Hong Kong Med J 2021;27:Epub 1 Apr 2021
Hong Kong Academy of Medicine. CC BY-NC-ND 4.0
ORIGINAL ARTICLE
Findings from the first public COVID-19 temporary test centre in Hong Kong
Will LH Leung, MB, ChB, FHKAM (Family Medicine)1; Ellen LM Yu, BSc (Stat & Fin), MSc (Epi & Biostat)2; SC Wong, MNurs3; M Leung, PhD4; Larry LY Lee, MB, BS (NSW), FHKAM (Emergency Medicine)5; KL Chung, MB, BS, FHKAM (Emergency Medicine)6; Vincent CC Cheng, MB, BS, MD7
1 Department of Family Medicine and Primary Health Care, Kowloon West Cluster, Hospital Authority, Hong Kong
2 Clinical Research Centre, Princess Margaret Hospital, Hong Kong
3 Infection Control Team, Queen Mary Hospital, Hong Kong West Cluster, Hospital Authority, Hong Kong
4 Central Nursing Department, Hospital Authority, Hong Kong
5 Department of Accident and Emergency, Tin Shui Wai Hospital, Hong Kong
6 Quality & Safety Division, Hospital Authority, Hong Kong
7 Department of Microbiology, Queen Mary Hospital, Hong Kong
Corresponding author: Dr Will LH Leung (llh864@ha.org.hk)
Abstract
Introduction: The Hospital Authority of Hong
Kong Special Administrative Region established a
coronavirus disease 2019 (COVID-19) temporary
test centre at the AsiaWorld-Expo from March 2020
to April 2020, which allowed high-risk individuals to
undergo early assessment of potential severe acute
respiratory syndrome coronavirus 2 (SARS-CoV-2)
infection. This study reviewed the characteristics
and outcomes of individuals who attended the centre
for COVID-19 testing.
Methods: This retrospective cross-sectional
study collected epidemiological and clinical data.
The primary outcome was a positive or negative
SARS-CoV-2 test result, according to reverse
transcription polymerase chain reaction analyses of
pooled nasopharyngeal and throat swabs collected at
the centre. The relationships of clinical characteristics
with SARS-CoV-2 positive test results were assessed
by multivariable binary logistic regression.
Results: Of 1258 attendees included in the analysis,
86 individuals tested positive for SARS-CoV-2
infection (positivity rate=6.84%; 95% confidence
interval [CI]=5.57%-8.37%). Of these 86 individuals,
40 (46.5%) were aged 15 to 24 years and 81 (94.2%)
had a history of recent travel. Symptoms were
reported by 86.0% and 96.3% of individuals with
positive and negative test results, respectively. The
clinical characteristics most strongly associated with
a positive test result were anosmia (adjusted odds
ratio [ORadj]=8.30; 95% CI=1.12-127.09) and fever (ORadj=1.32; 95% CI=1.02-3.28).
Conclusion: The temporary test centre
successfully helped identify individuals with
COVID-19 who exhibited mild disease symptoms.
Healthcare providers should carefully consider
the epidemiological and clinical characteristics
of COVID-19 to arrange early testing to reduce
community spread.
New knowledge added by this study
- A temporary test centre during the coronavirus disease 2019 (COVID-19) pandemic was effective for the identification of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among individuals who exhibited mild disease symptoms.
- At the temporary test centre at AsiaWorld-Expo, a greater proportion of infected individuals were aged 15 to 24 years (46.5%), compared with the proportion (26.7%) in a previously described age-matched population in Hong Kong, presumably because of the targeted testing strategy used at the centre.
- In our relatively healthy population of individuals with mild disease symptoms and epidemiological linkage to COVID-19, 6.84% had positive test results.
- In some individuals, COVID-19 causes mild initial symptoms despite its high infectivity; thus, there is a need for early identification of individuals with SARS-CoV-2 who exhibit mild symptoms.
- The temporary test centre was successful in identifying infected individuals in a large-scale, high-turnover setting, thereby reducing the testing burden in secondary and tertiary healthcare facilities.
