Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations
Abstract
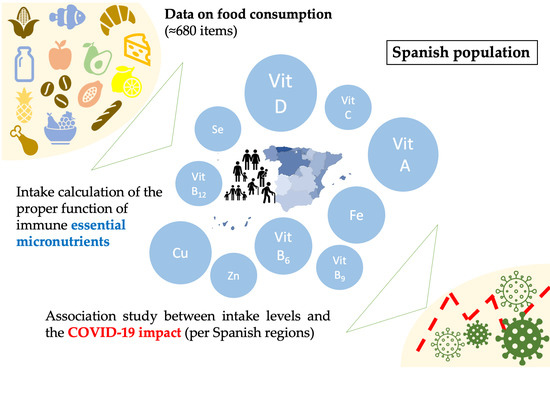
:1. Introduction
2. Materials and Methods
2.1. Selection of Nutrients
2.2. Micronutrient Intake in Spain and Spanish Regions (Autonomous Communities)
2.3. Epidemiological Indicators of COVID-19 in Spain
2.4. Data Calculation, Presentation, and Statistical Analyses
3. Results
3.1. Epidemiological Situation during the Exponential Phase of the Second Wave of COVID-19 in the Regions of Spain
3.2. Intake of Essential Micronutrients for the Immune System at the National Level
3.3. Micronutrient Intake in the Spanish Autonomous Communities
3.4. Association between the Intake of Micronutrients and COVID-19 Indicators in the ACs
3.5. Analysis of Main Contributing Foods by ACs and Their Relationship with Epidemiological Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 16 May 2022).
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Gombart, A.F.; Pierre, A.; Maggini, S. A Review of Micronutrients and the Immune System–Working in Harmony to Reduce the Risk of Infection. Nutrients 2020, 12, 236. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of a health claim related to vitamin D and contribution to the normal function of the immune system pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2015, 13, 4096. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin D and normal function of the immune system and inflammatory response (ID 154, 159), maintenance of normal muscle function (ID 155) and maintenance of normal cardiovascular functi. EFSA J. 2010, 8, 1468. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to vitamin B6 and protein and glycogen metabolism (ID 65, 70, 71), function of the nervous system (ID 66), red blood cell formation (ID 67, 72, 186), function of the immune system (ID 68). EFSA J. 2009, 7, 1225. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to zinc and function of the immune system (ID 291, 1757), DNA synthesis and cell division (ID 292, 1759), protection of DNA, proteins and lipids from oxidative damage (ID 294, 1758), mainte. EFSA J. 2009, 7, 1229. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to iron and formation of red blood cells and haemoglobin (ID 249, ID 1589), oxygen transport (ID 250, ID 254, ID 256), energy-yielding metabolism (ID 251, ID 1589), function of the immune s. EFSA J. 2009, 7, 1215. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to copper and protection of DNA, proteins and lipids from oxidative damage (ID 263, 1726), function of the immune system (ID 264), maintenance of connective tissues (ID 265, 271, 1722), ene. EFSA J. 2009, 7, 1211. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to copper and reduction of tiredness and fatigue (ID 272), maintenance of the normal function of the nervous system (ID 1723), maintenance of the normal function of the immune system (ID 17. EFSA J. 2011, 9, 2079. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to selenium and protection of DNA, proteins and lipids from oxidative damage (ID 277, 283, 286, 1289, 1290, 1291, 1293, 1751), function of the immune system (ID 278), thyroid function (ID 2. EFSA J. 2009, 7, 1220. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef] [PubMed]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake, Disparity between the Reported Consumption and the Level Needed for Adequacy and Food Sources of Calcium, Phosphorus, Magnesium and Vitamin D in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 168. [Google Scholar] [CrossRef]
- Olza, J.; Aranceta-Bartrina, J.; González-Gross, M.; Ortega, R.; Serra-Majem, L.; Varela-Moreiras, G.; Gil, Á. Reported Dietary Intake and Food Sources of Zinc, Selenium, and Vitamins A, E and C in the Spanish Population: Findings from the ANIBES Study. Nutrients 2017, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Aparicio-Ugarriza, R.; Olza, J.; Aranceta-Bartrina, J.; Gil, Á.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; González-Gross, M. Dietary intake and food sources of niacin, riboflavin, thiamin and vitamin B6 in a representative sample of the spanish population. The anthropometry, intake, and energy balance in Spain (ANIBES) study. Nutrients 2018, 10, 846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partearroyo, T.; De Lourdes Samaniego-Vaesken, M.; Ruiz, E.; Olza, J.; Aranceta-Bartrina, J.; Gil, Á.; González-Gross, M.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G. Dietary sources and intakes of folates and Vitamin B12 in the Spanish population: Findings from the ANIBES study. PLoS ONE 2017, 12, e189230. [Google Scholar] [CrossRef] [Green Version]
- Goñi, I.; Hernández-Galiot, A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA) DRV Finder. Available online: https://www.efsa.europa.eu/en/interactive-pages/drvs (accessed on 21 July 2020).
