Prevalence and risk factors for postinfectious cough in discharged patients with coronavirus disease 2019 (COVID-19)
Introduction
Up to December 18, 2021, coronavirus disease 2019 (COVID-19) infection has been reported in more than two hundred million cases around the world. Given the epidemic has been going on for an extended period of time, an incredibly increasing number of studies (1-7) involving the epidemiology, transmission pattern, immune mechanism, clinical manifestation, radiological features and clinical drug trials, have been trying to reveal the whole picture of this novel disease. As more and more patients recovered from the acute infection of COVID-19, increasing attention has been directed to the long-term outcome of the disease, which is now referred to as “post-acute sequelae of SARS-CoV-2 infection” (PASC).
Numerous studies have confirmed that cough is one of the most common symptoms of COVID-19, which occurred in 40–80% of patients with COVID-19 during infectious period (8-11). Evidence from other viral infection caused by rhinovirus and adenovirus suggested that postinfectious cough was common (12,13), and some even developed into chronic cough (12,14,15). Another survey conducted on H1N1 influenza found that postinfectious cough occurred in 8.5% of patients and 2.8% of patients developed chronic cough (16). In online surveys, cough was reported in 20–64% of patients 2–6 months after the onset of symptoms of COVID-19 (17-23) and 2.5–23.3% after 6 months (24-27). The results of the prevalence of cough in the long-term follow-up are quite different. In addition, there is no study exploring the risk factors associated with postinfectious cough in patients with COVID-19. In this study, we conducted an 8-month follow-up with a uniform questionnaire in discharged patients with COVID-19, to clarify the prevalence and risk factors of postinfectious cough in COVID-19. We present the following article in accordance with the STROBE reporting checklist (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-876/rc).
Methods
Participants
This observational follow-up was based on our previous study conducted at First People’s Hospital of Jingzhou and Xiangyang Central Hospital from January 19, 2020, to May 22, 2020 (28). 129 patients with confirmed SARS-CoV-2 infection by real-time PCR in the previous study received a follow-up at about 8 months after discharge from October 2020 to December 2020. The age of patients ranged from 19 to 84 years. The inclusion and diagnosis procedure were described before and disease severity was categorized according to the WHO interim guidance. Patients were discharged after fulfilment of all standard criteria in Diagnosis and Treatment of Novel Coronavirus Pneumonia (Trial version 5 revised) released by China’s National Health Commission (29). Follow-up was conducted mainly by telephone since most patients were unable to revisit the hospital due to the epidemic. To reduce information bias, the telephone follow-up was performed by the same physician in each center. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The First People’s Hospital of Jingzhou (42016803-T) and Xiangyang Central Hospital (2020-001). Only oral informed consent was obtained and recorded in electronic medical records from patients after a full explanation of this study.
Data collection
Baseline demographics, comorbidities, smoking history were extracted from the medical record. Patients returned to hospitals’ outpatient clinics or received telephone interview 8 months after discharge. All patients were asked to complete a uniform questionnaire including items on current symptoms, cough duration, Cough Visual Analog Scales (VAS), cough severity and cough frequency during hospitalization and after discharge. VAS is a 100 mm scale on which patients indicate the severity of cough. Cough severity level was scaled as mild, moderate, and severe and evaluated by patients. Cough frequency was categorized into four scales as scale 1 (a little of the time), scale 2 (some of the time), scale 3 (a good bit of the time) and scale 4 (most of the time). Cough severity and cough frequency were decided subjectively by patients. In this study, we defined postinfectious cough as a cough last for more than 3 weeks and persistent cough as a continuous cough from the onset of COVID-19 infection until the end of follow-up. Patients with a cough persistent for no more than 3 weeks or no cough throughout the disease were regarded as having no postinfectious cough.
Assessment
Spirometry and pulmonary diffusion capacity tests were performed following the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines in a part of patients. Variables with values below the lower limit of normal (LLN) or <80% of predicted were considered abnormal (30). Chest CT imaging analysis was performed by two radiographers with over 10-year experience who were blind to clinical and laboratory findings.