- Gatekeeping healthcare providers should carefully consider the epidemiological and clinical manifestations of COVID-19 and be vigilant in arranging appropriate early testing to reduce community spread.
Introduction
Patients with coronavirus disease 2019 (COVID-19),
including those with mild or no symptoms, may
readily transmit the disease given the high person-to-person infectivity in the latent period of
COVID-19; this transmission could threaten
public health.1 Severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2), the causative virus
of COVID-19, replicates efficiently in the upper
respiratory tract and appears to cause delayed onset of
symptoms; therefore, COVID-19 poses considerable
challenges to the health system.2 3 Thus, there is a
need to rapidly identify infected individuals who
exhibit only mild symptoms. In March to April 2020,
the Hospital Authority, Hong Kong, established a
temporary test centre (TTC) at the AsiaWorld-Expo
(AWE), which is within the Hong Kong International
Airport complex on Lantau Island. The AWE TTC
offered tests for individuals with mild symptoms
among those arriving at the airport, as well as those
engaged in home quarantine in Hong Kong, for the
early detection of SARS-CoV-2 infection that could
be managed by early isolation and intervention.4
Asymptomatic individuals were tested at a different
facility within AWE operated by the Department of
Health. This study reviewed the characteristics and
outcomes of individuals who attended the AWE
TTC for COVID-19 testing.
Methods
This retrospective cross-sectional study evaluated the characteristics and outcomes of individuals who
attended the AWE TTC during its operation from
20 March 2020 to 19 April 2020. All individuals who
attended the AWE TTC were included, with the
exception of patients who were transferred out of the
AWE TTC to accident and emergency departments
before they could undergo COVID-19 testing.
Infection control measures implemented at the AWE
TTC were reported previously.5 Ethics approval was
obtained from the Kowloon West Cluster Research
Ethics Committee, Hospital Authority.
Clinical characteristics assessed in this study
were fever, chills, cough, runny nose, sore throat,
vomiting, diarrhoea, fatigue, myalgia, headache,
anosmia, history of hypertension, history of diabetes
mellitus, history of chronic respiratory disease, and
history of malignancy. Epidemiological parameters
assessed in this study were age, sex, district of
residence, travel history, occupational exposure,
contact history, and clustering history. These data
were collected using a standard clinical assessment
template by duty medical officers in the Clinical
Management System of the Hospital Authority.
The primary outcome was positive or negative
SARS-CoV-2 test results, according to reverse
transcription polymerase chain reaction analyses of
pooled nasopharyngeal and throat swabs collected
at the AWE TTC.
The positivity rate with 95% confidence interval
(CI) was calculated. Demographic and clinical
characteristics of individuals with positive and
negative test results were compared using Pearson’s
Chi squared test, Fisher’s exact test, or the Mann-Whitney U test, as appropriate. Adjusted odds ratios
with 95% Wald CIs were derived using multivariable
binary logistic regression to assess the associations
of clinical characteristics with SARS-CoV-2 positive
test results. Partially standardised beta coefficients
were used to compare the strengths of associations
between individual clinical characteristics and
SARS-CoV-2 test results; a greater absolute value
of the partially standardised beta coefficient was
indicative of a stronger association. Ridge regression
was performed to implement penalisation for
management of the sparse data bias elicited by the
low prevalences of some clinical characteristics.6 7
The tuning parameter λ was identified as the optimal
value that resulted in minimal error via 10-fold
cross-validation; as the tuning parameter λ became
larger, the estimated odds ratio decreased towards
a value of 1. Bootstrapping was used to construct
95% CIs with 100 bootstrap replications. Statistical
analyses were performed using R version 3.6.1 with
“glmnet” and “boot” packages. A P value of <0.05 was
considered statistically significant.
Results
In total, 1286 individuals attended the AWE TTC for COVID-19 testing (Table 1). Of these 1286 attendees,
1258 were included in the analysis after the exclusion
of three attendees with important missing data
and 25 attendees who were immediately referred
to regional accident and emergency departments because of severe symptoms requiring investigation
or therapy beyond the capacity of the AWE TTC.
