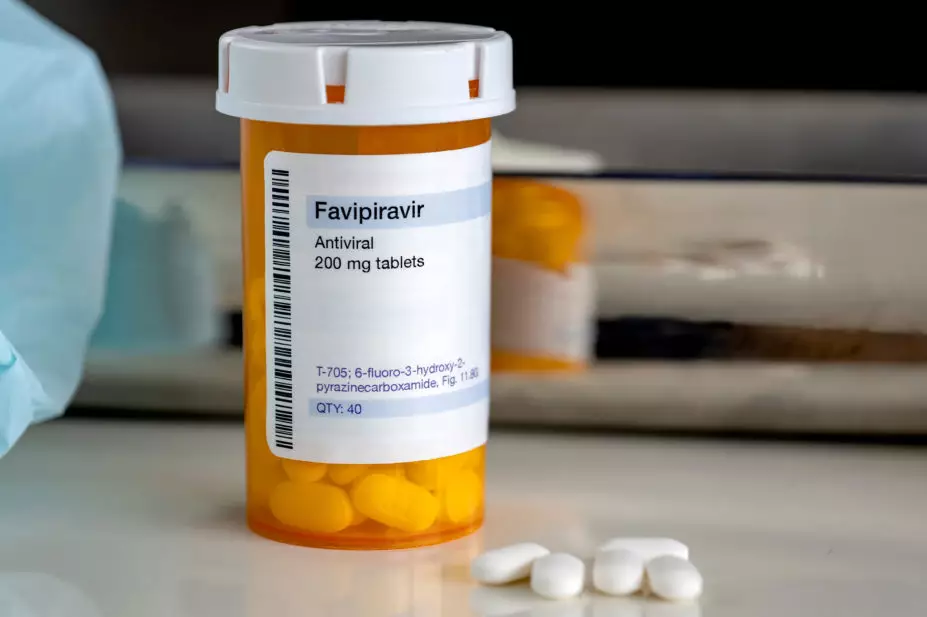
In April 2021, it was announced that the antiviral favipiravir would be investigated in the UK as part of the Platform Randomised trial of INterventions against COVID-19 In older peoPLE (PRINCIPLE) — the world’s largest clinical trial of possible COVID-19 treatments for recovery at home and in other non-hospital settings.
Pfizer is currently testing its oral antiviral PF-07321332 in up to 2,660 healthy adult participants, aged 18 years and over, who live in the same household as an individual with a confirmed symptomatic COVID-19 infection, as well as testing it in another trial in non-hospitalised, symptomatic adult patients. The drug is designed to block the activity of a key enzyme needed for the coronavirus to multiply, and will be administered along with a low dose of the protease inhibitor ritonavir.
An antiviral from Merck, molnupiravir, is also being studied in a late-stage trial in non-hospitalised patients to see if it reduces the risk of hospitalisation or death. Early trial data has shown it to be safe and effective in cutting down the time that patients test positive for COVID-19.
READ MORE: Everything you need to know about the COVID-19 therapy trials