Abstract
Background:
Coronavirus disease 2019 (COVID-19) is caused by an infection in the respiratory tract leading to extrapulmonary manifestations, including dysregulation of the immune system and hepatic injury.Objectives:
Given the high prevalence of viral hepatitis and a few studies carried out on severe acute respiratory syndrome coronavirus 2 and hepatitis B virus (HBV), this study investigated the impact of COVID-19 on chronic hepatitis B (CHB) patients in the northeast region of Iran.Methods:
In this cross-sectional study, the blood samples were collected from 93 CHB patients registered in the Patient Detection Data Bank of Golestan University of Medical Sciences, Gorgan, Iran, and 62 healthy individuals as controls. Reverse transcription-polymerase chain reaction was adopted to detect COVID-19 infection in all the participants’ nasopharyngeal samples. All the participants were subjected to anti-hepatitis C virus, anti-hepatitis delta virus, and liver function tests. Then, HBV deoxyribonucleic acid load was detected in CHB patients. The collected data were analyzed by statistical tests using SPSS software (version 20). A P-value less than 0.05 was considered statistically significant.Results:
In this study, 14% (13/93) and 32.25% (20/62) of CHB patients and control individuals were infected with COVID-19, respectively. The mean age of CHB patients was 39.69 ± 19.58 years, and 71% of them were female. The risk of developing COVID-19 in healthy controls was observed to be 2.3 times higher than in patients with CHB (0.95% confidence interval: 1.242 - 4.290). On the other hand, the mean values of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase in CHB patients superinfected with COVID-19 were higher than other participants. Out of 35.4% of patients with viral hepatitis B that were taking antiviral drugs, only 5.4% had COVID-19.Conclusions:
Although CHB infection did not predispose COVID-19 patients to more severe outcomes, the data of this study suggest that antiviral agents also decreased susceptibility to COVID-19 infection. Alternatively, careful assessment of hepatic manifestations and chronic viral hepatitis infections in COVID-19 patients can lead to more favorable health outcomes.Keywords
1. Background
Coronavirus disease 2019 (COVID-19) is a newly arrived respiratory disease that brings about severe acute syndromes in the respiratory system. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first detected in Wuhan, China, has spread to other parts of the world at an uncontrolled pace and has become a major public health threat ever since (1, 2). The knowledge-gaining process regarding the pathogenesis of SARS-CoV-2 is still considered an ongoing issue (3). The SARS-CoV-2 that is attached to the angiotensin-converting enzyme-2 receptors is widely detected in various parts of the human body, such as the pulmonary alveolar epithelial cells, the endothelium, and the gastrointestinal tract, and might bring about this multisystem disease (4). It is frequently reported that other organs and cell types are also impaired in addition to the pulmonary system in patients suffering from the ailment (3, 5). This is evidenced by the increments in leukocytopenia, alanine aminotransferase (ALT), aspartate aminotransferase (AST), thrombocytopenia, and lactate dehydrogenase levels in COVID-19 patients (6, 7).
The evidence has emphasized that COVID-19 would induce liver injury (8), where over 60% of COVID-19 patients underwent hepatic dysfunction, especially those suffering from the severe status of the disease (9). Therefore, the infection caused by chronic hepatitis B (CHB) was reported to be a recognized and independent risk factor for the progression of the illness to acute respiratory distress syndrome (6, 8, 10). Studies have indicated that 2 - 11% of COVID-19 patients suffer from underlying chronic liver disease (11). Viral coinfection in COVID-19 patients might cause more complications during the recovery period of the patients (12).
Viral hepatitis annually claims approximately 1.34 million deaths worldwide; therefore, it is known as a major reason of mortality around the world. It was reported that most mortality and morbidity caused by viral hepatitis are due to chronic hepatitis B and C (13). Hepatitis B virus (HBV), which is considered a prototypical member of the Hepadnaviridae family, is widely distributed worldwide (14), especially in Iran, and the global prevalence of hepatitis B surface antigen (HBsAg) was estimated to be about 3.9% (15, 16). Although commercially available vaccines have proven to be effective in reducing HBV infection, HBV is still known as a major public health threat since almost 300 million individuals are struggling with chronic infection worldwide. The HBV is also considered to be the main reason for liver cirrhosis and hepatocellular carcinoma leading to numerous mortalities every year (17-19). Furthermore, hepatitis C virus (HCV) infection is regarded as a global health issue bringing about sequelae from chronic infection. It has been estimated that 3 - 4 million individuals are annually infected with this virus, which puts the patients at risk of developing liver disease, including cirrhosis and liver cancer, in chronic conditions (20).