These severe symptoms included shortness of breath
(n=8); high fever (n=5); chest discomfort (n=5);
acute gastrointestinal symptoms (n=4); and acute ear, nose, throat symptoms (n=3). Finally, 1242
individuals were involved in the analysis because
16 individuals attended the AWE TTC twice due to
ongoing or changing symptoms; the remaining 1226
individuals attended the AWE TTC only once for
testing. Among the 1258 included tests, five showed
indeterminate results during the first sampling, while
subsequent re-tests revealed negative results; thus,
there were 86 positive SARS-CoV-2 results with
a positivity rate of 6.84% (95% CI=5.57%-8.37%).
During the study period, the maximum number of
attendees (n=79) was recorded on 30 March 2020
and the highest daily number of positive test results
(n=8) was recorded on 6 April 2020. Attendees
with positive test results were all admitted to public
hospitals through central coordination for further
clinical assessment and treatment.
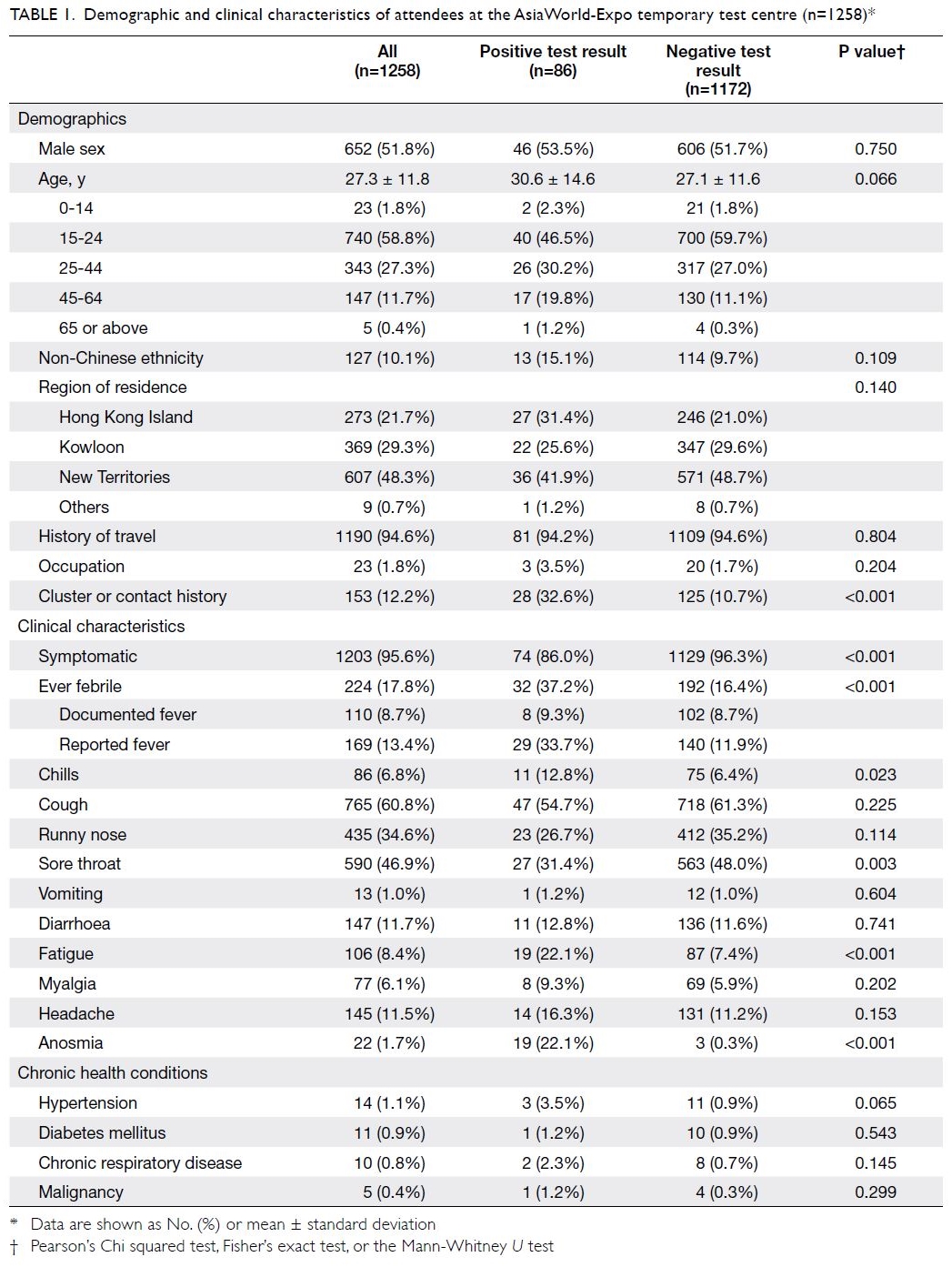
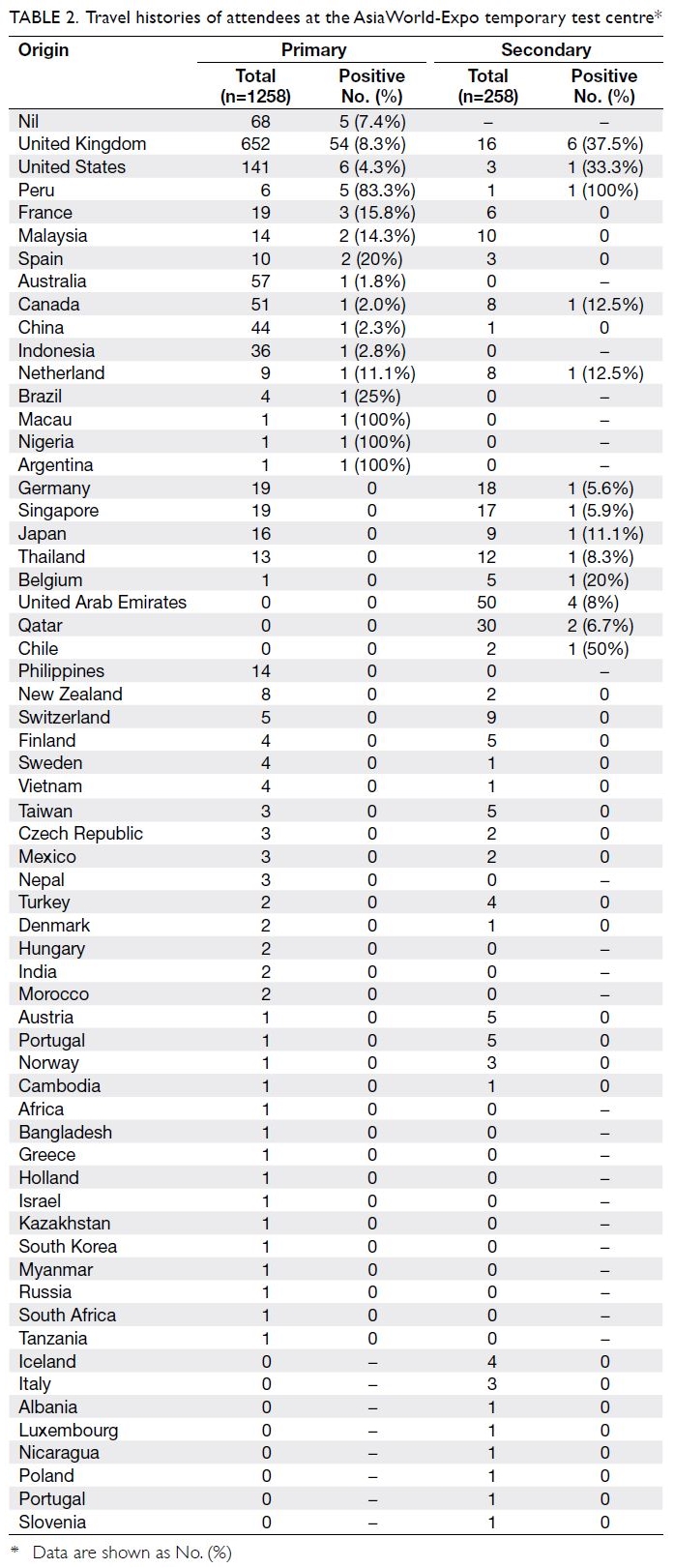
Table 1. Demographic and clinical characteristics of attendees at the AsiaWorld-Expo temporary testing centre (n=1258)*
Most attendees were aged 15 to 24 years
(740/1258, 58.8%). Furthermore, most attendees
(n=1190, 94.6%) were incoming travellers from
the United Kingdom, the United States, Canada,
Australia, and other parts of the world (Table 2).
