Abstract
Biological cardiac injury related to the Severe Acute Respiratory Syndrome Coronavirus-2 infection has been associated with excess mortality. However, its functional impact remains unknown. The aim of our study was to explore the impact of biological cardiac injury on myocardial functions in patients with COVID-19. 31 patients with confirmed COVID-19 (CoV+) and 16 controls (CoV−) were prospectively included in this observational study. Demographic data, laboratory findings, comorbidities, treatments and myocardial function assessed by transthoracic echocardiography were collected and analysed in CoV+ with (TnT+) and without (TnT−) elevation of troponin T levels and compared with CoV−. Among CoV+, 13 (42%) exhibited myocardial injury. CoV+/TnT + patients were older, had lower diastolic arterial pressure and were more likely to have hypertension and chronic renal failure compared with CoV+/TnT−. The control group was comparable except for an absence of biological inflammatory syndrome. Left ventricular ejection fraction and global longitudinal strain were not different among the three groups. There was a trend of decreased myocardial work and increased peak systolic tricuspid annular velocity between the CoV− and CoV + patients, which became significant when comparing CoV− and CoV+/TnT+ (2167 ± 359 vs. 1774 ± 521%/mmHg, P = 0.047 and 14 ± 3 vs. 16 ± 3 cm/s, P = 0.037, respectively). There was a decrease of global work efficiency from CoV− (96 ± 2%) to CoV+/TnT− (94 ± 4%) and then CoV+/TnT+ (93 ± 3%, P = 0.042). In conclusion, biological myocardial injury in COVID 19 has low functional impact on left ventricular systolic function.
Similar content being viewed by others
Introduction
The pathophysiology of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection is still poorly understood. Although lung involvement has all the usual characteristics of a severe acute respiratory syndrome, the natural history of infection goes beyond the simple respiratory failure. The intense systemic inflammatory stimuli that occurs after the viral replication phase is a major contributor to the systemic effects of the disease [1] and their consequences in terms of morbidity and mortality.
Myocardial injury, manifested by an increase in troponin, has been reported in 8 to 20% of infected patients [2,3,4], reaching 31% in the most severe patients [2, 5], and is associated with excess mortality [4, 5]. Although myocardial injury has been linked to the severity of inflammation [5], the exact mechanism of the myocardial injury that may accompany the infection remains unknown. Many arguments suggest a type 2 myocardial infarction via an oxygen supply/demand imbalance mechanism alone attributable to the cytokine storm. Alternatively, cardiovascular risk factors such as diabetes and hypertension, as well as coronary or cerebral cardiovascular history, have been associated with severe forms of infection [6,7,8]. Consequently, cytokine storm could lead to atherosclerotic plaque instability and rupture [9] (i.e. type 1 myocardial infraction). On the other hand, the supposed affinity of the virus for the myocardial angiotensin-converting enzyme 2 receptors [10] could lead to direct inflammation of the myocardium as suggested by several articles reporting acute/fulminant myocarditis [11,12,13].
However, to date, although there is a link between increased troponin and myocardial dysfunction through the increase in natriuretic peptides [5], the real segmental and global functional impact of the infection on the myocardium remains unknown. Complete cardiac data on infected patients, such as electrocardiography, echocardiography, coronary angiography and magnetic resonance imaging are missing [9].
The objective of this study was to perform cardiac imaging phenotype of Coronavirus Disease 19 (COVID-19) infected patients to assess the impact of the infection on myocardial work and function.