Patients infected with SARS-CoV-2 are diagnosed with impaired liver function and the common symptoms of the infection (20, 21). The evidence has indicated that the liver injury in those with HBV and SARS-CoV-2 superinfection follows a similar pattern and degree to that of inpatients with SARS-CoV-2 alone (21). However, COVID-19 infection was suggested to be a possible cause of liver damage and induction of HBV activity in patients struggling with hepatitis B (22, 23). It has been shown that the effects of COVID-19 are not associated with HBV infection despite the elevated liver enzymes in some patients (2, 23). Overall, it can be claimed that SARS-CoV-2/HBV superinfection has no profound influence on the progression of COVID-19 in terms of severity, mortality, and hospitalization (22, 23). Nevertheless, patients with severe COVID-19 and HBV superinfection simultaneously might encounter HBV reactivation because interleukin-1 and interleukin-6 are administered to control cytokine storm and reduce the immune-mediated multiorgan injury in COVID-19 patients (24-26).
Moreover, infection with SARS-CoV-2 with HBV or HCV can impair liver function; therefore, hepatitis infection might increase susceptibility to and severity of COVID-19 infection (2). However, it should be noted that hepatitis infection showed no considerable improvement or suppressive influence on the inflammatory response induced by SARS-CoV-2, as evidenced by a “muted” induction of an innate immune response in the liver tissues with chronic hepatitis infection (27). In addition, studies on patients with mixed infection of SARS-CoV-2, HBV, and HCV revealed that such superinfection could cause delayed antibody response (28). As SARS-CoV-2 and viral hepatitis, such as HBV, HCV, and hepatitis delta virus (HDV), can cause liver damage, it is urgently required to improve our knowledge about the menace of SARS-CoV-2 infection in those suffering from CHB and other hepatitis infections.
2. Objectives
This study aimed to reveal whether CHB patients are predisposed to severe COVID-19 infection by scrutinizing the serologic markers and clinical manifestations.
3. Methods
3.1. Patients
A total of 93 CHB patients and 62 healthy individuals as controls (HBsAg negative) were included within September 2020 and January 2021 in this cross-sectional study (Table 1). The CHB patients were registered in the Patient Detection Data Bank of Golestan University of Medical Sciences, Gorgan, Iran, as chronic HBV patients. The demographic information and antiviral administration records of the participants were registered.
Inclusion Criteria for Participating in the Study
| Group | HBsAg | HBs-Ab | HBc-Ab | HBe-Ag | HBe-Ab | SARS-CoV-2 RT-PCR |
|---|---|---|---|---|---|---|
| SARS-CoV-2 and chronic hepatitis B infected patients | Positive | Negative | Positive | Positive/negative | Positive/negative | Positive |
| Chronic hepatitis B monoinfected patients | Positive | Negative | Positive | Positive/negative | Positive/negative | Negative |
| SARS-CoV-2 monoinfected patients | Negative | Negative | Negative | Negative | Negative | Positive |
| SARS-CoV-2 and HBV-negative patients | Negative | Negative | Negative | Negative | Negative | Negative |
3.2. Serological Markers and Biochemical Assessments
The blood samples (6 mL) of the subjects were obtained under standardized conditions in sterile tubes. After the separation of plasma by centrifugation (3000 rpm for 5 minutes), multiple biochemical indicators, such as ALT, AST, and alkaline phosphatase (ALP), were evaluated by automatic serum biochemical analyzers (Pars Azmoon Kit, Pars Azmoon Co., Iran). Afterward, the measurement of HBV serological markers, such as HBsAg, hepatitis B surface antibody, hepatitis B envelope antigen (HBe-Ag), hepatitis B envelope antibody, hepatitis B core antibody, and anti-HCV and anti-HDV antibodies, was carried out based on the enzyme-linked immunosorbent assay technique (Pishtaz Teb, Iran).