- Annual Data of the Panel of Food Consumption in Households. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/series-anuales/default.aspx (accessed on 11 January 2021).
- Update no 235 of the Coronavirus Disease (COVID-19). Health Ministry of the Spanish Government. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_203_COVID-19.pdf. (accessed on 11 January 2021).
- Update no 203 of the Coronavirus Disease (COVID-19). Health Ministry of the Spanish Government. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_235_COVID-19.pdf. (accessed on 11 January 2021).
- Estudio ENE-COVID: Cuarta Ronda Estudio Nacional de Sero-Epidemiología de la Infección por SARS-COV-2 en España. 2020. Available online: https://www.sanidad.gob.es/gabinetePrensa/notaPrensa/pdf/15.12151220163348113.pdf. (accessed on 11 January 2021).
- STHDA—Home. Available online: http://www.sthda.com/english/ (accessed on 2 July 2020).
- European Food Safety Authority (EFSA). Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, e15121E. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Authority (EFSA). Scientific Opinion on Dietary Reference Values for energy. EFSA J. 2013, 11, 3005. [Google Scholar] [CrossRef] [Green Version]
- Gamero-de-Luna, E.J.; Gamero-Estévez, E. Mutaciones, variantes y cepas de SARS-CoV-2. Med. Fam. Semer. 2021, 47, 208–209. [Google Scholar] [CrossRef]
- Faurschou, A.; Beyer, D.M.; Schmedes, A.; Bogh, M.K.; Philipsen, P.A.; Wulf, H.C. The relation between sunscreen layer thickness and vitamin D production after ultraviolet B exposure: A randomized clinical trial. Br. J. Dermatol. 2012, 167, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Fayet-Moore, F.; Brock, K.E.; Wright, J.; Ridges, L.; Small, P.; Seibel, M.J.; Conigrave, A.D.; Mason, R.S. Determinants of vitamin D status of healthy office workers in Sydney, Australia. J. Steroid Biochem. Mol. Biol. 2019, 189, 127–134. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Dietary reference values for vitamin D. EFSA J. 2016, 14, e04547. [Google Scholar] [CrossRef]
- Carr, A.C.; Gombart, A.F. Multi-Level Immune Support by Vitamins C and D during the SARS-CoV-2 Pandemic. Nutrients 2022, 14, 689. [Google Scholar] [CrossRef] [PubMed]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- Alipio, M. Vitamin D Supplementation Could Possibly Improve Clinical Outcomes of Patients Infected with Coronavirus-2019 (COVID-2019). SSRN Electron. J. 2020. Available online: https://www.scienceopen.com/document?vid=403e39be-0c0f-4d35-afd0-5af7953bbfad. (accessed on 11 January 2021). [CrossRef]
- Daneshkhah, A.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. The Role of Vitamin D in Suppressing Cytokine Storm in COVID-19 Patients and Associated Mortality. medRxiv 2020. Available online: https://www.medrxiv.org/node/81465.external-links.html (accessed on 11 January 2021).