A history of travel to the United Kingdom was
significantly associated with positive test results
(69.8% of positive test results vs 51.9% of negative
test results; P=0.001). Cluster or contact history
was reported by 32.6% of attendees with positive
test results and 10.7% of attendees with negative
test results (P<0.001). The most frequently reported
symptoms among all attendees were cough (60.8%),
sore throat (46.9%), and runny nose (34.6%).
Symptoms were reported by 86.0% and 96.3% of
individuals with positive and negative test results,
respectively.
The clinical characteristics most strongly
associated with a positive test result were anosmia
(ridge regression adjusted odds ratio [ORadj]=8.30;
95% CI=1.12-127.09) and fever (ORadj=1.32;
95% CI=1.02-3.28) [Table 3]. Sore throat was
significantly associated with a negative test
result (ORadj= 0.86; 95% CI=0.36-0.99). Other
characteristics (ie, cough, runny nose, fatigue,
headache, myalgia, vomiting, chills, and diarrhoea)
did not show significant associations with positive
or negative test results, according to ridge regression
analysis.
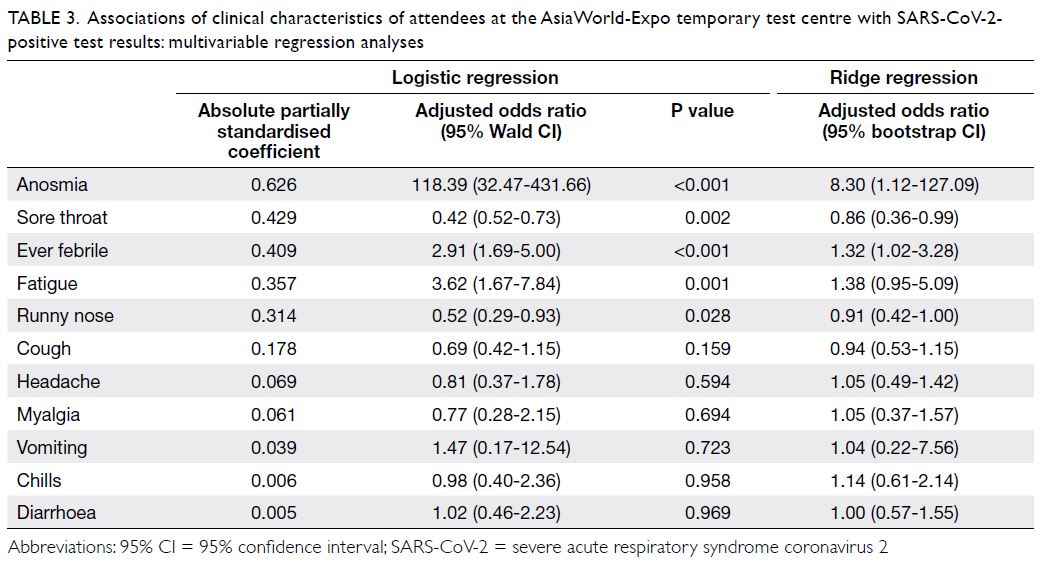
Table 3. Associations of clinical characteristics of attendees at the AsiaWorld-Expo temporary testing centre with SARS-CoV-2-positive test results: multivariable regression analyses
Discussion
To the best of our knowledge, this is the first study in
Hong Kong to explore the clinical characteristics of
attendees at a public TTC established by the Hospital
Authority in response to a worldwide pandemic.
