Cautious Evaluation of Weak SARS-CoV-2 Positive Cases on Xpert Xpress SARS-CoV-2: Formulating a Testing Algorithm in a Hospital Setup
Article Information
Tavisha Dama1*, Shashikala Shivaprakash1, Neetu Biyani1 and Pratiksha Chheda1
1Molecular Diagnostics, Laboratory Medicine, Sir HN Reliance Foundation Hospital, Mumbai, Maharashtra, India
*Corresponding Author: Tavisha Dama, Laboratory Medicine, Sir HN Reliance Foundation Hospital, Mumbai, Maharashtra, India.
Received: 14 October 2022; Accepted: 02 November 2022; Published: 25 November 2022
Citation: Tavisha Dama, Shashikala Shivaprakash, Neetu Biyani and Pratiksha Chheda. Cautious Evaluation of Weak SARS-CoV-2 Positive Cases on Xpert Xpress SARS-CoV-2: Formulating a Testing Algorithm in a Hospital Setup. Archives of Clinical and Medical Case Reports 6 (2022): 747-751.
View / Download Pdf Share at FacebookAbstract
Objectives: Due to the sensitivity of the Xpert Xpress SRAS-CoV-2 kit (LOD-0.0200 PFU/ml) and the presence of residual viral RNA from past infections, reporting of higher Ct values is a challenge. Formulating a testing algorithm became important in order to maintain infection control measures in our facility.
Materials and Methods: A retrospective analysis of 747 cases that were tested, between July 2020 to September 2021, by both Xpert Xpress SARS-Cov-2 kit and COVID-19 one-step RT-PCR kit (Meril Diagnostics) (reference method) was carried out. Samples with Ct≥36 on Xpert Xpress SARS-CoV-2 were evaluated further to rule out false positives by correlating with infection history and follow-up data. Data were analysed using Microsoft Excel’s built in Data Analysis Tool Pack capability.
Results: When compared with an ICMR approved kit (COVID-19 onestep RT-PCR kit), gold standard, the Xpert Xpress SARS-COV-2 kit was found to have POA of 70.6% (95% CI: 67.2% to 73.8%), PPA of 100% (95% CI: 97.9% to 100%) and NPA of 61.2% (95% CI: 57% to 65.2%). For cases with Ct ranging from 15-35, results were 100% concordant between Xpert Xpress SARS-CoV-2 and rt-PCR (n=388), however, there was a decrease in concordance as the Ct values increased on Xpert Xpress SARS-CoV-2 (288 cases with Ct≥36). Further evaluation of discordant cases (n=212) revealed 21.2% (45/212) had infection history. Of the remaining 167 cases, follow up swabs were obtained for 42.5% (71/167), out of which 8.4% (6/71) were found to have active infections.
Conclusions: Xpert Xpress SARS-Cov-2 kit can be used as a point of care device in units where urgent results are important. Using an arbitrary cutoff of Ct-36, a testing algorithm was devised where samples with Ct>36 on Xpert Xpress SARS-CoV-2
Keywords
COVID-19; infection; Viral Transport Medium; Xpert Xpress SRAS-CoV-2 kit
Article Details
1. Introduction
COVID-19 (Coronavirus disease 2019) is an infectious respiratory disorder caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). The symptoms are variable but mostly include fever, dry cough, breathing difficulties, fatigue and loss of sense of smell and taste. The first case was identified in Wuhan, China in December 2019 which then went on to spreading worldwide leading to a pandemic. Due to the pandemic and the chaos that followed, several companies designed kits to detect the SARS-CoV-2 virus from nasal, nasopharyngeal, oropharyngeal swabs in viral transport medium (VTM). The kits designed detect either the viral antigen by rapid chromatographic immunoassay technique or the viral RNA by various techniques including real-time rt-PCR reverse transcription polymerase chain reaction), NAAT (nucleic acid amplification test), isothermal amplification, etc. Our hospital being a COVID-19 free facility operationally, extreme infection control measures were undertaken. Contact tracing was carried out for any staff/patient that tested positive for SARS-CoV-2. Upon admission into ER or before admission into the hospital for other procedures, dialysis, etc. all patients were tested for SARS-CoV-2. If positive, they were shifted to an attached facility dedicated for the treatment and management of COVID-19 patients. Testing was carried out using real-time rt-PCR for the qualitative detection of SARS-CoV-2 viral RNA, considered as the gold standard and used for the confirmed diagnosis of COVID-19. Several point of care tests have been designed to get faster results in a hospital setup. Among these, the Cepheid Xpert Xpress SARS-CoV-2 kit (Xpert) has been widely used partly due to the availability of the GeneXpert platform in most healthcare centres and minimal expertise required. The Xpert Xpress SARS-CoV-2 kit makes use of automated real-time reverse transcription technique to rapidly amplify the pansarbecovirus E gene (envelope) and the N2 gene region of the N gene (nucleocapsid) specific to SARS-CoV-2. Studies have been carried out that have shown a correlation between Ct value and infectivity where patients with Ct>34 have been shown to be less likely to spread infection as it was not possible to culture the virus in such cases [1, 2]. Studies have also been carried out that compared the Xpert Xpress SARS-CoV-2 assay with rt-PCR and found discrepancy in results at higher Ct values [3]. Thus, samples with higher Ct values need to be evaluated with caution. This study aimed to evaluate the utility of the Xpert Xpress SARS-CoV-2 in routine clinical setting as a point of care device considering real-time rt-PCR assay, using a kit approved by the Indian Council of Medical Research (ICMR), as a reference method. Due to sensitivity of the Xpert Xpress SARS-CoV-2 (LOD-0.0200 PFU/ml) and the presence of residual RNA from previous infections, reporting of higher Ct values with Xpert Xpress SARS-CoV-2 was evaluated to come up with a testing algorithm in our facility.