- Grant, W.B.; Al Anouti, F.; Boucher, B.J.; Dursun, E.; Gezen-Ak, D.; Jude, E.B.; Karonova, T.; Pludowski, P. A Narrative Review of the Evidence for Variations in Serum 25-Hydroxyvitamin D Concentration Thresholds for Optimal Health. Nutrients 2022, 14, 639. [Google Scholar] [CrossRef]
- Karonova, T.L.; Chernikova, A.T.; Golovatyuk, K.A.; Bykova, E.S.; Grant, W.B.; Kalinina, O.V.; Grineva, E.N.; Shlyakhto, E.V. Vitamin D Intake May Reduce SARS-CoV-2 Infection Morbidity in Health Care Workers. Nutrients 2022, 14, 505. [Google Scholar] [CrossRef]
- Benedik, E. Sources of vitamin D for humans. Int. J. Vitam. Nutr. Res. 2022, 92, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertoldi, G.; Gianesello, L.; Calò, L.A. ACE2, Rho kinase inhibition and the potential role of vitamin D against COVID-19. Aliment. Pharmacol. Ther. 2020, 52, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that vitamin d supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Bonet, M.L.; Ribot, J.; Galmés, S.; Serra, F.; Palou, A. Carotenoids and carotenoid conversion products in adipose tissue biology and obesity: Pre-clinical and human studies. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2020, 1865, 158676. [Google Scholar] [CrossRef]
- Kańtoch, M.; Litwińska, B.; Szkoda, M.; Siennicka, J. Importance of vitamin A deficiency in pathology and immunology of viral infections. Rocz. Panstw. Zakl. Hig. 2002, 53, 385–392. [Google Scholar]
- Maggini, S.; Wintergerst, E.S.; Beveridge, S.; Hornig, D.H. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br. J. Nutr. 2007, 98, S29–S35. [Google Scholar] [CrossRef]
- Chew, B.P. Role of Carotenoids in the Immune Response. J. Dairy Sci. 1993, 76, 2804–2811. [Google Scholar] [CrossRef]
- Toti, E.; Oliver Chen, C.Y.; Palmery, M.; Valencia, D.V.; Peluso, I. Non-provitamin A and provitamin A carotenoids as immunomodulators: Recommended dietary allowance, therapeutic index, or personalized nutrition? Oxid. Med. Cell. Longev. 2018, 2018, 20. [Google Scholar] [CrossRef]
- Sarohan, A.R. COVID-19: Endogenous Retinoic Acid Theory and Retinoic Acid Depletion Syndrome. Med. Hypotheses 2020, 144, 110250. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I.; Alrushud, N.; Alnuwaysir, H.; Elkhatib, R.; Shoukri, M.; Aldayel, F.; Bakheet, R.; Almozaini, M. Essential metals, vitamins and antioxidant enzyme activities in COVID-19 patients and their potential associations with the disease severity. Biometals 2022, 35, 125–145. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999, 58, 719–727. [Google Scholar] [CrossRef] [Green Version]
- Villamor, E.; Mbise, R.; Spiegelman, D.; Hertzmark, E.; Fataki, M.; Peterson, K.E.; Ndossi, G.; Fawzi, W.W. Vitamin A Supplements Ameliorate the Adverse Effect of HIV-1, Malaria, and Diarrheal Infections on Child Growth. Pediatrics 2002, 109, e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trasino, S.E. A role for retinoids in the treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1765–1767. [Google Scholar] [CrossRef]
- Chen, K.-H.; Wang, S.-F.; Wang, S.-Y.; Yang, Y.-P.; Wang, M.-L.; Chiou, S.-H.; Chang, Y.-L. Pharmacological development of the potential adjuvant therapeutic agents against coronavirus disease 2019. J. Chin. Med. Assoc. 2020. ahead of print. [Google Scholar] [CrossRef]
- Midha, I.K.; Kumar, N.; Kumar, A.; Madan, T. Mega doses of retinol: A possible immunomodulation in Covid-19 illness in resource-limited settings. Rev. Med. Virol. 2020, 2, 2204. [Google Scholar] [CrossRef]