Statistical analysis
Continuous data conformance to normal distribution was described by mean ± standard deviation and an independent t-test was used to compare differences between groups, otherwise median (interquartile range) and Manny-Whitney U-test were used. For categorical data, frequency counts and percentages were used in descriptive analysis. The Chi-square test and Fisher’s exact test were used in group comparison. The association between postinfectious cough and clinical manifestations was assessed by binary logistic regression analysis corrected for age and sex. A two-sided P value <0.05 was considered statistically significant. For missing data, we deleted cases with missing data of a certain variable when analyzing the variable.
Results
Among 301 confirmed cases enrolled in the previous study, 129 were successfully followed up, the mean (standard deviation) age was 51.5 (14.9) years and 57 (44.2%) were male (Table 1). The reasons for loss of follow-up were inability to contact patients or patient refusal of follow-up. In 123 patients who have been classified according to disease severity, the numbers of mild, moderate, severe and critical cases on admission were 1 (0.8%), 107 (87.0%), 12 (9.8%) and 3 (2.4%) respectively, 6 patients were not classified due to lack of origin information on admission since they were transferred from other hospitals. The median (interquartile range) follow-up time was 8.1 (7.9–8.5) months after discharge (Table 1).
Table 1
| Clinical manifestation | Total (n=129) | Postinfectious cough (n=27) | No postinfectious cough (n=102) | P value |
|---|---|---|---|---|
| Age (y), mean (SD) | 51.5 (14.9) | 47.4 (12.9) | 52.6 (15.3) | 0.106 |
| Gender, male | 57 (44.2) | 11 (40.7) | 46 (35.7) | 0.828 |
| Duration of hospitalization (day) | 24.0 (11.0–32.0) | 26.0 (18.0–33.0) | 23.0 (8.8–31.0) | 0.612 |
| Severity on admission | ||||
| Mild | 1/123 (0.8) | 0/26 (0.0) | 1/97 (1.0) | 0.622 |
| Moderate | 107/123 (87.0) | 25/26 (96.2) | 82/97 (84.5) | |
| Severe | 12/123 (9.8) | 1/26 (3.8) | 11/97 (11.3) | |
| Critical severe | 3/123 (2.4) | 0/26 (0.0) | 3/97 (3.1) | |
| Symptoms | ||||
| Fever | 107 (83.0) | 23 (85.2) | 84 (82.4) | 0.999 |
| Cough | 102 (79.1) | 27 (100.0) | 75 (73.5) | NA |
| Sputum | 60 (46.5) | 18 (66.7) | 42 (41.2) | 0.029 |
| Dyspnea | 52 (40.3) | 12 (44.4) | 40 (39.2) | 0.663 |
| Headache | 43 (33.3) | 12 (44.4) | 31 (30.4) | 0.126 |
| Fatigue | 66 (51.2) | 16 (59.3) | 50 (49.0) | 0.391 |
| Myalgia | 52 (40.3) | 11 (40.7) | 41 (40.2) | 0.999 |
| Rhinobyon | 41 (31.8) | 11 (40.7) | 30 (29.4) | 0.352 |
| Sore throat | 44 (34.1) | 13 (48.2) | 31 (30.4) | 0.110 |
| Chest tightness | 23 (17.8) | 6 (22.2) | 17 (16.7) | 0.338 |
| Anorexia | 51 (39.5) | 12 (44.4) | 39 (38.2) | 0.659 |
| Nausea | 45 (34.9) | 14 (51.9) | 31 (30.4) | 0.044 |
| Vomit | 43 (33.3) | 14 (51.9) | 29 (28.4) | 0.037 |
| Diarrhea | 44 (34.1) | 14 (51.9) | 30 (29.4) | 0.040 |
| Chronic respiratory disease | 8 (6.2) | 5 (18.5) | 3 (2.9) | 0.010 |
| Smoking history, n=108 | ||||
| Non-smoker | 91/108 (84.3) | 20/27 (74.1) | 71/81 (87.7) | 0.016 |
| Current smoker | 9/108 (8.3) | 6/27 (22.2) | 3/81 (3.7) | |
| Ex-Smoker | 8/108 (7.4) | 1/27 (3.7) | 7/81 (8.6) |
Data are presented as mean (standard deviation), median (interquartile range) or n/N (%). N represents the number of patients with available data. COVID-19, coronavirus disease 2019; SD, standard deviation; NA, not available.
