Abstract
Purpose
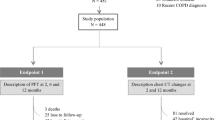
The aim of this study was to assess respiratory function at the time of clinical recovery, 6 weeks, 6 months, and 12 months after discharge in patients surviving to COVID-19 pneumonia.
Methods
Our case series consisted of 13 hospitalized patients with COVID-19 pneumonia.
Results
Baseline pulmonary function tests were 55.7 ± 15.6 for FEV1%, 68.6 ± 16.0 for FVC%, and 1.2 ± 0.1 for FEV1/FVC%. Although pulmonary function showed a small improvement after 6 weeks, patients experienced a more significant improvement after 6 and 12 months in FEV1% (95.4 ± 13.7 and 107.2 ± 16.5, respectively; p < 0.001), FVC% (91.3 ± 14.5, and 105.9 ± 15.6, respectively; p < 0.001), and FEV1/FVC% values (1.04 ± 0.04, and 1.01 ± 0.05, respectively; p < 0.001).
Conclusion
COVID-19 pneumonia may result in significant alterations in lung function, with a mainly restrictive pattern, partly persisting at 6 weeks after recovery from acute phase, but significantly improving during a 12-month follow-up period.
Similar content being viewed by others
Introduction
COVID-19 pneumonia is known to carry a high risk of severe course and intensive care unit admission, but it may also lead to long-term burdening sequelae. Therefore, it is particularly important to explore COVID-19 clinical characteristics, which may help to manage properly its consequences in the post-acute phase. It is worth noting that evidence about pulmonary function tests among COVID-19 patients was very limited until few months ago when it was shown that six-week respiratory rehabilitation may improve respiratory function, quality of life and anxiety of older patients [1]. We recently reported that COVID-19 pneumonia may result in clinically relevant alterations in pulmonary function tests, with a restrictive pattern in 10 out of 13 patients at the time of hospital discharge [2]. Additionally, pulmonary function was found to improve at 6-week follow-up, but some degree of restrictive alteration still persisted [2]. Long-lasting alterations in pulmonary function, including restrictive syndrome and reduced diffusing capacity of the lungs for carbon monoxide (DLCO), were also observed in a variable proportion of patients surviving to COVID-19 pneumonia during a 30-day to 6-month follow-up period [3,4,5].
In the present study, we aimed at extending follow-up until 12 months to investigate long-term changes in pulmonary function among patients surviving to COVID-19 pneumonia.
Methods
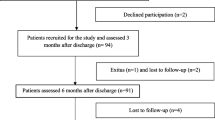
Our study included 13 adult patients with COVID-19 bilateral pneumonia admitted to the respiratory acute care ward at IRCCS INRCA hospital in Merate (Lombardy, Italy), between March 14 and April 14, 2020. Methods were previously described [2]. Briefly, inclusion criteria were the ability to provide written informed consent to participate in the study and perform pulmonary function tests correctly. The study was approved by the Ethics Committee of the IRCCS INRCA.
COVID-19 bilateral pneumonia was diagnosed by positive polymerase chain reaction (PCR) testing on nasopharyngeal swab and presence of bilateral lung infiltrates on chest X-ray upon admission.
Patient history, body mass index (BMI), smoking habit, signs and symptoms, complete laboratory panel and setting transitions were collected.
Chest high-resolution computed tomography (CT), spirometry, 2-min walking test and arterial blood gas analysis at the time of clinical recovery (i.e., the day before discharge) were included in the study. Clinical recovery was defined by the presence of all of the following: absence of fever for at least 48 h, PaO2 greater than 60 mmHg on arterial blood gas testing on room air, and negative C-reactive protein (CRP) on two consecutive blood samples performed at least 48 h apart.
Two-minute walking test was performed on room air under the supervision of a respiratory therapist in the patients' room. Nocturnal pulse oximetry was also recorded on room air. PalmSAT 2500 pulse oximeters were used for recordings (Nonin, USA).
Arterial blood gases analysis was performed regularly during hospitalization to monitor oxygen requirements by Cobas b 123 (Roche, Switzerland) point-of-care testing system.
Pulmonary function tests were performed using Microlab portable spirometer (Viasys Healthcare, USA). Forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio were included in the analysis. Correct performance of forced expiration was ensured by medical personnel who observed the patients at security distance to minimize the risk of infection due to droplet spreading.
At the time of hospital discharge, all patients received the prescription of a home low-intensity physical exercise program [6], consisting of daily sit–stand and walking exercises during 6 weeks. Data about respiratory drugs taken during follow-up period were also collected. Pulmonary function tests were repeated 6 weeks, 6 months and 12 months after discharge. During 6- and 12-month follow-up visits, 2-min walked distance was also recorded and included in the analysis. Lung CT scan was also repeated at 6-month follow-up.