3.3. Viral Nucleic Acid Extraction and Viral Load Test of Hepatitis B
Nucleic acid was isolated from plasma using a viral deoxyribonucleic acid (DNA) extraction kit (Payesh Gene Rasti, Iran). The HBV viral load in 200 μL of the HBsAg+ participants’ plasma was determined by an HBV PCR kit (Altona, Germany).
3.4. Diagnosis of Severe Acute Respiratory Syndrome Coronavirus 2 Ribonucleic Acid
The diagnosis of COVID-19 infection was conducted in all cases using real-time reverse transcription-polymerase chain reaction (RT-PCR) to detect SARS-CoV-2 ribonucleic acid in nasopharyngeal swab specimens (Pishtaz Teb, Iran). Some patients were clinically diagnosed based on their lung imaging features, especially computed tomography (CT), and enrolled by the census method, and the results were compared between the patients and controls.
3.5. Statistical Analysis
The continuous variables, such as demographic data, are presented as median ± standard deviation or frequencies. The chi-square test or Fisher’s exact test was used for the comparison of categorical variables. A P-value less than 0.05 was considered statistically significant. All data analyses were performed using SPSS software (version 20; SPSS Inc., Chicago, IL, USA). The flow diagram of the experimental design is shown in Figure 1.
Flow diagram of the experimental design
4. Results
4.1. Basic Characteristics
Among 93 CHB patients and 62 healthy controls who participated in this study, 65.2% were female. The mean age of the participants was 35.77 ± 20.27 years. There were no statistically significant differences between the age (P = 0.2) and gender (P = 0.4) of all participants with COVID-19 infection.
4.2. Comparisons of Laboratory Information Among Participants
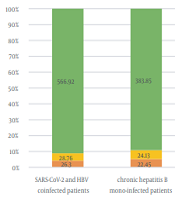
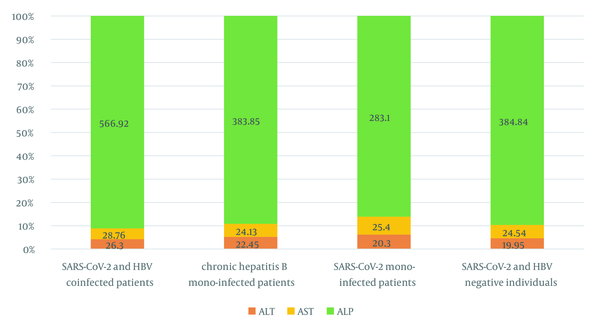
Based on the results of the RT-PCR test, only 14% (13/93) of participants with CHB were RT-PCR COVID-19 positive (cycle threshold [CT] < 30). According to the assay, 18.2% and 18.75% of the patients were positive for RT-PCR SARS-CoV-2 with anti-HCV and anti-HDV antibodies, respectively (Table 2). On the other hand, in a 55-year-old married CHB female subject, anti-HCV, anti-HDV, and RT-PCR SARS-CoV-2 (CT < 30) were simultaneously positive. Alternatively, this patient was hospitalized for 3 days and took antiviral drugs for 1 year. The results of liver function tests revealed that the mean value of ALP in CHB superinfection COVID-19 patients was 403.68 ± 313.12, which was higher than other participants and exceeded the normal range (80 - 360 U/I). On the other hand, the mean values of ALT and AST in CHB superinfection COVID-19 patients were 26.30 ± 11.79 and 28.76 ± 9.33, respectively, and higher than other participants (ref. range > 30) (Figure 2).