Methods
Study participants
Patients admitted to Rangueil Hospital of Toulouse University for dyspnea or chest pain with suspected COVID-19 disease were included in this prospective cohort study from April 6, 2020, to May 1, 2020. Data were collected in consecutive patients referred to either the medical unit or the intensive care unit for COVID-19. All patients underwent collection specimens from the upper respiratory tract by real-time reverse transcriptase–polymerase chain reaction (RT-PCR) testing for the SARS-COV-2, chest computed tomography (CT), cardiac biomarkers, including high-sensitivity troponin T (hs-TnT) and NT-proBNP, electrocardiography and transthoracic echocardiography (TTE) at admission, as part of the standard of care. Diagnosis of COVID-19 was retained in the presence of an evocative chest CT and a positive RT-PCR for COVID-19 or an evocative chest CT in the presence of a negative RT-PCR for COVID-19 after ruling out other common respiratory viral and bacterial infections [14]. Biological cardiac injury was defined as blood levels of hs-TnT above the 99th -percentile upper reference limit, regardless of new abnormalities in electrocardiography and TTE. Patients were then categorized into 3 groups based on the presence of COVID-19 infection and/or biological cardiac injury: patients without COVID-19 infection and without biological cardiac injury (CoV−), patients with COVID-19 infection without biological cardiac injury (CoV+/TnT− and patients with COVID-19 infection with biological cardiac injury (CoV+/TnT+).
Patients with history of heart disease and atrial fibrillation were excluded from the study.
The vital status of the patients was collected 3 months after discharge from the hospital by a phone call.
The study has been notified to national ethics committees as appropriate (protocol number ID-RCB: 2020-A00852-37, positive endorsement of the protection of persons committee West IV dated April 8, 2020) and to the French Data Protection Agency. The study has been registered with Clinicaltrials.gov NCT04358952. The investigation conforms to the principles outlined in the Declaration of Helsinki. All patients or their beneficiaries were informed of the study and gave their consent.
Data collection
The demographic characteristics (age and sex), clinical data (symptoms, comorbidities, treatments) and laboratory findings for patients during hospitalization were collected from electronic medical records and entered into the database by 2 investigators. Cardiovascular risk factors included hypertension, diabetes, hypercholesterolemia, previous or current smoking and obesity (defined by a body mass index ≥ 30 kg/m²). Arterial blood pressure was measured and collected at the time of TTE. Electrocardiography and TTE were performed by 3 investigators and analyzed by 2 independent investigators blinded to the clinical characteristics of the patients.
Transthoracic echocardiography
Transthoracic echocardiography was performed with either a Vivid E95 or Vivid S70 ultrasound system (GE Healthcare) using a 3.5 MHz transducer, allowing archiving acquisitions for a deferred analysis. Doppler, M-mode and two-dimensional gray scale echocardiography including the three standard apical views (four-, three- and two-chambers) using high frame rates (> 60 frames/s), pulsed-Doppler transmitral inflow and left ventricular outflow and pulsed-Doppler tissue imaging lateral mitral and tricuspid annular velocities were performed for each patient with simultaneous arterial blood pressure recording.
Images analysis
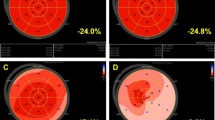
Image analysis was performed offline using the offline EchoPAC V.202 software (GE Medical Systems). Two-dimensional and Doppler echocardiography measurements and quantification were performed according to the American Society of Echocardiography and the European Association of Cardiovascular Imaging guidelines [15, 16]. The following measurements were collected: diastolic parameters, including peak early (E) and late (A) diastolic mitral inflow velocities, E/A ratio, deceleration time, lateral mitral annular diastolic velocity (Ea), peak systolic tricuspid annular velocity and tricuspid annular plane systolic excursion (TAPSE). All Doppler measurements were made over three cardiac cycles and averaged. Left ventricular end-diastolic and -systolic volumes, and ejection fraction were measured using the modified biplane Simpson’s method from apical two- and four-chambers views. Longitudinal strain (LS) was calculated using two-dimensional speckle tracking from greyscale images and the automated function imaging technique from the apical four-chambers, two-chambers and three-chambers views [17]. Strain values from all segments were averaged to obtain the global LS. Myocardial work was calculated from left ventricular LS and an estimated left ventricular pressure curve as proposed by Russell et al. [18]. After calculating global LS, indicating the time of aortic and mitral valve events by