Descriptive data were presented as mean ± SD for continuous variables or number (percentage) for categorical ones. Paired data t test was used when appropriate. Statistical analysis was carried out by SPSS V.24 statistical software package (SPSS for Windows V24, SPSS Inc., Chicago, IL, USA).
Results
Overall, patients enrolled in the study were aged 57.8 ± 10.0 years (range 34–73 years), almost exclusively male (12 patients, 92.3%) and no smokers. Most frequent comorbidities were obesity (7/13), hypertension (3/13), coronary artery disease (1/13), asthma (1/13), diabetes (1/13), atrial fibrillation (1/13), dementia (1/13) and stroke (1/13). Length of hospital stay was 29.5 ± 9.8 days.
At the time of clinical recovery, average PaO2 was 68.8 ± 7.1 mmHg, with mild to moderate hypoxemia in 7 out of 13 patients, while PaCO2 was 35.5 ± 3.6 mmHg, SaO2 95.9 ± 1.7%, HCO3 24.5 ± 1.5 mmol/l and pH 7.4 ± 0.01. The 2-min walked distance was 134.4 ± 61.6 m, and values less than 100 m were observed in 4 patients. A limited burden of dyspnea (Borg scale 2.5 ± 1.8) and fatigue (Borg scale 1.5 ± 1.7) was observed. Nevertheless, average nocturnal SaO2 was 91.2 ± 1.8%, with clinically relevant night-time hypoxemia in 7 out of 13 patients.
Figure 1 shows pulmonary function variables of patients studied. Baseline pulmonary function tests were 55.7 ± 15.6 for FEV1%, 68.6 ± 16.0 for FVC%, and 1.2 ± 0.1 for FEV1/FVC%. After 6 weeks, a significant improvement in FEV1% and FVC% values was observed (FEV1%: 68.6 ± 16.0, FVC%: 64.3 ± 13.5, p < 0.05), while increase in FEV1/FVC% was not significant (1.1 ± 0.1, p = 0.1). Compared to baseline values, patients experienced a more significant improvement after 6 and 12 months in FEV1% (95.4 ± 13.7 and 107.2 ± 16.5, respectively; p < 0.001), FVC% (91.3 ± 14.5, and 105.9 ± 15.6, respectively; p < 0.001), and FEV1/FVC% values (1.04 ± 0.04, and 1.01 ± 0.05, respectively; p < 0.001). Similar findings were observed when using absolute values of spirometric parameters (Fig. 1). Additionally, performance in 2MWD improved from 134.4 ± 61.6 at baseline, to 168 ± 30.4 at 6 months (p 0.11) to 183 ± 35.2 (p = 0.03). Only one patient affected by asthma was treated with inhaled beclomethasone/formoterol (already regularly taken before admission for COVID-19 pneumonia) during follow-up period. Baseline CT scan at the time of clinical recovery showed persistent multifocal ground glass opacities in 12 patients, crazy paving in 6 patients, linear opacities in 7 patients and consolidation pattern associated to multifocal ground glass opacities in 5 patients. After 6 months, lung parenchymal fibrosis bands were observed in 9 out of 13 patients, while multifocal ground glass opacities were still observed in only 6 patients.
Discussion
Our study shows that COVID-19 pneumonia may result in clinically relevant restrictive pattern alterations in pulmonary function tests in 10 out of 13 patients at the time of hospital discharge. During follow-up, pulmonary function improved significantly. Even if some degree of restrictive alteration still persisted at 6-week follow-up, a more evident improvement in pulmonary function could be observed at 6- and 12-month follow-up visits.
Our findings further suggest that survivors to COVID-19 pneumonia should be carefully screened for pulmonary function and rehabilitation needs at the end of acute phase, and eventually referred to specific care pathways to monitor and manage clinically relevant sequelae during follow-up.
Interestingly, recovery of pulmonary function of COVID-19 patients seems to be quietly different from that observed in other atypical pneumonias. Pulmonary function tests were found to improve significantly in the first 3 months but with no further significant improvement from 3 to 6 months after discharge among survivors to severe influenza A (H1N1) pneumonia [7], and other studies showed a complete normalization of pulmonary function 6 months after H1N1-related acute respiratory distress syndrome (ARDS) [8]. At variance, about 80% of survivors to ARDS not caused by influenza A H1N1 had reduced diffusing capacity, 20% had airway obstruction, and 20% had restrictive pattern 12 months after recovery [9].