Early identification and early containment have been
critical strategies adopted by the Centre for Health
Protection, Hong Kong to address the pandemic.
Locally, the first imported case in an individual with a
history of travel outside mainland China was reported
on 4 March 2020. This was followed by a large number of imported cases involving returning travellers,
including 245 students from the United Kingdom
and the United States who had positive test results;
the maximum number of cases (n=65) was reported
on 27 March 2020.8 The establishment of a TTC
was a crucial public health intervention to address
the influx of returning overseas travellers during the
worldwide spread of COVID-19 beginning in March
2020. Given the potential transmission of COVID-19
among individuals with relatively mild clinical
symptoms, early identification of SARS-CoV-2
infection by reverse transcription polymerase
chain reaction testing is crucial for reducing
disease spread.9 The AWE TTC was equipped with
extensive testing capacity for the target population
of individuals with mild disease symptoms.
Among AWE TTC attendees, the majority
of positive test results were recorded in young
individuals (aged 15-24 years; 40 of 86 cases), who
comprised 46.5% of total attendees with positive
test results. Notably, this proportion was greater
than the proportion reported by the Centre for
Health Protection concerning individuals in the
same age-group (289 of 1084 cases; 26.7%) among all
COVID-19 cases in Hong Kong during the study
period. This is potentially attributable to the targeted
testing strategy that focused on incoming overseas
students, which was implemented after the Hong
Kong Government announced compulsory testing
and quarantine for all arriving travellers beginning
on 19 March 2020.10 We previously reported that
most individuals could be tested on-site; moreover,
the AWE TTC fulfilled its gatekeeping role by
reducing the number of hospital admissions by 36 patients per day during its 31 days of operation.5
Primary care providers and emergency
physicians have performed important gatekeeping
roles in the early identification of individuals with
COVID-19. However, a local Family Physician survey
revealed that this gatekeeping task is challenging
because of the non-specific and mild disease
manifestations in many individuals with SARS-CoV-2
infections.11 In Hong Kong, among 1084 confirmed
cases reported between January 2020 and May 2020,
symptoms were reported by 859 (79.2%) affected
patients. The five most common symptoms reported
by Hong Kong patients with COVID-19 included
cough (436, 50.8%), fever (428, 49.8%), sore throat
(174, 20.3%), headache (98, 11.4%), and runny nose
(97, 11.3%). The remaining 225 patients (20.8%)
were asymptomatic.8 An early study of 41 patients in
Wuhan, published in January 2020, revealed that the
most common symptoms at onset of illness were fever
(98%), cough (76%), and myalgia or fatigue (44%).12
A multicentre study in Shanghai reported that the
most common symptoms among 1004 patients with
positive test results were fever (84%), cough (62%),
and fatigue (25%).13 Our study reviewed the clinical
characteristics and outcomes of relatively healthy
individuals in Hong Kong whose demographic
characteristics were similar to those of the general
practice population; we found that the three most
common symptoms among infected individuals were
cough, fever, and sore throat (Table 1), consistent
with the findings in a local study by the Centre for
Health Protection.6 In addition to the usual upper
respiratory tract symptoms, our results showed
that fever and anosmia were strongly associated with positive test results. These findings provide
important guidance for gatekeeping physicians to
carefully consider symptoms such as anosmia (a
relatively uncommon symptom in primary care
consultations), which was present in 22.1% of our
attendees with positive test results and only 0.3% of
attendees with negative test results. Evidence of such
symptoms should alert clinicians to the potential
presence of COVID-19. A study performed in South
Korea revealed that acute olfactory disturbance was
present in 15.3% of patients (488/3191) in the early
stage of COVID-19. Its prevalence was significantly
more common among female patients and younger
individuals (P=0.01 and P<0.001, respectively).14 A
study performed in the Netherlands showed that
anosmia was present in 47% of individuals with
positive test results and was strongly associated
with SARS-CoV-2 positivity (odds ratio=23.0;
95% CI=8.2%-64.8%).15 In our study, the ORadj for
anosmia was 8.30 (95% CI=1.12-127.09), indicating
a strong association between anosmia and a positive
test result. However, this result should be interpreted
cautiously, considering the potential for over- or