echocardiography and inserting values of arterial blood pressure, the software derived non-invasive pressure-strain loop. Peak systolic left ventricular pressure was assumed to be equal to the peak systolic blood pressure. Strain and pressure data were synchronized using the onset R wave on electrocardiogram as a common time reference. Myocardial work was calculated by integrating the power over time from mitral valve closure until mitral valve opening. Wasted work was defined by the work performed by the myocardium during segmental elongation, and constructive work by the work performed during segmental shortening represented. During isovolumetric relaxation, this definition was reversed such that myocardial work during shortening was considered wasted work and work during lengthening was considered constructive work. Work efficiency was then calculated as constructive work divided by the sum of constructive and wasted works. A “bull’s-eye” plots illustrating segmental and global LS, work index and work efficiency were automatically generated.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test and expressed as mean ± standard deviation. Laboratory findings and number of cardiovascular risk factors were not normally distributed, and results are, therefore, presented as medians with interquartile ranges (IQR). Nominal values were expressed as numbers and percentages. Group comparisons were made using nonparametric Kruskal–Wallis or Mann-Whitney rank sum tests when appropriate for continuous variables; and χ2 or Fischer exact tests when appropriate for categorical variables. Relationships between variables were assessed using Spearman correlation analysis and expressed by R. Differences were considered statistically significant for P-values of < 0.05. All analyses were performed using standard statistical software SPSS version 20 (SPSS Inc., Chicago, Illinois).
Results
We excluded 6 and 2 patients with history of heart disease and atrial fibrillation, respectively; 5 patients because of inadequate image quality for speckle tracking analysis, and 4 patients for prone position ventilation. Finally, 31 patients with COVID-19 and 16 controls were included for final analysis.
Baseline characteristics
Table 1 presents demographic characteristics by group according to the presence of SARS-CoV-2 infection and/or biological cardiac injury. Among the 31 patients with COVID-19 included in the study, all had bilateral pneumonia on chest computed tomography; 21 (68%) required invasive mechanical ventilation and were admitted to intensive care unit (ICU). 13 (42%) exhibited myocardial injury as indicated by elevated hs-TnT levels. hs-TnT ranged from 15 to 79 µg/L. On admission, none showed evidence of acute myocardial infarction, thromboembolic diseases, or chronic liver disease. No difference was found concerning cardiovascular risk factors: 1 [0–2], 0 [0–2] and 0 [1,2,3] among CoV−, CoV+/TnT− and CoV+/TnT+, respectively (P = 0.076). Patients CoV+/TnT + were older, had lower diastolic arterial pressure and were more likely to have hypertension and chronic renal failure, which were associated with a trend for presenting diabetes. Inotropic and/or vasopressor support was present in 4 (22%) CoV+/TnT− and 5 (38%) CoV+/TnT + patients (P = 0.433). Biologically, in addition to the cardiac injury, CoV+/TnT + had higher level of NT-proBNP and lower estimated glomerular filtration rate (CKD-epi), as well as lower hemoglobin level. Finally, nine (50%) CoV+/TnT− and 12 (92%) CoV+/TnT + patients were admitted in ICU (P = 0.020). No patient died during hospitalization and all patients were still alive 3 months after hospital discharge. The control group was broadly comparable except for characteristics defining infection by SARS-CoV2 (clinical symptoms, CRP and lymphocyte count).
Cardiac phenotype by transthoracic echocardiography
Transthoracic echocardiographic findings according to the presence of SARS-CoV-2 infection and/or biological cardiac injury are presented in Table 2. No patients had wall motion abnormality.
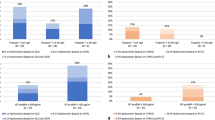
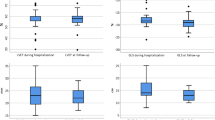
Left ventricular end-diastolic volume index, ejection fraction and global longitudinal strain by speckle tracking were not different among the 3 groups. No patient exhibited right ventricular dilatation. There was a trend of decreased myocardial work and increased peak systolic tricuspid annular velocity between the CoV− and CoV + group (P = 0.047), which became significant when comparing CoV− and CoV+/TnT+ (P = 0.037). There was a decrease of global work efficiency between CoV− and then CoV+/TnT+ (P = 0.007). The left ventricular diastolic function did not discriminate between groups, except for the lateral Ea wave, which was lower in CoV+/TnT + patients when compared to CoV+/TnT− patients (P = 0.008). A summary of the main echocardiographic findings according to the presence of SARS-CoV-2 infection and/or biological cardiac injury is presented in Fig. 1.