2. Materials and Methods
A retrospective analysis on a total of 747 nasopharyngeal and oropharyngeal swabs from inpatients and outpatients tested by both Xpert Xpress SARS-CoV-2 kit on Genexpert Dx Platform (closed system) and real-time rt-PCR (open system), between July 2020 to September 2021 was carried out. The swabs were transported in Viral Transport Medium (VTM) (HiMedia, India). RNA extraction for the rt-PCR assay was carried out using automated extraction platform KingFisher Flex system using the MagMax II Viral/Pathogen nucleic acid isolation kit (Thermo Fisher, Massachusettes, United States). PCR was carried out using an ICMR approved kit, COVID-19 one-step RT-PCR kit (Meril Diagnostics), targets the ORF (FAM) and N (HEX) genes. It also targets the RNAase P (ROX) gene which is an endogenous internal control to check for accuracy in sampling and extraction. The cycling reaction was carried out either using Rotor Gene Q (Qiagen, Hilden, Germany) or Quant Studio 5 (Foster City, California, United States) thermal cyclers. Samples with Ct>35 by rt-PCR were considered negative (as per manufacturer’s protocol). The Xpert Xpress SARS-CoV-2 kit targets N2 and E genes and includes a sample processing control (SPC), checks for adequate processing of the sample, monitors the presence of potential inhibitors and ensures that the RT-PCR reaction conditions (temperature and time) are appropriate for the amplification reaction. A probe check control (PCC) is additionally present to verify reagent rehydration, PCR tube filling, etc. The results are obtained automatically from the software as positive (N2 gene amplification present, E gene amplification present/absent), presumptive positive (only E gene amplification present) or negative (N2 and E gene amplification absent). Both the rt-PCR kit and the Xpert Xpress SARS-CoV-2 kit amplify N gene as the common gene. Samples with Ct≥36 by Xpert Xpress SARS-CoV-2 were evaluated further by comparison with rt-PCR results. The presumptive positive cases were retested (as per manufacturer’s protocol), if the retest result was presumptive positive again, the results were confirmed by rt-PCR. Percent overall agreement (POA), positive percent agreement (PPA), negative percent agreement (NPA), negative predictive value (NPV) and positive predictive value (PPV) were calculated. Data were analysed using Microsoft Excel’s built in Data Analysis Tool Pack.
3. Results
A total of 747 cases tested by both Xpert Xpress SARS-CoV-2 and rt-PCR were analyzed and the results were compared using rt-PCR as the reference method.
3.1 Comparison with Reference Method
Out of a total of 747 samples analysed by Xpert Xpress, 388 (51.94%) were found to be positive with Ct values ranging from 15 to 44, 334 (44.71%) were clear negatives with no amplification of either N2 or E genes and 25 (3.34%) were presumptive positive (E gene Ct value ranging from 38 to 42). All the presumptive positive cases were negative by rt-PCR. The 334 cases that were clearly negative by Xpert Xpress SARS-CoV-2 were also negative by rt-PCR (100% concordance). Of the 388 cases that were positive by Xpert Xpress SARS-CoV-2, 176 (48.36%) cases were positive and 212 (54.63%) were negative by rt-PCR. The percent overall agreement (POA) between the two methods was found to be 70.6% (95% CI: 67.2% to 73.8%) (Table 1). The overall positive percent agreement (PPA) was found to be 100% (95% CI: 97.9% to 100%) and the overall negative percent agreement (NPA) was found to be 61.2% (95% CI: 57% to 65.2%). A negative predictive value (NPV) of 100% and a positive predictive value (PPV) of 45.36% (95% CI: 42.77% to 47.98%) were observed.