| Group | Age | Gender | HBsAg | HBs-Ab | HBc-Ab | RT-PCR Result for COVID-19 | Total | Anti-HCV Antibodies | Anti-HDV Antibodies | |
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | |||||||||
| SARS-CoV-2 and chronic hepatitis B infected patients | 43.23 ± 18 | 5/13 (38.4) | 8/13 (61.53) | Positive | Negative | Positive | Positive (CT < 30) | 13/93 (14) | 2/11 (18.2) | 3/16 (18.75) |
| Chronic hepatitis B monoinfected patients | 39.11 ± 19 | 22/80 (27.5) | 58/80 (72.5) | Positive | Negative | Positive | Negative (CT > 30) | 80/93 (86) | 9/11 (81.8) | 13/16 (81.25) |
| SARS-CoV-2 monoinfected patients | 33.5 ± 14 | 10/20 (50) | 10/20 (50) | Negative | Positive or Negative | Negative | Positive (CT < 30) | 20/62 (32.25) | - | - |
| SARS-CoV-2 and HBV-negative patients | 28.17 ± 22 | 17/42 (40.4) | 25/42 (59.5) | Negative | Positive or Negative | Negative | Negative (CT > 30) | 42/62 (62.74) | - | - |
Comparison of liver function enzymes between chronic hepatitis B and Coronavirus disease 2019 patients
4.3. Relation Between Clinical Manifestations of Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Viral Hepatitis Patients
Serum HBV DNA loads were tested in CHB superinfection COVID-19 and CHB patients, and both had detectable serum HBV DNA. Furthermore, the mean value of HBV viral load in CHB patients (133484421 ± 27820140) was higher than in COVID-19 and HBV superinfected patients (44649695 ± 14118193). Totally, no mortalities occurred during the entire study period, and 9% (3/33) of the patients with SARS-CoV-2 were hospitalized for an average of 3 days. Of all the patients in the SARS-CoV-2-HBV infected group, only one of two cases in the SARS-CoV-2- HBV-HCV group was hospitalized for 2 days in the intensive care unit (ICU). Of the entire SARS-CoV-2 positive patients (control group), 33.4% (11/33) of patients were hospitalized for an average of 7 days, and 3 of 11 cases in this group were hospitalized for 5 days in the ICU (Table 3). By comparing the above-mentioned results, 30% (10/33) of CHB patients who were infected with SARS-CoV-2 were not hospitalized, which was higher than the group of SARS-CoV-2 single infected (27%). As a result, it was observed that having a hepatitis virus infection somewhat reduced the chances of getting COVID-19 infection and the hospitalization process; however, it was not statistically significant (95% confidence interval [CI]: 0.051 - 1.17).
Association Between Coronavirus Disease 2019 Infection in Chronic Hepatitis B Patients and Hepatitis B Surface Antigen Negative Patients
| COVID-19 Positive Patients (n = 33); HBsAg | No. (%) |
|---|---|
| HBsAg positive patients | 13 (39.4) |
| Hospitalized during the study | 3 (9) |
| HBsAg negative patients | 20 (60.6) |
| Hospitalized during the study | 11 (33.4) |
The majority of COVID-19 patients were provided with supportive medication that was comprised of oxygen with hydroxychloroquine, hydroxychloroquine, Kaletra, and antibiotics. The most common antibiotics used for inpatients were vancomycin, tavanex, azithromycin, and meropenem.
According to the data collected on taking medication in patients with hepatitis B, 15.1% (5/33) of all the patients were treated with the antiviral drug, and only one case was hospitalized (Table 3). The results of the present analysis revealed that the risk of developing COVID-19 in healthy individuals was 2.3 times higher than in CHB patients (0.95% CI: 1.242 - 4.290). Antiviral treatment in CHB patients, including tenofovir and entecavir, resulted in a decreased occurrence of SARS-CoV-2 positivity (adjusted odds ratio = 0.862; 0.95% CI, 0.257 - 2.884); nevertheless, the treatment did not bring about severe clinical consequences of COVID-19. There were no statistically significant differences between antiviral treatment and COVID-19 (P > 0.05). Out of 33 patients whose COVID-19 was confirmed by laboratory tests and RT-PCR, all of them underwent lung CT scans. Based on the results, 15.1% of SARS-CoV-2 and CHB infected patients and 36.3% of SARS-CoV-2 monoinfected patients had pulmonary involvement, and 24.2% of the two groups had normal CT scans.
5. Discussion
The COVID-19-related hepatic complications are particularly troublesome among patients with HCV, HBV, or HBV/HCV coinfection with preexisting liver complications. Considering the high pressure brought about by hepatitis B worldwide, the effect of SARS-CoV-2 infection on HBV patients should be evaluated. This study summarized the data of 93 chronic HBV patients and 62 patients with SARS-CoV-2/HBV superinfection and without HBV. This study showed that the mean values of ALT and AST in superinfected HBV/SARS-CoV-2 patients were 26.30 ± 11.79 and 28.76 ± 9.33, respectively, and higher than HBV/COVID-19 monoinfected patients (> 30). Li et al. reported that out of seven COVID-19 patients aged 33 - 49 years with chronic HBV infection, six patients were male, two patients had HBV-related cirrhosis, and 1 patient was positive for serum HBe-Ag (29). However, in the present study, most participants were female, with a mean age of 35.77 ± 20.27 years. In addition, 13.93% of all the cases were superinfected with HBV/SARS-CoV-2.