Finally, hs-TnT level was correlated with global work efficiency (R=-0.383, P = 0.009) but not with left ventricular ejection fraction (R = 0.160, P = 0.234), global LS (R = 0.154, P = 0.212) or global myocardial work (R = − 0.236, P = 0.114) (Fig. 2). NT-proBNP level was not correlated with any of left ventricular systolic function parameters (data not shown). Furthermore, there was a trend for a slight correlation between C-reactive protein and hs-TnT level (R = 0.287, P = 0.051).
Discussion
This is the first study showing that SARS-CoV-2 infection have little impact on the heart. Even in the presence of cardiac injury, direct systolic function parameters such as left ventricular ejection fraction or global are not altered. Only myocardial work indices are impaired by the SARS-CoV-2 infection with a trend to decrease of global myocardial work in case of cardiac injury, as well as a decrease in global work efficiency, parameters that depend on the afterload. Finally, we show a trend for increase of right ventricular systolic function assessed by peak systolic tricuspid annular velocity between controls and patients with COVID-19, particularly in case of cardiac injury. Despite evidences showing the negative prognostic impact of biological cardiac injury (i.e. increased troponin) in the presence of SARS-CoV-2 infection [5, 19, 20], there are, to date, no data on the functional impact of this injury.
Although there may have been warnings of an association between ST-segment elevation myocardial infarction and COVID-19 [21], the epidemiological series suggested rather a decrease in the number of acute myocardial infarction to a decrease in the use of specialized channels [22, 23]. Despite biological cardiac injury, our study did not find electrocardiographic signs of acute coronary syndrome or echocardiographic wall motion abnormality. It appears that the increase in troponin is only a consequence of the severity of the inflammatory syndrome and systemic impairment [19], and that heart failure is not at the forefront. However, the fact remains that patients with concomitant cardiac disease and COVID-19 have a poor prognosis compared with patients without a history of cardiac disease. The higher mortality is not necessarily driven by cardiac etiology [24] and reflects incompetent hemodynamic adaptation. Our study shows that the direct effects on the myocardium are weak, with in particular an absence of repercussions on left ventricular ejection fraction and global LS. The effects of SARS-CoV-2 infection are manifested at most by an alteration in left ventricular relaxation as shown by the decrease of the lateral Ea velocity. In contrast, cardiac involvement is accompanied by a decrease in myocardial work indexes. This occurs in patients with many cardiovascular risk factors and more severe infection as shown by mechanical ventilation, decreased diastolic blood pressure, impaired renal function and strong biological inflammatory syndrome.
Right ventricular dilatation [25] and systolic function [26] have been reported as prognostic factors in COVID-19 patients. The right ventricle is known to be sensitive to afterload [27] and its dilatation and alteration of systolic function are most often related to an increase in systolic pulmonary pressures. As reported in the study by Li and co-authors [26], it reflects the severity of the acute respiratory distress syndrome associated with the SARS-CoV2 infection and explain its prognostic impact. On the other hand, the high frequency of coagulopathies and thromboembolic events, including pulmonary embolism [28], reported in COVID-19 patients could explain the prognostic impact of right ventricular dilatation and systolic function and D-dimer [29, 30]. On the contrary, in our study, we show an absence of right ventricle dilatation including in patients with acute respiratory distress syndrome. We found a tendency to improve right ventricular systolic function in COVID-19 patients, particularly in case of cardiac injury, through the increase of the tricuspid annular S-wave. This finding could simply be the consequence of an initial phase of right ventricle compensation for lung damage in COVID-19.