under-reporting of the symptom at a cross-sectional
encounter, the co-existence of other conditions
that may lead to olfactory disturbance, and the
timing of illness presentation. The probability of
identifying an infected individual depends on the
incubation period and the proportion of individuals
with subclinical disease.16 Symptoms alone might
not be reliable for diagnosis. Early testing is critical
for the early identification of both symptomatic
and asymptomatic individuals. This approach has
been particularly essential with worsening disease
spread, which has required stricter infection control
measures since July 2020.17 18
Of the 1286 AWE TTC attendees, 25 (1.94%)
with severe symptoms were immediately transferred
to the accident and emergency departments;
these attendees did not undergo testing at the
AWE TTC. The inclusion and exclusion criteria
used in this TTC could be useful for planning and
implementation efforts (in terms of referral criteria)
if similar centres must be established in future
emergency circumstances. Notably, this type of
centre is considered safe and efficient for screening
to reduce community disease spread19 and could be
more readily implemented to manage an infectious
disease, compared with vaccination and effective
antiviral therapy.20
The strengths of this study were its large sample
size and centralised setting that allowed coverage
of the entire Hong Kong population (regardless of
residential location) with elevated COVID-19 risk,
including those arriving at the airport and those
under home quarantine; all AWE TTC attendees
exhibited mild disease symptoms similar to those
of potentially infected individuals encountered in primary care settings. The limitations of this study
included its retrospective data collection based on
electronic health records. Investigators could not
verify the reported conditions of the AWE TTC
attendees or recover any important missing data.
Nevertheless, all AWE TTC attendees were assessed
by physicians with a standard questionnaire for
documentation of demographics and symptoms;
they were also tested by reverse transcription
polymerase chain reaction analyses of standard
pooled nasopharyngeal and throat swabs, which
provided clear positive and negative results that
facilitated data analysis. Another limitation of the
study involved its cross-sectional study design. The
epidemiological information and clinical symptoms
collected during patient assessment at the AWE TTC
might not be identical to those of post-admission
situations because the patients’ conditions might
have changed in a manner dependent on the timing
of presentation. For example, a study of 1099 patients
in China found that fever was present in 43.8% of
patients on admission, but was present in 88.7%
of patients during hospitalisation.21 Importantly,
the present study could not offer predictive
value or relative risk projection on the basis of
its epidemiological and clinical findings. Further
studies with a longitudinal design may provide useful
epidemiological and clinical insights.
Conclusion
In some individuals, COVID-19 causes mild initial
symptoms despite its high infectivity; thus, there is
a need for early identification of individuals with
SARS-CoV-2 infection who exhibit mild symptoms.
The establishment of a TTC was successful in
identifying infected individuals in a large-scale,
high-turnover setting, thereby reducing the testing
burden in secondary and tertiary healthcare
facilities. Gatekeeping healthcare providers should
carefully consider the epidemiological and clinical
manifestations of COVID-19 and be vigilant in
arranging appropriate early testing to reduce
community spread.
Author contributions
Concept or design: WLH Leung, ELM Yu.
Acquisition of data: WLH Leung.
Analysis or interpretation of data: WLH Leung, ELM Yu.
Drafting of the manuscript: WLH Leung.
Critical revision of the manuscript for important intellectual content: All authors.
Acquisition of data: WLH Leung.
Analysis or interpretation of data: WLH Leung, ELM Yu.
Drafting of the manuscript: WLH Leung.
Critical revision of the manuscript for important intellectual content: All authors.
All authors had full access to the data, contributed to the study, approved the final version for publication, and take
responsibility for its accuracy and integrity.
Conflicts of interest
All authors have disclosed no conflicts of interest.
Acknowledgement
The authors acknowledge all workers involved in the setup and operation of the temporary test centre at AsiaWorld-Expo, Hong Kong.
Funding/support
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
This study was approved by the Kowloon West Cluster
Research Ethics Committee, Hospital Authority [Ref KW/EX-20-085(148-09)]. The Ethics Committee waived the need for
patient consent for this retrospective study.