- Li, R.; Wu, K.; Li, Y.; Liang, X.; Fai Tse, W.K.; Yang, L.; Lai, K.P. Revealing the targets and mechanisms of vitamin A in the treatment of COVID-19. Aging 2020, 12, 15784–15796. [Google Scholar] [CrossRef]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Saeed, F.; Nadeem, M.; Ahmed, R.S.; Tahir Nadeem, M.; Arshad, M.S.; Ullah, A. Studying the impact of nutritional immunology underlying the modulation of immune responses by nutritional compounds—A review. Food Agric. Immunol. 2016, 27, 205–229. [Google Scholar] [CrossRef] [Green Version]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized Folic Acid in Plasma Is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, Z.; Dokoohaki, M.H.; Negahdaripour, M.; Dehdashti, M.; Zolghadr, H.; Moghadami, M.; Masoom Masoompour, S.; Zolghadr, A.R. The role of folic acid in the management of respiratory disease caused by COVID-19. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Singh, Y.; Gupta, G.; Kazmi, I.; Al-Abbasi, F.A.; Negi, P.; Chellappan, D.; Dua, K. SARS CoV-2 aggravates cellular metabolism mediated complications in COVID-19 infection. Dermatol. Ther. 2020, 33, e13871. [Google Scholar] [CrossRef]
- Hayden, M.R.; Tyagi, S.C. Impaired Folate-Mediated One-Carbon Metabolism in Type 2 Diabetes, Late-Onset Alzheimer’s Disease and Long COVID. Medicina 2021, 58, 16. [Google Scholar] [CrossRef]
- Acosta-Elias, J.; Espinosa-Tanguma, R. The Folate Concentration and/or Folic Acid Metabolites in Plasma as Factor for COVID-19 Infection. Front. Pharmacol. 2020, 11, 1062. [Google Scholar] [CrossRef]
- Uta, M.; Neamtu, R.; Bernad, E.; Mocanu, A.G.; Gluhovschi, A.; Popescu, A.; Dahma, G.; Dumitru, C.; Stelea, L.; Citu, C.; et al. The Influence of Nutritional Supplementation for Iron Deficiency Anemia on Pregnancies Associated with SARS-CoV-2 Infection. Nutrients 2022, 14, 836. [Google Scholar] [CrossRef]
- Topilski, I.; Flaishon, L.; Naveh, Y.; Harmelin, A.; Levo, Y.; Shachar, I. The anti-inflammatory effects of 1,25-dihydroxyvitamin D3 on Th2 cellsin vivo are due in part to the control of integrin-mediated T lymphocyte homing. Eur. J. Immunol. 2004, 34, 1068–1076. [Google Scholar] [CrossRef]
- Maggini, S. Feeding the immune system: The role of micronutrients in restoring resistance to infections. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 1–21. [Google Scholar] [CrossRef]
- de Almeida Brasiel, P.G. The key role of zinc in elderly immunity: A possible approach in the COVID-19 crisis. Clin. Nutr. ESPEN 2020, 38, 65–66. [Google Scholar] [CrossRef]
- Tuerk, M.J.; Fazel, N. Zinc deficiency. Curr. Opin. Gastroenterol. 2009, 25, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, T.J.M.; Gonçalves, S.E.A.B.; Guarnieri, A.; Risegato, R.C.; Guimarães, M.P.; de Freitas, D.C.; Razuk-Filho, A.; Junior, P.B.B.; Parrillo, E.F. Association between Low Zinc Levels and Severity of Acute Respiratory Distress Syndrome by New Coronavirus (SARS-CoV-2). Nutr. Clin. Pract. 2020, 36, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Joachimiak, M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl. Trop. Dis. 2021, 15, e0008895. [Google Scholar] [CrossRef] [PubMed]
- Finzi, E. Treatment of SARS-CoV-2 with high dose oral zinc salts: A report on four patients. Int. J. Infect. Dis. 2020, 99, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Abdelmaksoud, A.A.; Ghweil, A.A.; Hassan, M.H.; Rashad, A.; Khodeary, A.; Aref, Z.F.; Sayed, M.A.A.; Elsamman, M.K.; Bazeed, S.E.S. Olfactory Disturbances as Presenting Manifestation Among Egyptian Patients with COVID-19: Possible Role of Zinc. Biol. Trace Elem. Res. 2021, 199, 4101–4108. [Google Scholar] [CrossRef]
- Pormohammad, A.; Monych, N.; Turner, R. Zinc and SARS-CoV-2: A molecular modeling study of Zn interactions with RNA-dependent RNA-polymerase and 3C-like proteinase enzymes. Int. J. Mol. Med. 2020, 47, 326–334. [Google Scholar] [CrossRef]
- Wee, A.K.H. COVID-19′s toll on the elderly and those with diabetes mellitus—Is vitamin B12 deficiency an accomplice? Med. Hypotheses 2020, 146, 110374. [Google Scholar] [CrossRef]