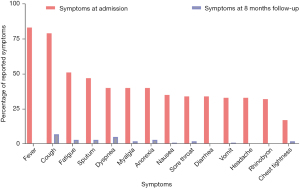
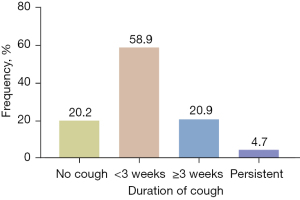
By the end of follow-up, 27 (20.9%) patients still had at least one symptom caused by COVID-19. The presenting symptoms included cough (6, 4.7%), dyspnea (6, 4.7%), sputum (4, 3.1%), fatigue (4, 3.1%), anorexia (4, 3.1%), chest tightness (3, 2.3%), myalgia (3, 2.3%), sore throat (2, 1.6%) and other symptoms (7, 5.4%) including anosmia, hypogeusia, insomnia and palpitation (Figure 1). Based on the duration of cough, 76 (58.9%) patients had acute cough (≤3 weeks after cough onset), and 27 (20.9%) patients had postinfectious cough, among which 6 (4.7%) patients developed into persistent cough (Figure 2). In patients with postinfectious cough, 5 (18.5%) had a history of chronic respiratory disease including chronic obstructive pulmonary disease (COPD), chronic cough and pulmonary tuberculosis, and all of them complained of more severe cough during COVID-19 infection. Six (22.2%) were current smokers, including current second-hand smokers (Table 1). Of 6 patients with persistent cough, 3 had a history of chronic respiratory disease among which 2 were current smokers, while the other 3 patients had no history of respiratory disease and none of them were current smokers. Of these 3 patients with persistent cough caused by COVID-19, the median score of VAS was 30, and all of them presented occasional mild dry cough which could be evoked by cold air, cigarette, smoke or perfume (data not shown).
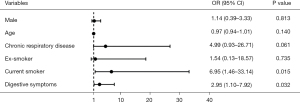
Compared with patients without postinfectious cough, the incidence of digestive symptoms including nausea (51.9% vs. 30.4%, P=0.044), vomit (51.9% vs. 28.4%, P=0.037) and diarrhea (51.9% vs. 29.4%, P=0.040) during hospitalization were higher in postinfectious cough group. In patients who had cough during hospitalization, the proportions of cough frequency were 48.1% and 84.0% on scale 1–2, 51.8% and 16.0% on scale 3–4 in patients with and without postinfectious cough respectively (P<0.001). For cough severity, 51.5% of patients had mild cough, 29.6% had moderate cough, 18.5% had severe cough in patients with postinfectious cough group, while the corresponding proportion were 85.3%, 13.3% and 1.3% in patients without postinfectious cough (P<0.001) (Table 2). The prevalence of a history of chronic respiratory disease was higher in the postinfectious cough group (18.5% vs. 2.9%, P=0.010). The proportion of current smoker was also higher in the postinfectious cough group (22.2% vs. 3.7%, P=0.016). In multivariate logistic regression analysis adjusted by age and gender, digestive symptoms on admission showed an odds ratio (OR) of 2.95 [95% confidence interval (CI): 1.10–7.92] and current smoking showed an OR of 6.95 (95% CI: 1.46–33.14) for postinfectious cough after COVID-19 infection (Figure 3). No significant association of age, gender, or history of chronic respiratory disease with postinfectious cough was observed.