References
1. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier
transmission of COVID-19. JAMA 2020;323:1406-7. Crossref
2. Heymann DL, Shindo N, WHO Scientific and Technical
Advisory Group for Infectious Hazards. COVID-19: what
is next for public health? Lancet 2020;395:542-5. Crossref
3. Chan JF, Yuan S, Kok KH, et al. A familial cluster of
pneumonia associated with the 2019 novel coronavirus
indicating person-to-person transmission: a study of a
family cluster. Lancet 2020;395:514-23. Crossref
4. Hong Kong SAR Government. Temporary test centres
speed up tests for people upon arrival. Available from:
https://www.info.gov.hk/gia/general/202003/19/P2020031900664.htm. Accessed 11 Jul 2020.
5. Wong SC, Leung M, Lee LL, Chung KL, Cheng VC.
Infection control challenge in setting up a temporary
test centre at Hong Kong International Airport for rapid
diagnosis of COVID-19 due to SARS-CoV-2. J Hosp Infect
2020;105:571-3. Crossref
6. Greenland S, Mansournia MA, Altman DG. Sparse data
bias: a problem hiding in plain sight. BMJ 2016;352:i1981. Crossref
7. Doerken S, Avalos M, Lagarde E, Schumacher M. Penalized
logistic regression with low prevalence exposures beyond
high dimensional settings. PloS One 2019;14:e0217057. Crossref
8. Lam HY, Lam TS, Wong CH, et al. The epidemiology of
COVID-19 cases and the successful containment strategy
in Hong Kong–January to May 2020. Int J Infect Dis
2020;98:51-8. Crossref
9. To KK, Yuen KY. Responding to COVID-19 in Hong Kong. Hong Kong Med J 2020;26:164-6. Crossref
10. Hong Kong SAR Government. Compulsory quarantine law
gazetted. Available from: https://www.news.gov.hk/eng/20
20/03/20200318/20200318_211807_723.html. Accessed 11
Jul 2020.
11. Yu EY, Leung WL, Wong SY, Liu KS, Wan EY, HKCFP
Executive and Research Committee. How are family
doctors serving the Hong Kong community during the
COVID-19 outbreak? A survey of HKCFP members. Hong
Kong Med J 2020;26:176-83. Crossref
12. Huang C, Wang Y, Li X, et al. Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China.
Lancet 2020;395:497-506. Crossref
13. Mao B, Liu Y, Chai YH, et al. Assessing risk factors
for SARS-CoV-2 infection in patients presenting with
symptoms in Shanghai, China: a multicentre, observational
cohort study. Lancet Digit Health 2020;2:e323-30. Crossref
14. Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of
acute loss of smell or taste in COVID-19 patients. J Korean
Med Sci 2020;35:e174. Crossref
15. Tostmann A, Bradley J, Bousema T, et al. Strong associations
and moderate predictive value of early symptoms for
SARS-CoV-2 test positivity among healthcare workers, the
Netherlands, March 2020. Euro Surveill 2020;25:2000508. Crossref
16. Gostic K, Gomez AC, Mummah RO, Kucharski AJ,
Lloyd-Smith JO. Estimated effectiveness of symptom and
risk screening to prevent the spread of COVID-19. Elife
2020;9:e55570. Crossref
17. Hong Kong SAR Government. Social distancing rules to be
tightened. Available from: https://www.news.gov.hk/eng/2020/07/20200709/20200709_175812_722.html. Accessed
11 Jul 2020.
18. Hong Kong SAR Government. Government further tightens
social distancing measures. Available from: https://www.info.gov.hk/gia/general/202007/27/P2020072700650.htm.
Accessed 27 Jul 2020.
19. Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through
screening center for COVID-19: a safe and efficient
screening system against massive community outbreak. J
Korean Med Sci 2020;35:e123. Crossref
20. Peto J. Covid-19 mass testing facilities could end the
epidemic rapidly. BMJ 2020;368:m1163. Crossref
21. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics
of coronavirus disease 2019 in China. N Engl J Med
2020;382:1708-20. Crossref