- Tan, C.W.; Ho, L.P.; Kalimuddin, S.; Cherng, B.P.Z.; Teh, Y.E.; Thien, S.Y.; Wong, H.M.; Tern, P.J.W.; Chandran, M.; Chay, J.W.M.; et al. Cohort study to evaluate the effect of vitamin D, magnesium, and vitamin B12 in combination on progression to severe outcomes in older patients with coronavirus (COVID-19). Nutrition 2020, 79–80, 111017. [Google Scholar] [CrossRef]
- Jagielski, P.; Łuszczki, E.; Wnęk, D.; Micek, A.; Bolesławska, I.; Piórecka, B.; Kawalec, P. Associations of Nutritional Behavior and Gut Microbiota with the Risk of COVID-19 in Healthy Young Adults in Poland. Nutrients 2022, 14, 350. [Google Scholar] [CrossRef]
- Gupta, U.C.; Gupta, S.C. Selenium in soils and crops, its deficiencies in livestock and humans: Implications for management. Commun. Soil Sci. Plant Anal. 2000, 31, 1791–1807. [Google Scholar] [CrossRef]
- Jones, G.D.; Droz, B.; Greve, P.; Gottschalk, P.; Poffet, D.; McGrath, S.P.; Seneviratne, S.I.; Smith, P.; Winkel, L.H.E. Selenium deficiency risk predicted to increase under future climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 2848–2853. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| DRV for Adult Population | Intake Median (% DRV) | Published Intake Median (% DRV) | Accomplishment (%) [13] | |
|---|---|---|---|---|
| Vitamin D (µg/day) | AI: 15 | 2.46 (16.2%) | 2.60 (17.3%) [14] | 14.1 |
| Vitamin A (µg/day) | PRI: 650 (w)/750 (m) | 277 (39.9%) | 477 (68.1%) [15] | 77.2 |
| Vitamin C (mg/day) | PRI: 95 (w)/110 (m) | 95.0 (91.9%) | 71.3 (69.6%) [15] | 110 |
| Vitamin B6 (mg/day) | AI: 1.6 (w)/1.7 (m) | 1.40 (83.2%) | 1.44 (87.3%) [16] | 112 |
| Vitamin B9 (µg/day) | PRI: 330 | 238 (71.1%) | 160 (48.4%) [17] | 74.9 |
| Vitamin B12 (µg/day) | PRI: 4 | 5.23 (132%) | 4.20 (105%) [17] | 128 |
| Zinc (mg/day) | PRI *: 10.1 (w)/10.9 (m) | 4.42 (38.6%) | 7.70 (67.0%) [14] | 81.2 |
| Iron (mg/day) | PRI: 11 | 7.96 (72.8%) | 10.5 (95.7%) [16] | 111 |
| Copper (mg/day) | PRI: 1.3 (w)/1.6 (m) | 0.62 (43.5%) | 1.00 (69.0%) [18] | 115 |
| Selenium (µg/day) | AI: 70 | 92.3 (131%) | 72.0 (103%) [15] | 108 |
| ACs | Prevalence (P) | Incidence -45 (I-45) | Incidence (I) | Δ Incidence (ΔI) | Mortality (M) | I + M (Z-score) |
|---|---|---|---|---|---|---|
| Canary Islands | 3.8 | 445.9 | 768.4 | 1.7 | 12.4 | −2.5 |
| Galicia | 4.5 | 617.1 | 1063.6 | 1.7 | 31.8 | −1.8 |
| Principality of Asturias | 6.1 | 367.4 | 922.2 | 2.5 | 38.2 | −1.8 |
| Valencian Community | 5.7 | 584.6 | 1075.8 | 1.8 | 34.4 | −1.8 |
| Andalusia | 7.1 | 450.5 | 1263.3 | 2.8 | 27.0 | −1.8 |
| Balearic Islands | 6.3 | 881.7 | 1468.2 | 1.7 | 30.6 | −1.5 |
| Region of Murcia | 6.1 | 630.5 | 1958.2 | 3.1 | 18.9 | −1.3 |
| Cantabria | 6.3 | 868.9 | 1462.8 | 1.7 | 42.7 | −1.3 |
| Extremadura | 8.0 | 585.0 | 1505.8 | 2.6 | 63.3 | −0.9 |
| Catalonia | 11.6 | 1561.4 | 2502.0 | 1.6 | 77.7 | 0.2 |
| Basque Country | 8.2 | 1578.6 | 2610.2 | 1.7 | 92.0 | 0.6 |
| Castilla y León | 12.6 | 1372.0 | 2916.8 | 2.1 | 143.6 | 1.9 |
| Aragon | 11.7 | 2191.6 | 3659.7 | 1.7 | 118.7 | 2.0 |
| Castilla-La Mancha | 16.1 | 1367.9 | 2821.0 | 2.1 | 164.2 | 2.2 |
| CC of Navarre | 14.3 | 1770.4 | 4281.3 | 2.4 | 102.1 | 2.2 |
| La Rioja | 8.2 | 2014.2 | 3566.9 | 1.8 | 139.5 | 2.3 |
| Community of Madrid | 18.6 | 2283.9 | 4393.5 | 1.9 | 153.4 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galmés, S.; Palou, A.; Serra, F. Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations. Nutrients 2022, 14, 2254. https://doi.org/10.3390/nu14112254
Galmés S, Palou A, Serra F. Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations. Nutrients. 2022; 14(11):2254. https://doi.org/10.3390/nu14112254
Chicago/Turabian StyleGalmés, Sebastià, Andreu Palou, and Francisca Serra. 2022. "Suboptimal Consumption of Relevant Immune System Micronutrients Is Associated with a Worse Impact of COVID-19 in Spanish Populations" Nutrients 14, no. 11: 2254. https://doi.org/10.3390/nu14112254