Table 2
| Assessment of cough | Total (n=129) | Postinfectious cough (n=27) | No postinfectious cough (n=102) | P value |
|---|---|---|---|---|
| VAS (mm), n=89 | 21.0 (10.5–51.5) | 42.5 (20.0–72.0) | 19.0 (0.0–48.0) | 0.001 |
| Frequency of cough | (n=102) | (n=27) | (n=75) | |
| A little of the time (scale 1) | 55 (53.9) | 6 (22.2) | 49 (65.3) | <0.001 |
| Some of the time (scale 2) | 21 (20.6) | 7 (25.9) | 14 (18.7) | |
| A good bit of the time (scale 3) | 21 (20.6) | 9 (33.3) | 12 (16.0) | |
| Most of the time (scale 4) | 5 (4.9) | 5 (18.5) | 0 | |
| Severity of cough | (n=102) | (n=27) | (n=75) | |
| Mild (scale 1) | 78 (76.5) | 14 (51.9) | 64 (85.3) | <0.001 |
| Moderate (scale 2) | 18 (17.6) | 8 (29.6) | 10 (13.3) | |
| Severe (scale 3) | 6 (5.9) | 5 (18.5) | 1 (1.3) |
Data are presented as median (interquartile range) or n (%). COVID-19, coronavirus disease 2019; VAS, Visual Analog Scales.
Totally 41 patients received pulmonary function test at the follow-up, 36 (87.8%) patients had at least one abnormal variable. 13 (31.71%) and 3 (7.32%) patients had the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) below LLN, while 6 (14.63%) patients had decreased maximum mid expiratory flow (MMEF). 21 (51%) and 29 (70.7%) patients showed decreased total lung capacity (TLC) and diffusing capacity of the lung for carbon monoxide (DLCO) respectively. No significant difference between the postinfectious cough group and the no postinfectious cough group in all indices mentioned above (data not shown). Chest CT was performed on 50 patients, 39 (78.0%) had at least one type of abnormality. The most common abnormality of chest CT at the follow-up was focal fibrosis (16, 32.0%). Compared to patients without postinfectious cough, more patients showed interstitial infiltration (8.3% vs. 2.6%) in the postinfectious cough group (data not shown).
Discussion
To our knowledge, this is the first study that analyses the prevalence of acute cough, postinfectious cough and persistent cough in COVID-19 with a follow-up of 8 months after discharge. Our study showed that symptoms of COVID-19 significantly reduced long after discharge, however, approximately one-fifth of patients still presented with at least one symptom, including cough, dyspnea, fatigue and anorexia at the follow-up. The incidence of symptoms in our study is significantly lower compared with previous studies (17,18,21-23,26,27). We attributed it to a longer period after discharge, which also implied that COVID-19 infection would rarely cause severe and long-term sequelae.
The prevalence of acute cough and persistent cough caused by COVID-19 in our study were 58.9% and 4.7% respectively. A previous study found that the prevalence of persistent cough was 18% (95% CI: 12–24%), with follow-up duration ranging from 6 weeks to 4 months (31). However, the prevalence varied widely among studies, and is presumably associated with patient characteristics. A recent study involving 1,950 hospitalized COVID-19 patients in Madrid showed that one year after discharge, the prevalence of post-COVID-19 cough was as low as 2.5%. However, in that study, only 28.1% of patients had cough at hospital admission (26) which was much lower than that in our study (79%) and other studies in China (8,32-35). Compared with studies from European and American countries, the prevalence of post-COVID-19 cough in Asian countries seemed to be lower (20–41.9% vs. 27.5–29%) (20-27,36). It is unclear whether the ethnic difference contributed to the prevalence of cough. The prevalence of persistent cough in previous studies enrolling mild to moderate patients was less than 10%, while it was more than 30% in more critical severe patients (37-39). Therefore, besides the follow-up duration, the severity of disease on admission may be a non-negligible reason for the different outcomes. Due to the minority of severe patients in our study, we are not able to explore whether the severity of disease is an independent risk factor for persistent cough in COVID-19 infection. Overall, the prevalence of postinfectious cough especially persistent cough is relatively low compared with other COVID-19 symptoms. The C-fibers innervating conducting airways have been identified in inducing cough and reflex-mediated bronchospasm. However, some evidence implies the C-fibers innervating the distal airways may inhibit cough (11,40,41). Therefore, we speculate the possible reason for the low incidence of postinfectious and persistent cough post COVID-19 is that SARS-CoV-2 infection mainly damages distal airway and alveoli innervated by C-fibers preferentially regulating the bronchia contraction rather than cough upon activation (40,42). That may also explain the inconsistency of a relatively high incidence of decreased lung function and persistent abnormalities in chest CT but low prevalence of persistent cough.
