Published online Mar 25, 2022. doi: 10.5501/wjv.v11.i2.98
Peer-review started: October 16, 2021
First decision: December 16, 2021
Revised: December 19, 2021
Accepted: February 10, 2022
Article in press: February 10, 2022
Published online: March 25, 2022
Several mechanisms may explain how exercise training mechanistically confers protection against coronavirus disease 2019 (COVID-19). Here we propose two new perspectives through which cardiorespiratory fitness may protect against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Physical exercise-activated adenosine monophosphate (AMP)-activated protein kinase (AMPK) signaling induces endothelial nitric oxide (NO) synthase (eNOS), increases NO bio-availability, and inhibits palmitoylation, leading to specific and immediate SARS-CoV-2 protection. AMPK signaling also induces angiotensin 1-7 release and enhances eNOS activation thus further mediating cardio- and reno-protection. Irisin, a myokine released from skeletal muscles during aerobic exercise, also participates in the AMPK/Akt-eNOS/NO pathway, protects mitochondrial functions in endothelial cells, and antagonizes renin angiotensin system proinflammatory action leading to reductions in genes associated with severe COVID-19 outcomes. Collectively, all the above findings point to the fact that increased AMPK and irisin activity through exercise training greatly benefits molecular processes that mediate specific, immediate, and delayed SARS-CoV-2 protection. Maintaining regular physical activity levels is a safe and affordable lifestyle strategy against the current and future pandemics and may also mitigate against obesity and cardiometabolic disease syndemics. Move more because a moving target is harder to kill.
Core Tip: Increased nitric oxide bio-availability through exercise training-induced activation of the master regulator of metabolism, the energy-sensing cellular enzyme adenosine monophosphate-activated protein kinase and irisin, the fat browning exercise hormone, released from skeletal muscles during aerobic exercise may mediate specific, immediate, and delayed severe acute respiratory syndrome coronavirus-2 protection. Move more because a moving target is harder to kill.
- Citation: Papadopoulos KI, Sutheesophon W, Aw TC. Too hard to die: Exercise training mediates specific and immediate SARS-CoV-2 protection. World J Virol 2022; 11(2): 98-103
- URL: https://www.wjgnet.com/2220-3249/full/v11/i2/98.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i2.98
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of the coronavirus disease 2019 (COVID-19), has to date (December 2021) infected over 270 million people worldwide and the death tally approaches 5.5 million[1]. Evolutionary evidence supports the survival of the fittest through natural selection for pathogen resistance, with effects mediated through younger age, lifestyle choices and importantly, genetics[2]. Epidemiological data support a lower COVID-19 incidence and severity in children and adolescents[3], individuals with high cardiorespiratory fitness (CRF) and muscle strength[4] as well as certain protective erythropoietin (EPO) augmenting genetic variants[3]. At the other end of the spectrum, inactivity, obesity, insulin resistance, diabetes, and hypertension, are associated with worse SARS-CoV-2 infection course and disproportionate COVID-19 mortality risk[5,6]. Public policies should promote increased physical activity and endeavor to increase the overall physical fitness in society by all available means. This is especially imperative for the population groups associated with worse SARS-CoV-2 prognosis[7]. The scope of this minireview is to focus on the mechanistical perspectives of two novel pathways, namely adenosine monophosphate (AMP)-activated protein kinase (AMPK) and irisin, through which exercise training may mitigate against SARS-CoV-2 infection and improve COVID-19 prognosis.
We conducted a PubMed literature search for publications in the English language since the start of the pandemic until September 2021, using the keywords: “AMPK”; “Irisin”; “physical exercise”; “renin angiotensin system (RAS)”; “angiotensin-converting enzyme 2 (ACE2)”; “nitric oxide (NO)”; “endothelial nitric oxide (NO) synthase (eNOS)”; “beta common receptor (βcR)”; “SARS-CoV-2”; and “COVID-19”. We noticed a veritable dearth of publications, especially when the keywords “eNOS”, “Irisin”, “AMPK” were used in different combinations together with “physical exercise” and “SARS-CoV-2 or COVID-19” which prompted us to focus on AMPK/eNOS and Irisin. Those pathways are known for their cardiometabolic, and vascular protective properties and suggest concrete mechanisms that offer immediate and delayed SARS-CoV-2 protection[8].
