A Case Series of SARS-CoV-2 Reinfection in Elite Athletes
Abstract
:1. Introduction
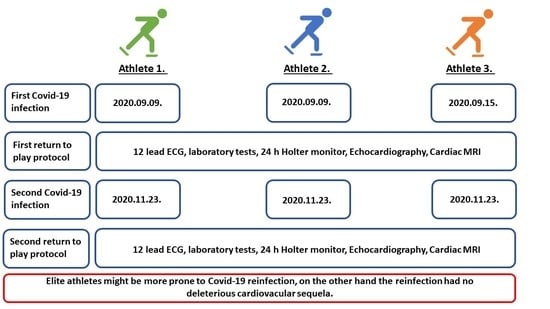
2. Case Presentations
3. Discussion
4. Conclusions
4.1. Practical Applications
4.2. Possible Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lauring, A.S.; Hodcroft, E.B. Genetic Variants of SARS-CoV-2-What Do They Mean? JAMA 2021, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Chakeri, A.; Ioannidis, J.P.; Richter, L.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Allerberger, F. SARS-CoV-2 re-infection risk in Austria. Eur. J. Clin. Investig. 2021, 51, e13520. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Molbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- Martinez, M.W.; Tucker, A.M.; Bloom, O.J.; Green, G.; DiFiori, J.P.; Solomon, G.; Phelan, D.; Kim, J.H.; Meeuwisse, W.; Sills, A.K.; et al. Prevalence of Inflammatory Heart Disease among Professional Athletes with Prior COVID-19 Infection Who Received Systematic Return-to-Play Cardiac Screening. JAMA Cardiol. 2021, 6, 745–752. [Google Scholar] [CrossRef]
- Wilson, M.G.; Hull, J.H.; Rogers, J.; Pollock, N.; Dodd, M.; Haines, J.; Harris, S.; Loosemore, M.; Malhotra, A.; Pieles, G.; et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: A practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020, 54, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Pillay, L.; Janse van Rensburg, D.C.C.; Jansen van Rensburg, A.; Ramagole, D.A.; Holtzhausen, L.; Dijkstra, H.P.; Cronje, T. Nowhere to hide: The significant impact of coronavirus disease 2019 (COVID-19) measures on elite and semi-elite South African athletes. J. Sci. Med. Sport 2020, 23, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.; Delgado, L.; Moreira, P.; Haahtela, T. Does exercise increase the risk of upper respiratory tract infections? Br. Med. Bull. 2009, 90, 111–131. [Google Scholar] [CrossRef] [Green Version]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Xiao, C.H.; Chen, L.F.; Li, Y. Recurrent SARS-CoV-2 RNA positivity and prolonged viral shedding in a patient with COVID-19: A case report. BMC Infect. Dis. 2021, 21, 1076. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Risch, S.; Doderer-Lang, C.; Caillard, S.; Fafi-Kremer, S. Long-term shedding of viable SARS-CoV-2 in kidney transplant recipients with COVID-19. Am. J. Transplant. 2021, 21, 2871–2875. [Google Scholar] [CrossRef] [PubMed]
- Addetia, A.; Crawford, K.H.D.; Dingens, A.; Zhu, H.; Roychoudhury, P.; Huang, M.L.; Jerome, K.R.; Bloom, J.D.; Greninger, A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Naaber, P.; Hunt, K.; Pesukova, J.; Haljasmagi, L.; Rumm, P.; Peterson, P.; Hololejenko, J.; Eero, I.; Jogi, P.; Toompere, K.; et al. Evaluation of SARS-CoV-2 IgG antibody response in PCR positive patients: Comparison of nine tests in relation to clinical data. PLoS ONE 2020, 15, e0237548. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, A.; Liu, J.; Martinot, A.J.; McMahan, K.; Mercado, N.B.; Peter, L.; Tostanoski, L.H.; Yu, J.; Maliga, Z.; Nekorchuk, M.; et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020, 369, 812–817. [Google Scholar] [CrossRef]
- Campbell, J.P.; Turner, J.E. Debunking the Myth of Exercise-Induced Immune Suppression: Redefining the Impact of Exercise on Immunological Health Across the Lifespan. Front. Immunol. 2018, 9, 648. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, M.; Pyne, D.B. Special feature for the Olympics: Effects of exercise on the immune system: Exercise effects on mucosal immunity. Immunol. Cell Biol. 2020, 78, 536–544. [Google Scholar] [CrossRef]
- Russell, M.W.; Moldoveanu, Z.; Ogra, P.L.; Mestecky, J. Mucosal Immunity in COVID-19: A Neglected but Critical Aspect of SARS-CoV-2 Infection. Front. Immunol. 2020, 11, 611337. [Google Scholar] [CrossRef]
- Surkova, E.; Nikolayevskyy, V.; Drobniewski, F. False-positive COVID-19 results: Hidden problems and costs. Lancet Respir. Med. 2020, 8, 1167–1168. [Google Scholar] [CrossRef]
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano. 2020, 14, 3822–3835. [Google Scholar] [CrossRef]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering from COVID-19 Infection. JAMA Cardiol. 2020, 6, 116–118. [Google Scholar] [CrossRef] [PubMed]
- Moulson, N.; Petek, B.J.; Drezner, J.A.; Harmon, K.G.; Kliethermes, S.A.; Patel, M.R.; Baggish, A.L.; Investigators, O. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation 2021, 144, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, L.; Cochet, H.; Mahida, S.; Blanchard S, S.; Benard, A.; Cariou, T.; Sridi-Cheniti, S.; Benhenda, S.; Doutreleau, S.; Cade, S.; et al. Resuming Training in High-Level Athletes after Mild COVID-19 Infection: A Multicenter Prospective Study (ASCCOVID-19). Sports Med. Open 2022, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Writing, C.; Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll Cardiol. 2022, 79, 1717–1756. [Google Scholar]
- Bauer, P.; Kraushaar, L.; Dorr, O.; Keranov, S.; Nef, H.; Hamm, C.W.; Most, A. Vascular alterations among male elite athletes recovering from SARS-CoV-2 infection. Sci. Rep. 2022, 12, 8655. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fülöp, G.Á.; Lakatos, B.; Ruppert, M.; Kovács, A.; Juhász, V.; Dér, G.; Tállay, A.; Vágó, H.; Kiss, B.; Merkely, B.; et al. A Case Series of SARS-CoV-2 Reinfection in Elite Athletes. Int. J. Environ. Res. Public Health 2022, 19, 13798. https://doi.org/10.3390/ijerph192113798
Fülöp GÁ, Lakatos B, Ruppert M, Kovács A, Juhász V, Dér G, Tállay A, Vágó H, Kiss B, Merkely B, et al. A Case Series of SARS-CoV-2 Reinfection in Elite Athletes. International Journal of Environmental Research and Public Health. 2022; 19(21):13798. https://doi.org/10.3390/ijerph192113798
Chicago/Turabian StyleFülöp, Gábor Áron, Bálint Lakatos, Mihály Ruppert, Attila Kovács, Vencel Juhász, Gábor Dér, András Tállay, Hajnalka Vágó, Boldizsár Kiss, Béla Merkely, and et al. 2022. "A Case Series of SARS-CoV-2 Reinfection in Elite Athletes" International Journal of Environmental Research and Public Health 19, no. 21: 13798. https://doi.org/10.3390/ijerph192113798