Finally, the slight functional impact of the biological cardiac injury on the myocardium, with regard to its prognostic impact, could be explained either by a type 2 myocardial infarction (oxygen supply/demand imbalance) as a consequence of the severity of the infection, or a direct inflammation of the myocardium without functional impact. Recent findings suggest that SARS-CoV-2 infection could facilitate the induction of endotheliitis in several organs as a direct consequence of viral involvement [31]. On the other hand, a recent autopsy study shows that the presence of large amounts of the virus in the heart is accompanied by a cytokine response without inflammatory cell infiltration [32]. Cardiac magnetic resonance imaging studies on patients recovered from COVID-19 with cardiac symptoms or unexplained elevated hs-TnT confirm the absence of left ventricular systolic dysfunction and suggest a mixed mechanism showing that cardiac injury in SARS-CoV-2 infection is associated with myocardial edema and then fibrosis [33, 34] with a heterogeneous pattern of late gadolinium enhancement including ischemic and non-ischemic patterns as myocarditis-like or non-specific mid-wall late gadolinium enhancement [35].
Limitations
The main limitation of our study is that it is a single-site and limited sample studies. However, it takes place in a teaching hospital of a wild suburban area. Moreover, all patients referred to either the medical or the intensive care units for COVID-19 were consecutively included. This study is therefore representative of a sample of the COVID-19 patients and is one of the few to describe the left ventricular functional impact of SARS-CoV2 acute phase infection using sensitive tools integrating after-load such as myocardial work indices.
Finally, our results are to be interpreted in the light of the low mortality of our population, which does not allow us to conclude that myocardial damage may be more pronounced in more severe patients. Furthermore, the absence of short-term impact does not allow to conclude on the absence of long-term consequences of cardiac injury during infection.
Conclusions
The cardiac injury associated with SARS-CoV-2 infection has a low functional impact on the myocardium, essentially reflected in a decrease in myocardial work indices, which are dependent on left ventricular after-load. The cardiac impact of SARS-CoV-2 infection appears to be related to cardiovascular risk factors and the severity of the sepsis.
Abbreviations
- CT:
-
Computed tomography
- hs-TnT:
-
High-sensitivity troponin T
- IQR:
-
Interquartile ranges
- LS:
-
Longitudinal strain
- TTE:
-
Transthoracic echocardiography
- RT-PCR:
-
Real-time reverse transcriptase–polymerase chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus-2
- TAPSE:
-
Tricuspid annular plane systolic excursion
References
Conti P, Ronconi G, Caraffa A et al (2020) Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 34:1
Huang C, Wang Y, Li X et al (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Lippi G, Lavie CJ, Sanchis-Gomar F (2020) Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. https://doi.org/10.1016/j.pcad.2020.03.001
Shi S, Qin M, Shen B et al (2020) Association of cardiac injury with mortality in hospitalized patients With COVID-19 in Wuhan, China. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.0950
Guo T, Fan Y, Chen M et al (2020) Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1017
Yang X, Yu Y, Xu J et al (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30079-5
Guan W-J, Ni Z-Y, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. https://doi.org/10.1056/NEJMoa2002032
Zhang J-J, Dong X, Cao Y-Y et al (2020) Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. https://doi.org/10.1111/all.14238
Bonow RO, Fonarow GC, O’Gara PT, Yancy CW (2020) Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1105
Zheng Y-Y, Ma Y-T, Zhang J-Y, Xie X (2020) COVID-19 and the cardiovascular system. Nat Rev Cardiol. https://doi.org/10.1038/s41569-020-0360-5
Hu H, Ma F, Wei X, Fang Y (2020) Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa190
Inciardi RM, Lupi L, Zaccone G et al (2020) Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.1096
Chen C, Zhou Y, Wang DW (2020) SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 45:23–232
World Health Organization (2020) WHO Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. WHO, Geneva