Several reviews have described numerous immune mechanisms which may explain how exercise training mechanistically confers protection against COVID-19. First, exercise downregulates the expression/activation of proinflammatory Toll-like receptors (TLR)[5]. Second, exercise training demonstrates an anti-inflammatory cytokine profile with increased levels of anti-inflammatory interleukin (IL)-10, IL-1 receptor antagonist (IL-1ra), and IL-37, which in turn inhibits the TLR-inflammation pathway and counteracts the inflammatory response induced by the inflammasomes[5]. In general, exercise promotes the recirculation of key immune cells and mediates an anti-inflammatory and antioxidant state through multiple mechanisms[5]. Effective rehabilitation programs for sarcopenia, could reduce inflammation and the need for IL-37 to exert its negative feedback to control the release of inflammatory cytokines[9].
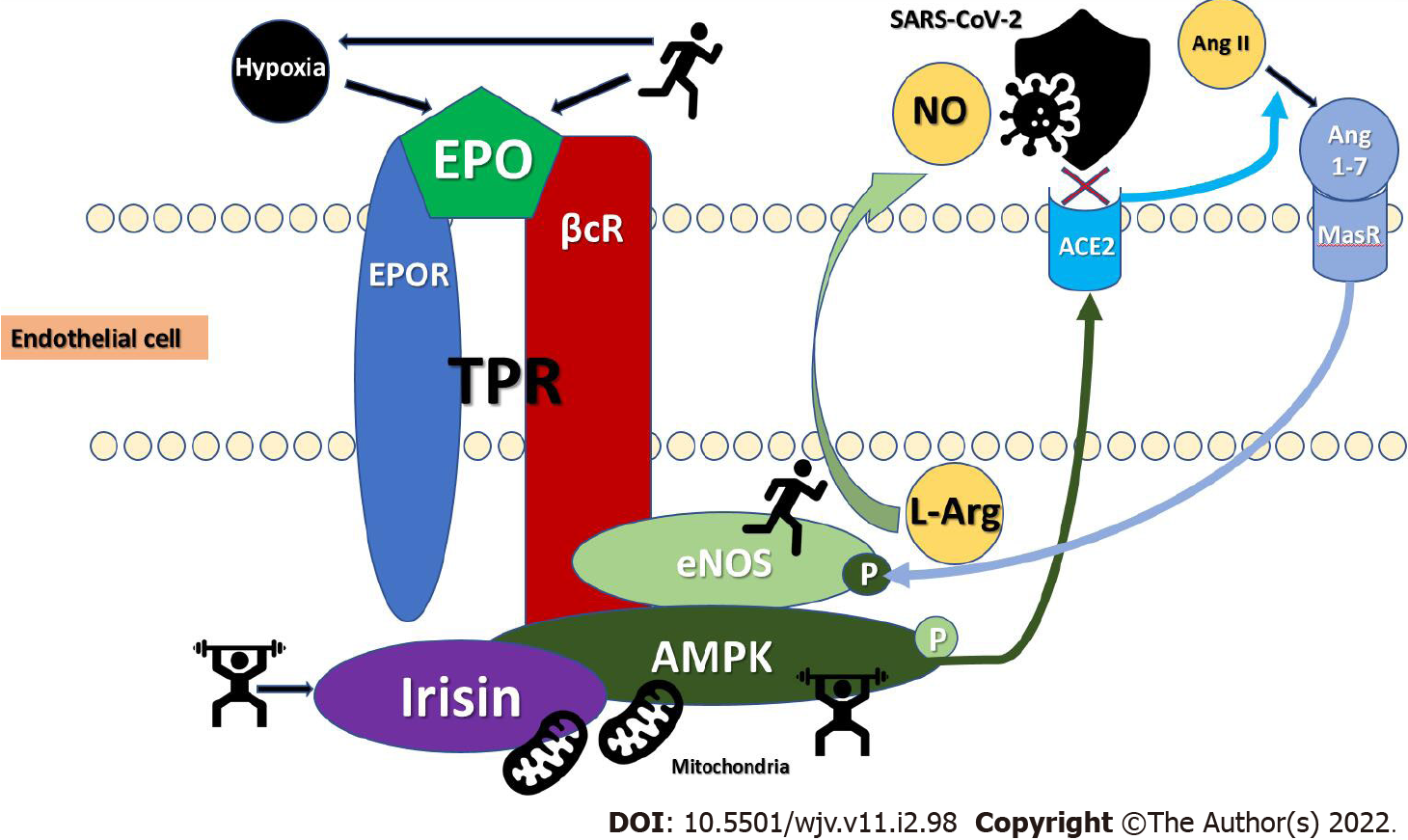
A more specific mechanism with immediate antiviral effects involves AMPK. We propose two new perspectives through which high CRF may protect from SARS-CoV-2. AMPK is an energy-sensing heterotrimeric enzyme, able to detect minute changes in cellular ADP and AMP as well as glucose availability[10]. Located in various cells and organs, AMPK modulates numerous downstream targets through switching phosphorylation on-off, including targets in the RAS[11]. AMPK is activated through several physiological and pathological conditions, such as hypoxia, caloric restriction, and physiological exercise but also via certain well known pharmacological agents as metformin, aspirin, canagliflozin, telmisartan, and herbal substances such as resveratrol, berberine, and quercetin[11,12]. Since activating AMPK has been shown to suppresses the Angiotensin II-induced vascular smooth muscle proliferative pathway and improve cardiometabolic disease, we believe that physical exercise-induced AMPK regulation of diverse cellular pathways is a reasonable mechanism in mediating both immediate and delayed SARS-CoV-2 protection (Figure 1)[11,13,14]. Physiological exercise induces AMPK activation as an important molecular mechanism of adaptation after physical activity. AMPK-eNOS phosphory