Zou et al. reported that 14 patients with simultaneous conditions of SARS-CoV-2 and chronic HBV suffered from liver complications with severe COVID-19 witnessed in patients suffering from a liver injury (30). However, this study did not observe more severe outcomes of COVID-19 infection in patients with HBV infection; nevertheless, 13.93% of all the cases were SARS-CoV-2 and chronic HBV coinfected patients, which is not in line with the results of the study by Zou et al. (30). The HBV superinfection did not increase the risk of disease severity or lead to a worse prognosis in COVID-19 infection. Kang et al. reported that in 204,418 patients tested for SARS-CoV-2, the occurrence of CHB was observed to be at a lower rate in those suffering from COVID-19 infection than in those who were SARS-CoV-2 negative (i.e., controls) (1). Kang et al. also mentioned that the occurrence of CHB led to a lower rate of SARS-CoV-2 positivity and was responsible for the appearance of severe clinical consequences of COVID-19 infection; however, it should be noted that this value was observed to be insignificant (1). In the present study, the percentage of SARS-CoV-2 positive patients was lower than SARS-CoV-2 negative in CHB patients, which is consistent with the results of the study by Kang et al. (1).
He et al. reported that out of 571 COVID-19 patients, 15 cases (2.6%) were observed to be HBV infected; nonetheless, only 3 cases (20%) were given an anti-HBV treatment (entecavir) (31). He et al. also witnessed milder manifestations of severe events; however, they did not analyze the effect of antiviral treatment on prognosis (31). The aforementioned results are consistent with the results of the current study regarding the anti-HBV therapy (34.40%) in HBV patients. Additionally, in a cohort study by Chen et al., out of 326 confirmed COVID-19 patients, 20 cases (6.1%) were reported to be HBV superinfected (32), which is in accordance with the results of the present study. However, in numerous other articles, HBV superinfection in the patients under study was not considerable, which is likely due to the fact that a smaller number of the patients were given antiviral medications, which might have influenced the results. In the present study, 35.40% of CHB patients had received antiviral treatment, and 5.4% of HBV- positive patients were HBV/SARS-CoV-2 superinfected. A recently published cohort investigation carried out on a larger scale in Spain indicated that the occurrence of COVID-19 infection in patients with CHB who were given tenofovir was reduced (0.4%), which shows the positive influence of tenofovir on SARS-CoV-2 (33).
The present study showed that AST and ALT levels in HBV/SARS-CoV-2 superinfected patients were higher than in other participants. Liu et al. reported that some of the COVID-19 patients that had or did not have the infection brought about by HBV experienced a higher occurrence of AST (9). Similar observations have reported impaired liver function in SARS patients, which is not in line with the results of the present study. Superinfection has a possible effect on the elevation of morbidity and mortality when pandemics occur. The estimated viral superinfection was reported as 12.58% (95% CI: 7.31 - 18.96). Blood viruses (95% CI: 8.57 - 16.93) had the most frequent viral superinfection; however, respiratory viruses (95% CI: 2.78 - 6.15) had less frequent viral superinfection (12).
Another study reported that 11.6% of COVID-19 patients had superinfection, and superinfection with respiratory viruses is common among COVID-19 patients (34). Superinfection with hepatitis viruses and SARS-CoV-2 is also quite controversial. A study demonstrated that SARS-CoV-2-HBV cases had mortality rates of 4.7% and 15% in cross-sectional and case report investigations, respectively; nevertheless, the occurrence of SARS-CoV-2-HCV cases showed an 8.3% mortality (7), which is not in line with the findings of the current study. The percentage of HBV/COVID-19 superinfection was reported to be 13.93%; nonetheless, the rates of SARS-CoV-2 or HBV alone were 20.62% and 80.93%, respectively.