Compared with those without postinfectious cough, patients with postinfectious cough showed more severe and frequent cough during hospitalization. Interestingly, our results also implicated that, patients with evident digestive symptoms including nausea, vomit and diarrhea during infection period are prone to developing postinfectious cough. Digestive symptoms have been reported in severe acute respiratory syndrome (SARS) and COVID-19 patients during and after infection (43-45). Although the pathophysiology of SARS-CoV-2 infection in gastrointestinal tract remains unclear, it has been identified that the virus invades target cells by binding to the angiotensin-converting-enzyme 2 (ACE2) receptors expressed in airway epithelial and gastrointestinal tract, which shares the similar pattern with SARS and Middle East Respiratory Syndrome (MERS) (43,46). The ACE2 gene has also been reported in a subset of human dorsal root ganglion sensory neurons in the thoracic ganglia (47), suggesting the possibility of direct infection of SARS-CoV-2 to sensory nerve. A previous study found the convergence of vagal afferents from the esophagus and respiratory tract in the brain stem, which is the cause of an esophageal-bronchial reflex (48). It might be possible that the central sensitization caused by the interaction of vagal afferents contributes to the prone to postinfectious cough in patients with digestive symptoms. Since the questionnaire did not investigate the duration and treatment of digestive symptoms in our study, it is not clear if cough symptom was parallel to digestive symptoms during the last 8 months after discharge. Whether postinfectious cough after COVID-19 is related to gastroesophageal reflux disease (GERD) or shares a similar mechanism with gastroesophageal reflux-related cough (GERC) remains to be further investigated.
Cigarette smoking is regarded as a major risk factor for respiratory and cardiovascular disease including airway infection. Data had shown that the prevalence of influenza was five times higher in smokers than that in non-smokers (49). Several studies implicated that smoking was significantly associated with more severe and increased mortality of COVID-19 (50-52), however, some studies reported inconsistent results (53). In our study, only 6 patients with persistent cough were current smokers among which 4 patients had no history of respiratory disease. Therefore, we speculated smoking is an important risk factor for postinfectious cough in COVID-19.
Decreased diffusion capacity was presented in most patients who also have comorbid decreased TLC and RV, indicating the injury of alveolar interstitium and a restrictive ventilatory deficit in COVID-19. It was identified in several studies that 10% to 52% of COVID-19 patients had decreased DLCO which was associated with total CT score, dyspnea score and duration of oxygen supplementation during hospitalization (36,54-56). And the high prevalence of lung diffusion impairment could persist for 6 months and one year after discharge (57). However, there was no clear association between cough and decreased diffusion capacity, with regard to the similar DLCO between patients with and without postinfectious cough in our study and the previous study (58). Whether the decline of pulmonary function would persist for a long time needs further and larger cohort studies. With regard to chest CT, a part of patients still presented abnormalities at the follow-up, which was consistent with some previous studies (59,60). We noticed that the proportion of local patchy shadowing and interstitial infiltration were higher in patients with postinfectious cough, suggesting the impairment of parenchyma and interstitium of lung caused by COVID-19 infection may exist for a long time. The pathogenesis of cough in interstitial lung diseases (ILD) or parenchyma and interstitium damage remains unclear. Lung fibrosis could increase cough reflex sensitivity in response to capsaicin (61) and mechanical stimulation of the chest wall (62). A generalized neuronal hypersensitivity caused by neuroinflammatory and neuroimmune mechanisms after the elimination of SARS-CoV-2 might also be an important consideration (31). Thus, a functional up-regulation of sensory neurons of the lung and inflammatory changes could both probably get involved (63).