Chronic exercise induces EPO elevation, a well-known neuroprotective hormone, which mediates COVID-19 protection[3]. EPO’s protective effects are mediated through AMPK-dependent signaling, leading to enhanced phosphorylation of the beta common receptor (βcR) and eNOS, increased βcR-AMPK-eNOS complex formation, NO production, increased NO bio-availability, and ultimately tissue protection (Figure 1)[24]. Elevated, protective EPO mRNA levels were recently reported to be 2.6 times higher in nasopharyngeal swab samples of adult SARS-CoV-2 patients that were asymptomatic or showing mild COVID-19 symptoms, as compared to a control group[25]. Patients with acute respiratory distress syndrome (ARDS) in a moderate-sized COVID-19 cohort showed lower soluble eNOS levels, implying that greater eNOS activity and the presumed increased NO synthesis probably prevent patients from serious lung complications[26]. Fluvoxamine, intensely investigated as a SARS-CoV-2 protective agent, also mediates its action through sigma-1 receptor (S1R) agonism that induces eNOS, albeit via phosphatidylinositol-3-kinase and protein kinase B signaling[27].
Moreover, AMPK signaling exerts beneficial effects through RAS by elevating the protective arm of ACE2 and angiotensin (Ang) 1-7 through the Mas receptor (MasR) (Figure 1)[11]. Phosphorylation of ACE2 by AMPK enhances the stability of ACE2 and increases Ang 1-7 and eNOS-derived NO bio-availability further sustaining increased, protective NO levels[28]. Reduced inflammatory responses in lung emphysema, mitigation of pulmonary hypertension and protection against lipopolysaccharide-induced acute lung injury and ARDS have been reported with increased AMPK signaling[28-30]. Later in the course of SARS-CoV-2 infection, AMPK/ACE2/Ang 1-7/MasR-induced NO-increase may be cardio-, and renoprotective through lower oxidative stress, apoptosis, and systemic inflammatory responses[11,31].