Mirzaie et al. reported a 4.7% mortality rate among the reported cases of SARS-CoV-2-HBV coinfection and the hospitalization of 86.4% (19 of 22) cases in the SARS-CoV-2-HCV group (7). Although this finding can be considered an indication of higher hospitalization risk among these patients, it also revealed that this condition is mainly observed among patients suffering from severe COVID-19 with hepatic comorbidities (7). Another study conducted by Mirzaie et al. indicated that 235 and 22 patients with SARS-CoV-2 were infected with HBV and HCV, respectively, and the majority of patients were male (7). The mean age of HBV patients was 49.8 years, and the mortality rate was 6%; however, the mean age in SARS-CoV-2-HCV patients was 62.8 years. This was further apparent in Mirzaie et al.’s findings, where 14.1% in the HBV group and 21.4% in the HCV group were transferred to the ICU, and severe COVID-19 was reported in 38.8% and 21.4% of HBV and HCV patients, respectively (7, 35).
The results of the above-mentioned studies were somewhat consistent with the findings of the present study. Of all of the patients in the SARS-CoV-2-HBV infected group, only three patients (9%) were hospitalized, and one of two cases in the SARS-CoV-2-HBV-HCV group were hospitalized for 2 days in the ICU. In other words, 18.2% of the SARS-CoV-2-HBV infected group were positive for HCV coinfection. In addition, 30% of CHB patients who were infected with SARS-CoV-2 were not hospitalized, which was higher than the group of SARS-CoV-2 single infected (27%). Nevertheless, in all 155 participants in the current study, no mortalities occurred during the entire study period. As a result, it was observed that having a hepatitis virus infection somewhat reduced the chances of getting COVID-19 and the hospitalization process; nonetheless, it was not statistically significant (95% CI: 0.051 - 1.17).
5.1. Conclusions
Due to the COVID-19 pandemic, there has been plausible negligence in mitigating viral hepatitis. On the other hand, the hepatic manifestations of COVID-19 are frequently observed among HBV patients. The results of this study revealed that the abnormalities detected in liver function could occur in COVID-19 patients suffering from the infection caused by CHB. Nevertheless, no patient showed severe liver injury when tested in hospitalization. In addition, the results indicated that the occurrence of COVID-19 infection in those suffering from CHB was not as much as that in public and that an antiviral treatment using tenofovir might reduce the risk of COVID-19 infection. The aforementioned findings showed that the immune condition of the host is to some extent influenced by CHB, which might affect the consequences of the infection brought about by SARS-CoV-2.
References
-
1.
Kang SH, Cho DH, Choi J, Baik SK, Gwon JG, Kim MY. Association between chronic hepatitis B infection and COVID-19 outcomes: A Korean nationwide cohort study. PLoS One. 2021;16(10). e0258229. [PubMed ID: 34610052]. [PubMed Central ID: PMC8491877]. https://doi.org/10.1371/journal.pone.0258229.
-
2.
Xiang TD, Zheng X. Interaction between hepatitis B virus and SARS-CoV-2 infections. World J Gastroenterol. 2021;27(9):782-93. [PubMed ID: 33727770]. [PubMed Central ID: PMC7941862]. https://doi.org/10.3748/wjg.v27.i9.782.
-
3.
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. [PubMed ID: 31978945]. [PubMed Central ID: PMC7092803]. https://doi.org/10.1056/NEJMoa2001017.
-
4.
Alqahtani SA, Buti M. COVID-19 and hepatitis B infection. Antivir Ther. 2020;25(8):389-97. [PubMed ID: 33616549]. https://doi.org/10.3851/IMP3382.
-
5.
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [PubMed ID: 32217556]. [PubMed Central ID: PMC7190011]. https://doi.org/10.1136/bmj.m1091.
-
6.
Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361(9371):1767-72. [PubMed ID: 12781535]. [PubMed Central ID: PMC7112410]. https://doi.org/10.1016/s0140-6736(03)13412-5.
-
7.
Mirzaie H, Vahidi M, Shokoohi M, Darvishian M, Sharifi H, Sharafi H, et al. COVID-19 among patients with hepatitis b or hepatitis C: A systematic review. Hepat Mon. 2021;20(11). https://doi.org/10.5812/hepatmon.111617.
-
8.
Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73(5):1231-40. [PubMed ID: 32553666]. [PubMed Central ID: PMC7295524]. https://doi.org/10.1016/j.jhep.2020.06.006.