However, there are some limitations of our study. Firstly, the follow-up was conducted by telephone in a part of patients, so only parts of patients who were able to revisit in clinic received pulmonary function test and chest CT. Secondly, a timely follow-up had not been conducted regularly after discharge, so the duration and severity of postinfectious cough and treatment after discharge could not be reached. Thirdly, most of patients included in this study were moderate, therefore the results may not be applicable to severe and critical severe patients.
Conclusions
Our 8-months follow-up study demonstrated that about one-fifth of COVID-19 patients develop postinfectious cough, but persistent cough is uncommon in rehabilitated patients. Current smoking including second-hand smoking and digestive symptoms in acute phase are risk factors for postinfectious cough after recovery from COVID-19.
Acknowledgments
We would like to thank all doctors and nurses in The First People’s Hospital of Jingzhou, and Xiangyang Central Hospital.
Funding: This study was funded by Incubative Project for Innovation Team of Guangzhou Medical University (GMU) (2017-159), and Clinical Research Foundation of GMU (2017-160). The funder had no role in this study.
Footnote
Provenance and Peer Review: This article was a standard submission for the series “Cough Section” published in Journal of Thoracic Disease. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-876/rc
Data Sharing Statement: Available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-876/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-21-876/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The First People’s Hospital of Jingzhou (42016803-T) and Xiangyang Central Hospital (2020-001). Oral informed consent was obtained and recorded in electronic medical records from patients after a full explanation of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Megha KB, Joseph X, Akhil V, et al. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine 2021;91:153712. [Crossref] [PubMed]
- Koelle K, Martin MA, Antia R, et al. The changing epidemiology of SARS-CoV-2. Science 2022;375:1116-21. [Crossref] [PubMed]
- Rousan LA, Elobeid E, Karrar M, et al. Chest x-ray findings and temporal lung changes in patients with COVID-19 pneumonia. BMC Pulm Med 2020;20:245. [Crossref] [PubMed]
- da Rosa Mesquita R, Francelino Silva LC Junior, Santos Santana FM, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr 2021;133:377-82. [Crossref] [PubMed]
- Zhao Y, Wang D, Mei N, et al. Longitudinal Radiological Findings in Patients With COVID-19 With Different Severities: From Onset to Long-Term Follow-Up After Discharge. Front Med (Lausanne) 2021;8:711435. [Crossref] [PubMed]
- Lamers MM, Haagmans BL. SARS-CoV-2 pathogenesis. Nat Rev Microbiol 2022;20:270-84. [Crossref] [PubMed]
- Dean N. Tracking COVID-19 infections: time for change. Nature 2022;602:185. [Crossref] [PubMed]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [Crossref] [PubMed]
- Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [Crossref] [PubMed]
- Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91-5. [Crossref] [PubMed]
- Tatar M, Webber SE, Widdicombe JG. Lung C-fibre receptor activation and defensive reflexes in anaesthetized cats. J Physiol 1988;402:411-20. [Crossref] [PubMed]
- Bush A, Floto RA. Pathophysiology, causes and genetics of paediatric and adult bronchiectasis. Respirology 2019;24:1053-62. [Crossref] [PubMed]
- Kantar A, Seminara M. Why chronic cough in children is different. Pulm Pharmacol Ther 2019;56:51-5. [Crossref] [PubMed]
- O'Connell F, Thomas VE, Studham JM, et al. Capsaicin cough sensitivity increases during upper respiratory infection. Respir Med 1996;90:279-86. [Crossref] [PubMed]