Finally, the impact of respiratory rehabilitation is worth of testing. Indeed, our patients underwent a simple home exercise program after discharge. However, respiratory rehabilitation can effectively improve respiratory function in older patients with COVID-19 [1], and recent evidence suggests that multidisciplinary respiratory rehabilitation may significantly improve respiratory function, blood gases and ability to exercise, even among patients with relevant preexisting cardiorespiratory comorbidities [10]. Thus, it is conceivable that a more intensive and multidisciplinary approach to respiratory rehabilitation may speed up recovery and reduce long-term burden of COVID-19 pneumonia, which will make necessary to rethink the pneumology services with an increase in the availability of respiratory rehabilitation units in the areas most violently affected by the pandemic.
The small sample size, the simple spirometric approach and the lack of DLCO and plethysmographic data are main limitations of the present study. Additionally, pulmonary function tests before COVID-19 infection are not available for our patients. Finally, smokers and women were not included in our study. Thus, it is desirable that future studies contribute to remove these caveats. Nevertheless, our results add to the present knowledge by showing that a home low-intensity exercise program may lead to a slow recovery of pulmonary function and further strengthening the need of prescribing more intensive multidisciplinary rehabilitation cycles in patients who have a slow recovery from COVID-19 [10].
In conclusion, COVID-19 pneumonia may result in significant alterations in lung function, with a mainly restrictive pattern, partly persisting at 6 weeks after recovery from acute phase, but significantly improving during a 6-month period. Low-intensity physical exercise may help to improve pulmonary function, but multidisciplinary pulmonary rehabilitation contributes to reduce recovery time and burden of COVID-19 pneumonia sequelae.
Data availability
Data are locally available for participating researchers and stored in the data repository of the IRCCS INRCA.
References
Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39:101166.
Fumagalli A, Misuraca C, Bianchi A, Borsa N, Limonta S, Maggiolini S, et al. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection. 2021;49:153–7.
Fortini A, Torrigiani A, Sbaragli S, Lo Forte A, Crociani A, Cecchini P, et al. COVID-19: persistence of symptoms and lung alterations after 3–6 months from hospital discharge. Infection. 2021.
Wu Q, Zhong L, Li H, Guo J, Li Y, Hou X, et al. A follow-up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J. 2021;2021:6692409.
Ordinola Navarro A, Cervantes-Bojalil J, Cobos Quevedo OJ, Avila Martinez A, Hernandez-Jimenez CA, Perez Alvarez E, et al. Decreased quality of life and spirometric alterations even after mild-moderate COVID-19. Respir Med. 2021;181:106391.
Chinese Association of Rehabilitation M, Respiratory Rehabilitation Committee of Chinese Association of Rehabilitation M, Cardiopulmonary Rehabilitation Group of Chinese Society of Physical M, Rehabilitation. Recommendations for respiratory rehabilitation of coronavirus disease 2019 in adult. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:308–14.
Hsieh MJ, Lee WC, Cho HY, Wu MF, Hu HC, Kao KC, et al. Recovery of pulmonary functions, exercise capacity, and quality of life after pulmonary rehabilitation in survivors of ARDS due to severe influenza A (H1N1) pneumonitis. Influenza Other Respir Viruses. 2018;12:643–8.
Toufen C Jr, Costa EL, Hirota AS, Li HY, Amato MB, Carvalho CR. Follow-up after acute respiratory distress syndrome caused by influenza a (H1N1) virus infection. Clinics (Sao Paulo). 2011;66:933–7.
Orme J Jr, Romney JS, Hopkins RO, Pope D, Chan KJ, Thomsen G, et al. Pulmonary function and health-related quality of life in survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2003;167:690–4.
Maniscalco M, Fuschillo S, Ambrosino P, Martucci M, Papa A, Matera MG, et al. Preexisting cardiorespiratory comorbidity does not preclude the success of multidisciplinary rehabilitation in post-COVID-19 patients. Respir Med. 2021;184:106470.
Funding
None.
Author information
Authors and Affiliations
Contributions
Study design-AF, CM, DC; Literature Search-AC, MDR, LS; Data Collection-AF, CM AB, NB, SL, SM, DRB, DC; Data Analysis-AC, MDR, LS; Drafting Paper-AF, CM, AC, MDR, LS, FL; Manuscript Reviewing-all authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare to have no conflict of interest/competing interest to disclose with this manuscript.
Ethics approval
The study was approved by the Ethics Committee of the Italian National Research Center on Aging (IRCCS INRCA), study #20008/2020 and deliberation #141/DGEN/2020.
Consent to participate
All patients signed a written informed consent to be enrolled in the study.
Rights and permissions
About this article
Cite this article
Fumagalli, A., Misuraca, C., Bianchi, A. et al. Long-term changes in pulmonary function among patients surviving to COVID-19 pneumonia. Infection 50, 1019–1022 (2022). https://doi.org/10.1007/s15010-021-01718-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01718-2