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270. https://doi.org/10.1093/ehjci/jev014
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 17:1321–1360. https://doi.org/10.1093/ehjci/jew082
Voigt J-U, Pedrizzetti G, Lysyansky P et al (2015) Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 16:1–11. https://doi.org/10.1093/ehjci/jeu184
Russell K, Eriksen M, Aaberge L et al (2013) Assessment of wasted myocardial work: a novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am J Physiol Heart Circ Physiol 305:H996–H1003. https://doi.org/10.1152/ajpheart.00191.2013
Fan H, Zhang L, Huang B et al (2020) Cardiac injuries in patients with coronavirus disease 2019: not to be ignored. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. https://doi.org/10.1016/j.ijid.2020.05.024
Wei J-F, Huang F-Y, Xiong T-Y et al (2020) Acute myocardial injury is common in patients with covid-19 and impairs their prognosis. Heart. https://doi.org/10.1136/heartjnl-2020-317007
Bangalore S, Sharma A, Slotwiner A et al (2020) ST-segment elevation in patients with Covid-19—a Case Series. N Engl J Med. https://doi.org/10.1056/NEJMc2009020
Solomon MD, McNulty EJ, Rana JS et al (2020) The Covid-19 pandemic and the incidence of acute myocardial infarction. N Engl J Med. https://doi.org/10.1056/NEJMc2015630
De Filippo O, D’Ascenzo F, Angelini F et al (2020) Reduced rate of hospital admissions for ACS during Covid-19 outbreak in northern Italy. N Engl J Med. https://doi.org/10.1056/NEJMc2009166
Inciardi RM, Adamo M, Lupi L et al (2020) Characteristics and outcomes of patients hospitalized for COVID-19 and cardiac disease in Northern Italy. Eur Heart J 41:1821–1829. https://doi.org/10.1093/eurheartj/ehaa388
Argulian E, Sud K, Vogel B et al (2020) Right ventricular dilation in hospitalized patients with COVID-19 infection. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2020.05.010
Li Y, Li H, Zhu S et al (2020) Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2020.04.014
Haddad F, Hunt SA, Rosenthal DN, Murphy DJ (2008) Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 117:1436–1448. https://doi.org/10.1161/CIRCULATIONAHA.107.653576
Fox SE, Akmatbekov A, Harbert JL et al (2020) Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med 8:681–686. https://doi.org/10.1016/S2213-2600(20)30243-5
Grillet F, Behr J, Calame P et al (2020) Acute pulmonary embolism associated with COVID-19 pneumonia detected by pulmonary CT angiography. Radiology. https://doi.org/10.1148/radiol.2020201544
Tang N, Li D, Wang X, Sun Z (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:844–847. https://doi.org/10.1111/jth.14768
Varga Z, Flammer AJ, Steiger P et al (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395:1417–1418
Lindner D, Fitzek A, Bräuninger H et al (2020) Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.3551
Huang L, Zhao P, Tang D et al (2020) Cardiac involvement in patients recovered from COVID-2019 identified using magnetic resonance imaging. JACC Cardiovasc Imaging. https://doi.org/10.1016/j.jcmg.2020.05.004
Puntmann VO, Carerj ML, Wieters I et al (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. https://doi.org/10.1001/jamacardio.2020.3557
Knight DS, Kotecha T, Razvi Y et al (2020) COVID-19: myocardial injury in survivors. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.120.049252
Acknowledgements
The investigators thank the staff of the departments of cardiology, radiology, geriatric, and anesthesiology and intensive care, and Louise Saitta (General Electric) for their technical support.
Funding
COCARDE is an investigator-initiated trial funded by department source (cardiology, geriatric, and anesthesiology and intensive care departments). General Electric supported the study by loaning a Vivid E95 ultrasound system for the inclusion period.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Olivier Lairez has received personal compensation for speaking with General Electric. The other co-authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lairez, O., Blanchard, V., Houard, V. et al. Cardiac imaging phenotype in patients with coronavirus disease 2019 (COVID-19): results of the cocarde study. Int J Cardiovasc Imaging 37, 449–457 (2021). https://doi.org/10.1007/s10554-020-02010-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-02010-4