-
9.
Liu R, Zhao L, Cheng X, Han H, Li C, Li D, et al. Clinical characteristics of COVID-19 patients with hepatitis B virus infection - a retrospective study. Liver Int. 2021;41(4):720-30. [PubMed ID: 33351265]. https://doi.org/10.1111/liv.14774.
-
10.
Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS-associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology. 2004;39(2):302-10. [PubMed ID: 14767982]. [PubMed Central ID: PMC7165792]. https://doi.org/10.1002/hep.20111.
-
11.
Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14(5):612-20. [PubMed ID: 32725453]. [PubMed Central ID: PMC7386238]. https://doi.org/10.1007/s12072-020-10078-2.
-
12.
Malekifar P, Pakzad R, Shahbahrami R, Zandi M, Jafarpour A, Rezayat SA, et al. Viral coinfection among COVID-19 patient groups: an update systematic review and meta-analysis. Biomed Res Int. 2021;2021:5313832. [PubMed ID: 34485513]. [PubMed Central ID: PMC8416381]. https://doi.org/10.1155/2021/5313832.
-
13.
Kazmi SK, Khan FMA, Natoli V, Hunain R, Islam Z, Costa A, et al. Viral hepatitis amidst COVID-19 in Africa: Implications and recommendations. J Med Virol. 2022;94(1):7-10. [PubMed ID: 34506635]. [PubMed Central ID: PMC8661579]. https://doi.org/10.1002/jmv.27330.
-
14.
Shi Y, Zheng M. Hepatitis B virus persistence and reactivation. BMJ. 2020;370:m2200. [PubMed ID: 32873599]. https://doi.org/10.1136/bmj.m2200.
-
15.
Mansour-Ghanaei F, Joukar F, Naghipour M, Hassanipour S, Yeganeh S, Sepehrimanesh M, et al. Epidemiologic profile of viral hepatitis B and C in North of Iran: results from PERSIAN Guilan Cohort Study (PGCS). BMC Res Notes. 2021;14(1):59. [PubMed ID: 33568187]. [PubMed Central ID: PMC7877021]. https://doi.org/10.1186/s13104-021-05474-2.
-
16.
Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428-30. [PubMed ID: 32145190]. [PubMed Central ID: PMC7129165]. https://doi.org/10.1016/S2468-1253(20)30057-1.
-
17.
Xia Y, Liang TJ. Development of direct-acting antiviral and host-targeting agents for treatment of hepatitis b virus infection. Gastroenterology. 2019;156(2):311-24. [PubMed ID: 30243618]. [PubMed Central ID: PMC6340783]. https://doi.org/10.1053/j.gastro.2018.07.057.
-
18.
Cooke GS, Andrieux-Meyer I, Applegate TL, Atun R, Burry JR, Cheinquer H, et al. Accelerating the elimination of viral hepatitis: a Lancet Gastroenterology & Hepatology Commission. Lancet Gastroenterol Hepatol. 2019;4(2):135-84. [PubMed ID: 30647010]. https://doi.org/10.1016/S2468-1253(18)30270-X.
-
19.
G. B. D. Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736-88. [PubMed ID: 30496103]. [PubMed Central ID: PMC6227606]. https://doi.org/10.1016/S0140-6736(18)32203-7.
-
20.
Pott H, Theodoro M, de Almeida Vespoli J, Senise JF, Castelo A. Mother-to-child transmission of hepatitis C virus. Eur J Obstet Gynecol Reprod Biol. 2018;224:125-30. [PubMed ID: 29597101]. https://doi.org/10.1016/j.ejogrb.2018.03.034.
-
21.
Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998-1004. [PubMed ID: 32170806]. [PubMed Central ID: PMC7228361]. https://doi.org/10.1111/liv.14435.
-
22.
ZhiLiang G. Study of the relationship SARS and hepatitis virus B. Chin J Clini Hepatol. 2003;19(6):342-3.
-
23.
Aghbash PS, Eslami N, Shirvaliloo M, Baghi HB. Viral coinfections in COVID-19. J Med Virol. 2021;93(9):5310-22. [PubMed ID: 34032294]. [PubMed Central ID: PMC8242380]. https://doi.org/10.1002/jmv.27102.
-
24.