- Atkinson SK, Sadofsky LR, Morice AH. How does rhinovirus cause the common cold cough? BMJ Open Respir Res 2016;3:e000118. [Crossref] [PubMed]
- Lin L, Yang ZF, Zhan YQ, et al. The duration of cough in patients with H1N1 influenza. Clin Respir J 2017;11:733-8. [Crossref] [PubMed]
- Goërtz YMJ, Van Herck M, Delbressine JM, et al. Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Res 2020;6:e00542-2020. [Crossref] [PubMed]
- What does COVID-19 recovery actually look like? An analysis of the prolonged COVID-19 symptoms survey by patient-led research team. London, UK: The COVID-19 Body Politic Slack Group, 2020. (accessed Sept 24, 2020). Available online: https://patientresearchcovid19.com/research/report-1/
- Sudre CH, Murray B, Varsavsky T, et al. Attributes and predictors of long COVID. Nat Med 2021;27:626-31. [Crossref] [PubMed]
- Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir Med 2020;174:106197. [Crossref] [PubMed]
- Ares-Blanco S, Pérez Álvarez M, Gefaell Larrondo I, et al. SARS-CoV-2 pneumonia follow-up and long COVID in primary care: A retrospective observational study in Madrid city. PLoS One 2021;16:e0257604. [Crossref] [PubMed]
- Bastola A, Nepal R, Shrestha B, et al. Persistent Symptoms in Post-COVID-19 Patients Attending Follow-Up OPD at Sukraraj Tropical and Infectious Disease Hospital (STIDH), Kathmandu, Nepal. Trop Med Infect Dis 2021;6:113. [Crossref] [PubMed]
- Tleyjeh IM, Saddik B, AlSwaidan N, et al. Prevalence and predictors of Post-Acute COVID-19 Syndrome (PACS) after hospital discharge: A cohort study with 4 months median follow-up. PLoS One 2021;16:e0260568. [Crossref] [PubMed]
- Chand S, Kapoor S, Naqvi A, et al. Long-Term Follow up of Renal and Other Acute Organ Failure in Survivors of Critical Illness Due to Covid-19. J Intensive Care Med 2022;37:736-42. [Crossref] [PubMed]
- Fumagalli C, Zocchi C, Tassetti L, et al. Factors associated with persistence of symptoms 1 year after COVID-19: A longitudinal, prospective phone-based interview follow-up cohort study. Eur J Intern Med 2022;97:36-41. [Crossref] [PubMed]
- Fernández-de-Las-Peñas C, Guijarro C, Plaza-Canteli S, et al. Prevalence of Post-COVID-19 Cough One Year After SARS-CoV-2 Infection: A Multicenter Study. Lung 2021;199:249-53. [Crossref] [PubMed]
- Shang YF, Liu T, Yu JN, et al. Half-year follow-up of patients recovering from severe COVID-19: Analysis of symptoms and their risk factors. J Intern Med 2021;290:444-50. [Crossref] [PubMed]
- Long L, Zeng X, Zhang X, et al. Short-term outcomes of COVID-19 and risk factors for progression. Eur Respir J 2020;55:2000990. [Crossref] [PubMed]
- Diagnosis and treatment of novel coronavirus pneumonia (Trial version 5 revised). National Health Commission of the PRC, 2020.
- Quanjer PH, Stanojevic S, Cole TJ, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J 2012;40:1324-43. [Crossref] [PubMed]
- Song WJ, Hui CKM, Hull JH, et al. Confronting COVID-19-associated cough and the post-COVID syndrome: role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir Med 2021;9:533-44. [Crossref] [PubMed]
- Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507-13. [Crossref] [PubMed]
- Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475-81. [Crossref] [PubMed]
- Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA 2020;323:1843-4. [Crossref] [PubMed]
- Wang Y, Luo S, Zhou CS, et al. Clinical and radiological characteristics of COVID-19: a multicentre, retrospective, observational study. Hong Kong Med J 2021;27:7-17. [PubMed]
- Liang L, Yang B, Jiang N, et al. Three-month Follow-up Study of Survivors of Coronavirus Disease 2019 after Discharge. J Korean Med Sci 2020;35:e418. [Crossref] [PubMed]
- Xiong Q, Xu M, Li J, et al. Clinical sequelae of COVID-19 survivors in Wuhan, China: a single-centre longitudinal study. Clin Microbiol Infect 2021;27:89-95. [Crossref] [PubMed]
- Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 2020;25:100463. [Crossref] [PubMed]
- Sadeghi H, Lumanglas AL, Baumbach WR, et al. Interaction of monoclonal antibodies with growth hormone-binding protein and its complex with growth hormone. J Endocrinol 1993;139:495-501. [Crossref] [PubMed]
- Chou YL, Mori N, Canning BJ. Opposing effects of bronchopulmonary C-fiber subtypes on cough in guinea pigs. Am J Physiol Regul Integr Comp Physiol 2018;314:R489-98. [Crossref] [PubMed]
- Stone RA, Worsdell YM, Fuller RW, et al. Effects of 5-hydroxytryptamine and 5-hydroxytryptophan infusion on the human cough reflex. J Appl Physiol (1985) 1993;74:396-401. [Crossref] [PubMed]
- Dicpinigaitis PV, Canning BJ. Is There (Will There Be) a Post-COVID-19 Chronic Cough? Lung 2020;198:863-5. [Crossref] [PubMed]
- Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767-72. [Crossref] [PubMed]
- Zhang J, Garrett S, Sun J. Gastrointestinal symptoms, pathophysiology, and treatment in COVID-19. Genes Dis 2021;8:385-400. [Crossref] [PubMed]
- Effenberger M, Grabherr F, Mayr L, et al. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 2020;69:1543-4. [Crossref] [PubMed]
- Hofmann H, Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol 2004;12:466-72. [Crossref] [PubMed]
- Shiers S, Ray PR, Wangzhou A, et al. ACE2 and SCARF expression in human dorsal root ganglion nociceptors: implications for SARS-CoV-2 virus neurological effects. Pain 2020;161:2494-501. [Crossref] [PubMed]
- Smith JA, Decalmer S, Kelsall A, et al. Acoustic cough-reflux associations in chronic cough: potential triggers and mechanisms. Gastroenterology 2010;139:754-62. [Crossref] [PubMed]
- Lawrence H, Hunter A, Murray R, et al. Cigarette smoking and the occurrence of influenza - Systematic review. J Infect 2019;79:401-6. [Crossref] [PubMed]
- Zhang H, Ma S, Han T, et al. Association of smoking history with severe and critical outcomes in COVID-19 patients: A systemic review and meta-analysis. Eur J Integr Med 2021;43:101313. [Crossref] [PubMed]
- Adrish M, Chilimuri S, Mantri N, et al. Association of smoking status with outcomes in hospitalised patients with COVID-19. BMJ Open Respir Res 2020;7:e000716. [Crossref] [PubMed]
- Farsalinos K, Barbouni A, Poulas K, et al. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis 2020;11:2040622320935765. [Crossref] [PubMed]
- Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med 2020;75:107-8. [Crossref] [PubMed]
- Smet J, Stylemans D, Hanon S, et al. Clinical status and lung function 10 weeks after severe SARS-CoV-2 infection. Respir Med 2021;176:106276. [Crossref] [PubMed]
- Lerum TV, Aaløkken TM, Brønstad E, et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J 2021;57:2003448. [Crossref] [PubMed]
- Shah AS, Wong AW, Hague CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax 2021;76:402-4. [Crossref] [PubMed]
- Huang L, Yao Q, Gu X, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet 2021;398:747-58. [Crossref] [PubMed]
- Zhao Y, Yang C, An X, et al. Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis 2021;112:173-82. [Crossref] [PubMed]
- Zhang S, Liu L, Yang B, et al. Clinical characteristics of 134 convalescent patients with COVID-19 in Guizhou, China. Respir Res 2020;21:314. [Crossref] [PubMed]
- Mandal S, Barnett J, Brill SE, et al. 'Long-COVID': a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2021;76:396-8. [Crossref] [PubMed]
- Hope-Gill BD, Hilldrup S, Davies C, et al. A study of the cough reflex in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2003;168:995-1002. [Crossref] [PubMed]
- Jones RM, Hilldrup S, Hope-Gill BD, et al. Mechanical induction of cough in Idiopathic Pulmonary Fibrosis. Cough 2011;7:2. [Crossref] [PubMed]
- Brown KK. Chronic cough due to chronic interstitial pulmonary diseases: ACCP evidence-based clinical practice guidelines. Chest 2006;129:180S-5S. [Crossref] [PubMed]