|
rtPCR results |
||||
|
Positive |
Negative |
Total |
||
|
Xpert Xpress SARS-CoV-2 |
Positive |
176 |
212 |
388 |
|
Negative |
0 |
334 |
334 |
|
|
Total |
176 |
546 |
722 |
|
|
Positive Predictive Agreement (PPA) |
100% (95% CI: 97.9% to 100%) |
|||
|
Negative Predictive Agreement (NPA) |
61.2% (95% CI: 57% to 65.2%) |
|||
|
Percent Overall Agreement (POA) |
70.6% (95% CI: 67.2% to 73.8%) |
|||
|
Positive Predictive Value (PPV) |
45.36% (95% CI: 42.77% to 47.98%) |
|||
|
Negative Predictive Value (NPV) |
100% |
|||
Table 1: Comparison between Xpert Xpress SARS-CoV-2 kit and rt-PCR (ICMR approved kit COVID-19 one-step RT-PCR kit, Meril Diagnostics).
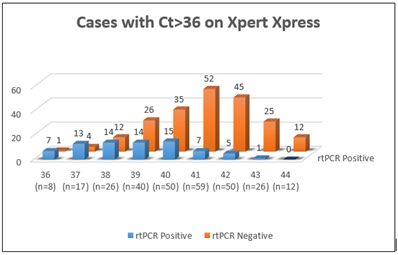
3.2 Evaluation of Cases with Ct≥36 on Xpert Xpress SARS-CoV-2
Due to the low NPA, the discordant cases i.e. the cases that were positive by Xpert Xpress SARS-CoV-2 and negative by rt-PCR were evaluated further. For samples with Ct < 36 (n=100) there was 100% concordance between Xpert Xpress SARS-CoV-2 and rt-PCR. For samples with Ct=36 (n=8) by Xpert Xpress SARS-CoV-2, 12.5% cases (1/8) showed negative result by rt-PCR. A total of 280 cases had Ct greater than 36. It was observed that for higher Ct values (Ct>36) the concordance between Xpert Xpress SARS-CoV-2 and rt-PCR kept decreasing. Figure 1 shows samples with Ct ≥36 (n=288) by Xpert Xpress SARS-CoV-2 and their corresponding results by rt-PCR. Out of these, 212 were clearly negative by rt-PCR. Each of these 212 cases was evaluated to explain the discrepancy in results. It is important to note that 45 of these 212 negative cases had a previous positive history indicating that the Ct value showing up by Xpert was that of residual viral RNA still present in those patients, further confirming the sensitivity of genexpert. The rest of the 167 discrepant cases were asked to submit a follow-up swab after 24-48 hours. Follow-up swabs were received for 71 cases for rt-PCR, out of which 6 were positive and 65 were negative.
4. Discussion
Due to its sensitivity and availability in most healthcare centres, the utility of Xpert Xpress SARS-CoV-2 was evaluated in a clinical setting as a point of care device. We used an open system (rt-PCR) as a reference method in our study and a comparison between the two revealed a POA of 70.6% (95% CI: 67.2% to 73.8%), PPA of 100% (95% CI: 97.9% to 100%) and NPA of 61.2% (95% CI: 57% to 65.2%). Other studies have been carried out that evaluated the efficacy of Cepheid Xpert Xpress SARS-CoV-2. Dinnes et. al. [4] conducted a review of rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection (18 studies, n=871). They reported a PPA of 77% (95% CI 69% to 84%) and a NPA of 100% for Xpert Xpress SARS-CoV-2 when compared with various rapid antigen and rt-PCR tests. Zhen et. al. [5] (n=108) compared three closed platforms: Cepheid Xpert Xpress SARS-CoV-2, Abbott ID NOW COVID-19 and GenMark ePlex SARS-CoV-2 and found that Xpert Xpress SARS-CoV-2 showed a PPA of 98.3% followed by ePlex (91.4%) and ID NOW (87.7%) when compared with Hologic Panther Fusion SARS-CoV-2 assay, all three assays showed a NPA of 100%. However, the cut off Ct value used for analysis in these studies was not known. Similarly, Moran et. al. [6] carried out a study on 103 specimens and observed a 99% agreement between Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. They found 1 sample that had a Ct value of 42 with Xpert Xpress SARS-CoV-2 and was negative with Roche cobas SARS-CoV-2. We found a 100% concordance between Xpert Xpress SARS-CoV-2 and rt-PCR for samples with Ct<36 and a discordance of 28.3% (212/747) observed at higher Ct values (>36) where cases that were positive by Xpert Xpress SARS-CoV-2 were negative by rt-PCR resulting in a low NPA. This can also be attributed to the difference in the limits of detection of both the methods [7]. It was observed that as the viral load decreased, a decrease in result concordance was observed. A study conducted by Lowe et. al. [8], on a small sample set (n=37), observed an overall concordance of 86.5 % (32/37) with 100 % concordance for samples with Ct values ranging between 30−33.9. The discordant samples with Ct≥34 was reported as 22.7 % (5/22). Another study carried out by Naina R et. al. [7] also commented on cases with higher Ct values where they also compared the Ct values between Xpert Xpress SARS-CoV-2 and rt-PCR and found that although the sensitivity (PPA) of the assay was 100%, the specificity (NPA) was 80%. It becomes difficult to tell if these higher Ct values are true positives with low viral particles or residual viral RNA. On evaluation of the discordant cases (all had Ct≥36), it was observed that 21.2% cases (45/212) had a history of COVID-19 indicating that the amplification was from the residual virus still present in these patients. Thus, Xpert Xpress SARS-CoV-2 was able to pick up residual virus for long durations after active infection. Of the 78.7% (167/212) cases that were asked for a fresh swab after 24-48 hours, follow-up swabs were received for only 42.5% (71/167) out of which 8.4% (6/71) were positive by rt-PCR and the rest were negative (65/71). This finding also indicated that cases of early infection were being detected by Xpert Xpress SARS-CoV-2 and only active infected cases were being detected by rt-PCR which can be helpful in mitigating the spread and implementing infection control measures in a timely manner.