Sonneveld MJ, Murad SD, van der Eijk AA, de Man RA. Fulminant liver failure due to hepatitis b reactivation during treatment with tocilizumab. ACG Case Rep J. 2019;6(12). e00243. [PubMed ID: 32042838]. [PubMed Central ID: PMC6946203]. https://doi.org/10.14309/crj.0000000000000243.
-
25.
Chen LF, Mo YQ, Jing J, Ma JD, Zheng DH, Dai L. Short-course tocilizumab increases risk of hepatitis B virus reactivation in patients with rheumatoid arthritis: a prospective clinical observation. Int J Rheum Dis. 2017;20(7):859-69. [PubMed ID: 28160426]. https://doi.org/10.1111/1756-185X.13010.
-
26.
Wong GL, Wong VW, Yuen BW, Tse YK, Yip TC, Luk HW, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72(1):57-66. [PubMed ID: 31499132]. https://doi.org/10.1016/j.jhep.2019.08.023.
-
27.
Suslov A, Boldanova T, Wang X, Wieland S, Heim MH. Hepatitis B virus does not interfere with innate immune responses in the human liver. Gastroenterology. 2018;154(6):1778-90. [PubMed ID: 29408639]. https://doi.org/10.1053/j.gastro.2018.01.034.
-
28.
Zhao J, Liao X, Wang H, Wei L, Xing M, Liu L, et al. Early virus clearance and delayed antibody response in a case of coronavirus disease 2019 (COVID-19) with a history of coinfection with human immunodeficiency virus type 1 and hepatitis C virus. Clin Infect Dis. 2020;71(16):2233-5. [PubMed ID: 32270178]. [PubMed Central ID: PMC7184426]. https://doi.org/10.1093/cid/ciaa408.
-
29.
Li Y, Li C, Wang J, Zhu C, Zhu L, Ji F, et al. A case series of COVID-19 patients with chronic hepatitis B virus infection. J Med Virol. 2020;92(11):2785-91. [PubMed ID: 32558945]. [PubMed Central ID: PMC7323302]. https://doi.org/10.1002/jmv.26201.
-
30.
Zou X, Fang M, Li S, Wu L, Gao B, Gao H, et al. Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV coinfection. Clin Gastroenterol Hepatol. 2021;19(3):597-603. [PubMed ID: 32553907]. [PubMed Central ID: PMC7294291]. https://doi.org/10.1016/j.cgh.2020.06.017.
-
31.
He Q, Zhang G, Gu Y, Wang J, Tang Q, Jiang Z, et al. Clinical characteristics of COVID-19 patients with pre-existing hepatitis B virus infection: a multicenter report. Am J Gastroenterol. 2021;116(2):420-1. [PubMed ID: 32925195]. [PubMed Central ID: PMC7505029]. https://doi.org/10.14309/ajg.0000000000000924.
-
32.
Chen L, Huang S, Yang J, Cheng X, Shang Z, Lu H, et al. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J Viral Hepat. 2020;27(12):1504-7. [PubMed ID: 32668494]. [PubMed Central ID: PMC7404861]. https://doi.org/10.1111/jvh.13362.
-
33.
Lens S, Miquel M, Mateos-Munoz B, Garcia-Samaniego J, Forns X. SARS-CoV-2 in patients on antiviral HBV and HCV therapy in Spain. J Hepatol. 2020;73(5):1262-3. [PubMed ID: 32673740]. [PubMed Central ID: PMC7357504]. https://doi.org/10.1016/j.jhep.2020.07.007.
-
34.
Davis B, Rothrock AN, Swetland S, Andris H, Davis P, Rothrock SG. Viral and atypical respiratory co-infections in COVID-19: a systematic review and meta-analysis. J Am Coll Emerg Physicians Open. 2020;1(4):533-48. [PubMed ID: 32838380]. [PubMed Central ID: PMC7323310]. https://doi.org/10.1002/emp2.12128.
-
35.
Khalili M, Karamouzian M, Nasiri N, Javadi S, Mirzazadeh A, Sharifi H. Epidemiological characteristics of COVID-19: a systematic review and meta-analysis. Epidemiol Infect. 2020;148. e130. [PubMed ID: 32594937]. [PubMed Central ID: PMC7343974]. https://doi.org/10.1017/S0950268820001430.