Irisin is a myokine, cleaved as a peptide hormone of 112 amino acids from fibronectin type III domain containing 5 in skeletal muscle and secreted during aerobic exercise[32]. Irisin is positively correlated with an active lifestyle and vigorous intensity physical activity[32]. Both aerobic and resistance exercise are associated with high irisin levels, especially in older age groups[32]. Irisin is involved in muscle hyper
Collectively, all the above findings point to the fact that increased AMPK and irisin activity with exercise training greatly benefits molecular processes that mediate specific, immediate, and delayed SARS-CoV-2 protection.
Evolution arms us with ingenious and adaptive defense structures - our immune system, musculature, and cardiovascular system. Increased CRF through regular aerobic exertion and resistance exercise, greatly benefits all the above systems promoting survival and longevity[5]. Regular physical exercise enhances vaccination response and immunoprotection[5]. Maintaining regular physical activity levels along with prudent and balanced nutrition are safe and affordable lifestyle strategies against the current and future pandemics. Physical exercise may also reverse insulin resistance, alleviate hypertension, and mitigate against obesity and cardiometabolic disease syndemics[39]. While observing social distancing, exercise is still possible in public indoor spaces or outdoors. Exercise prescription for vulnerable groups and free or subsidized use of digital technology with online platforms delivering exercise classes could be employed to achieve the recommended exercise guidelines. For greater health benefits, 300 min of aerobic activity is recommended along with strength training exercises for all major muscle groups at least two times a week[40]. “Work from home” directives along with time savings from daily commuting have potentially freed up time for exercise that can be achievable in the home environment. The beneficial effects of exercise training in communicable and non-communicable disease prevention must remain central when deciding appropriate public health policies and subsidies. Government bodies should heed the Damoclean warning in this pandemic of the excess mortality threatening over 500 million people affected with obesity and diabetes worldwide or risk new hecatombs. We may have to learn to live with the virus for many years to come. It is thus imperative, on an individual level, to devise personal strategies for exercise training that do not depend on access to public gymnasiums. The takeaway message is once again to move more because a moving target is harder to kill.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA, Wu QN S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2021 . [DOI] [Cited in This Article: ] |
| 2. | Karlsson EK, Kwiatkowski DP, Sabeti PC. Natural selection and infectious disease in human populations. Nat Rev Genet. 2014;15:379-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 282] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 3. | Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. Age and genotype dependent erythropoietin protection in COVID-19. World J Stem Cells. 2021;13:1513-1529. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (4)] |
| 4. | Af Geijerstam A, Mehlig K, Börjesson M, Robertson J, Nyberg J, Adiels M, Rosengren A, Åberg M, Lissner L. Fitness, strength and severity of COVID-19: a prospective register study of 1 559 187 Swedish conscripts. BMJ Open. 2021;11:e051316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Nieman DC, Wentz LM. The compelling link between physical activity and the body's defense system. J Sport Health Sci. 2019;8:201-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 562] [Cited by in F6Publishing: 576] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 6. | Gammone MA, D'Orazio N. COVID-19 and Obesity: Overlapping of Two Pandemics. Obes Facts. 2021;14:579-585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | O'Rourke RW, Lumeng CN. Pathways to Severe COVID-19 for People with Obesity. Obesity (Silver Spring). 