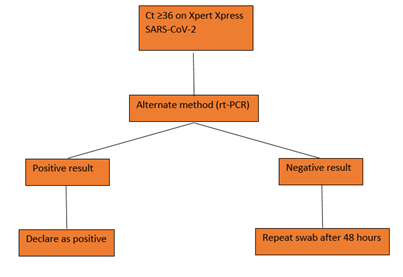
5. Conclusion
After gaining sufficient experience and coming across samples with varying viral burden of SARS-CoV-2 during the COVID-19 pandemic, it was challenging to report cases with low viral loads. Owing to the findings from this study, an algorithm (figure 2) was formulated for testing staff and patients upon admission into the ER or before admission into the hospital for other procedures in order to maintain the COVID-free status of the facility. We used an arbitrary cut-off of 36 where if cases with Ct> 36 were obtained by testing with Xpert Xpress SARS-CoV-2, the test was repeated by rt-PCR using the same swab. On repeating, a positive result was declared as positive, however, if on repeat a negative result was obtained then the patient was asked to submit a fresh swab after 48 hours. If the patient had a known COVID-19 history then they were asked to submit follow-up swabs for rt-PCR only and not for genexpert. This algorithm helped us in devising a testing strategy during subsequent waves which further helped in implementing infection control measures in a timely manner in our facility. Thus, it can be concluded that the Cepheid Genexpert system is an excellent point of care device to be used for the detection of SARS-CoV-2 and should be used in the emergency departments and ICUs where urgent reports are of utmost importance. It can also be concluded that COVID-19 history of a patient must be taken into consideration before using Xpert Xpress SARS-CoV-2 kit for detection. Cases with higher Ct values should be evaluated and confirmed using an alternate method.
References
- La Scola B, Le Bideau M, Andreani J, et al. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. European Journal of Clinical Microbiology & Infectious Diseases 39 (2020): 1059-1061.
- Rhee C, Kanjilal S, Baker M, et al. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectivity: When is it safe to discontinue isolation?. Clinical Infectious Diseases 72 (2021): 1467-1474.
- Rashmita Das R, Joshi S, Pednekar S, et. al. Comparison of Xpert Xpress SARS-CoV-2 assay and RT-PCR test in diagnosis of COVID-19. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) 20 (2021): 12-17.
- Dinnes J, Deeks JJ, Adriano A, et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database of Systematic Reviews (2020): CD013705.
- Zhen W, Smith E, Manji R, et al. Clinical evaluation of three sample-to-answer platforms for detection of SARS-CoV-2. J Clin Microbiol 58 (2020): e00783-20.
- Moran A, Beavis KG, Matushek SM, et al. 2020. Detection of SARS-CoV-2 by use of the Cepheid Xpert Xpress SARS-CoV-2 and Roche cobas SARS-CoV-2 assays. J Clin Microbiol 58 (2020): e00772-20.
- Rakotosamimanana N, Randrianirina F, Randremanana R, et al. GeneXpert for the diagnosis of COVID-19 in LMICs. Lancet Glob Health 8 (2020): e1457-e1458.
- Lowe CF, Matic N, Ritchie G, et al. Detection of low levels of SARS-CoV-2 RNA from nasopharyngeal swabs using three commercial molecular assays. Journal of Clinical Virology 128 (2020): 104387.


 Impact Factor: * 3.1
Impact Factor: * 3.1 CiteScore: 2.9
CiteScore: 2.9  Acceptance Rate: 11.01%
Acceptance Rate: 11.01%  Time to first decision: 10.4 days
Time to first decision: 10.4 days  Time from article received to acceptance: 2-3 weeks
Time from article received to acceptance: 2-3 weeks 