2021;29:645-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Inoue K, Fujie S, Hasegawa N, Horii N, Uchida M, Iemitsu K, Sanada K, Hamaoka T, Iemitsu M. Aerobic exercise training-induced irisin secretion is associated with the reduction of arterial stiffness via nitric oxide production in adults with obesity. Appl Physiol Nutr Metab. 2020;45:715-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | La Rosa F, Agostini S, Saresella M, Costa AS, Piancone F, Miglioli R, Trecate F, Clerici M. Deregulation of IL-37 and its miRNAs modulators in sarcopenic patients after rehabilitation. J Transl Med. 2021;19:172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov. 2019;18:527-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 369] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 11. | Liu J, Li X, Lu Q, Ren D, Sun X, Rousselle T, Li J, Leng J. AMPK: a balancer of the renin-angiotensin system. Biosci Rep. 2019;39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Myojo M, Nagata D, Fujita D, Kiyosue A, Takahashi M, Satonaka H, Morishita Y, Akimoto T, Nagai R, Komuro I, Hirata Y. Telmisartan activates endothelial nitric oxide synthase via Ser1177 phosphorylation in vascular endothelial cells. PLoS One. 2014;9:e96948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Carapeto PV, Aguayo-Mazzucato C. Effects of exercise on cellular and tissue aging. Aging (Albany NY). 2021;13:14522-14543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 165] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Kashiwagi S, Atochin DN, Li Q, Schleicher M, Pong T, Sessa WC, Huang PL. eNOS phosphorylation on serine 1176 affects insulin sensitivity and adiposity. Biochem Biophys Res Commun. 2013;431:284-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 16. | Tenopoulou M, Doulias PT. Endothelial nitric oxide synthase-derived nitric oxide in the regulation of metabolism. F1000Res. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Nosarev AV, Smagliy LV, Anfinogenova Y, Popov SV, Kapilevich LV. Exercise and NO production: relevance and implications in the cardiopulmonary system. Front Cell Dev Biol. 2014;2:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Yuan JX, Malhotra A, Manor U, Wang S, Yuan ZY, Shyy JY. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ Res. 2021;128:1323-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 259] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 19. | Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Järhult JD, Lennerstrand J, Lundkvist Å. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 20. | Akerström S, Gunalan V, Keng CT, Tan YJ, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Jackson CB, Farzan M, Chen B, Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol. 2022;23:3-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1176] [Cited by in F6Publishing: 1238] [Article Influence: 619.0] [Reference Citation Analysis (0)] |
| 22. | Wu Z, Zhang Z, Wang X, Zhang J, Ren C, Li Y, Gao L, Liang X, Wang P, Ma C. Palmitoylation of SARS-CoV-2 S protein is essential for viral infectivity. Signal Transduct Target Ther. 2021;6:231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 23. | Tanner JE, Alfieri C. The Fatty Acid Lipid Metabolism Nexus in COVID-19. Viruses. 2021;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Su KH, Yu YB, Hou HH, Zhao JF, Kou YR, Cheng LC, Shyue SK, Lee TS. AMP-activated protein kinase mediates erythropoietin-induced activation of endothelial nitric oxide synthase. J Cell Physiol. 2012;227:3053-3062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Mpekoulis G, Frakolaki E, Taka S, Ioannidis A, Vassiliou AG, Kalliampakou KI, Patas K, Karakasiliotis I, Aidinis V, Chatzipanagiotou S, Angelakis E, Vassilacopoulou D, Vassilaki N. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS One. 2021;16:e0253458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Vassiliou AG, Zacharis A, Keskinidou C, Jahaj E, Pratikaki M, Gallos P, Dimopoulou I, Kotanidou A, Orfanos SE. Soluble Angiotensin Converting Enzyme 2 (ACE2) Is Upregulated and Soluble Endothelial Nitric Oxide Synthase (eNOS) Is Downregulated in COVID-19-induced Acute Respiratory Distress Syndrome (ARDS). Pharmaceuticals (Basel). 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Papadopoulos KI, Sutheesophon W, Aw TC. Anti-SARS-CoV-2 Action of Fluvoxamine may be Mediated by Endothelial Nitric Oxide Synthase. Pharmacopsychiatry. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Dong J, Martin M, He M, Gongol B, Marin TL, Chen L, Shi X, Yin Y, Shang F, Wu Y, Huang HY, Zhang J, Zhang Y, Kang J, Moya EA, Huang HD, Powell FL, Chen Z, Thistlethwaite PA, Yuan ZY, Shyy JY. AMP-activated Protein Kinase Phosphorylation of Angiotensin-Converting Enzyme 2 in Endothelium Mitigates Pulmonary Hypertension. Am J Respir Crit Care Med. 2018;198:509-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 29. | Cheng XY, Li YY, Huang C, Li J, Yao HW. AMP-activated protein kinase reduces inflammatory responses and cellular senescence in pulmonary emphysema. Oncotarget. 2017;8:22513-22523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 30. | Wu YX, Wang YY, Gao ZQ, Chen D, Liu G, Wan BB, Jiang FJ, Wei MX, Zuo J, Zhu J, Chen YQ, Qian F, Pang QF. Ethyl ferulate protects against lipopolysaccharide-induced acute lung injury by activating AMPK/Nrf2 signaling pathway. Acta Pharmacol Sin. 2021;42:2069-2081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 31. | Pourbagher-Shahri AM, Farkhondeh T, Talebi M, Kopustinskiene DM, Samarghandian S, Bernatoniene J. An Overview of NO Signaling Pathways in Aging. Molecules. 2021;26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | Cosio PL, Crespo-Posadas M, Velarde-Sotres Á, Pelaez M. Effect of Chronic Resistance Training on Circulating Irisin: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Environ Res Public Health. 2021;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Jodeiri Farshbaf M, Alviña K. Multiple Roles in Neuroprotection for the Exercise Derived Myokine Irisin. Front Aging Neurosci. 2021;13:649929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | De Sousa RAL, Improta-Caria AC, Aras-Júnior R, de Oliveira EM, Soci ÚPR, Cassilhas RC. Physical exercise effects on the brain during COVID-19 pandemic: links between mental and cardiovascular health. Neurol Sci. 2021;42:1325-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 35. | Fu J, Han Y, Wang J, Liu Y, Zheng S, Zhou L, Jose PA, Zeng C. Irisin Lowers Blood Pressure by Improvement of Endothelial Dysfunction via AMPK-Akt-eNOS-NO Pathway in the Spontaneously Hypertensive Rat. J Am Heart Assoc. 2016;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 36. | Bi J, Zhang J, Ren Y, Du Z, Zhang Y, Liu C, Wang Y, Zhang L, Shi Z, Wu Z, Lv Y, Wu R. Exercise hormone irisin mitigates endothelial barrier dysfunction and microvascular leakage-related diseases. JCI Insight. 2020;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Chen RR, Fan XH, Chen G, Zeng GW, Xue YG, Liu XT, Wang CY. Irisin attenuates angiotensin II-induced cardiac fibrosis via Nrf2 mediated inhibition of ROS/ TGFβ1/Smad2/3 signaling axis. Chem Biol Interact. 2019;302:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 38. | de Oliveira M, De Sibio MT, Mathias LS, Rodrigues BM, Sakalem ME, Nogueira CR. Irisin modulates genes associated with severe coronavirus disease (COVID-19) outcome in human subcutaneous adipocytes cell culture. Mol Cell Endocrinol. 2020;515:110917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Di Ciaula A, Krawczyk M, Filipiak KJ, Geier A, Bonfrate L, Portincasa P. Noncommunicable diseases, climate change and iniquities: What COVID-19 has taught us about syndemic. Eur J Clin Invest. 2021;51:e13682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 40. | Flack KD, Hays HM, Moreland J, Long DE. Exercise for Weight Loss: Further Evaluating Energy Compensation with Exercise. Med Sci Sports Exerc. 2020;52:2466-2475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |