- 1Department of Psychology, University of Turin, Turin, Italy
- 2European Innovation Partnership on Active and Healthy Ageing, Brussels, Belgium
- 3Department of Humanities and Life Sciences, Scuola Universitaria Superiore Istituto Universitario di Studi Superiori (IUSS), Pavia, Italy
- 4Istituti Clinici Scientifici Maugeri IRCCS, Cognitive Neuroscience Laboratory of Pavia Institute, Pavia, Italy
- 5UOC Neuroradiologia -IRCCS Istituto Neurologico Carlo Besta, Milan, Italy
- 6Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Mondino Foundation, Pavia, Italy
The COVID-19 pandemic is a health issue leading older adults to an increased vulnerability to unfavorable outcomes. Indeed, the presence of physical frailty has recently led to higher mortality due to SARS-CoV-2 infection. However, no longitudinal studies have investigated the role of neuropsychogeriatric factors associated with lockdown fatigue in healthy cognitive aging. Eighty-one healthy older adults were evaluated for their neuropsychological characteristics, including physical frailty, before the pandemic (T0). Subsequently, 50 of them agreed to be interviewed and neuropsychologically re-assessed during the lockdown (T1) and immediately after it (T2). Moreover, during another home confinement, they performed a psychological screening (T3) to evaluate possible mood changes and fatigue. According to Fried's frailty criteria, at T0, 63% of the sample was robust, 34.5% pre-frail, and only 2.5% frail. Significantly, these subjects presented a decrease in handgrip strength and walking speed (29.6 and 6.1%, respectively). Results from Principal Component Analyses and multiple regression models highlighted the contribution of “cognitive” and “psychological” factors (i.e., attentive-executive performance and mood deflections) in explaining handgrip strength and gait speed. At T3, lockdown fatigue was explained by higher scores on the Beck Depression Inventory and lower scores on the Trail Making Test part A. Results from a moderated-mediation model showed that the effect of psychomotor speed on lockdown fatigue was mediated by depression, with a moderating effect of gait speed. Our findings highlight the complex interrelationship between cognitive, psychological, and physical factors in the emergence of pandemic fatigue in a carefully selected older population.
Introduction
The presence of a state of frailty, characterized by a clinical history of polypathology, has recently led to higher mortality due to the SARS-CoV-2 pandemic among the older population (Onder et al., 2020). Polypathological subjects suffer from two or more chronic diseases, which could lead to disability and higher mortality rates (Gómez-Salgado et al., 2019).
The increased susceptibility to epidemic effects is likely to be determined not only by existing comorbidity conditions, but also by reduced immunity, partly due to the physiological aging process (Gavazzi and Krause, 2002).
Lockdown measures play a major role in the containment of SARS-CoV-2 infection. However, isolation and social distancing are associated with cognitive decline, depression, and anxiety in the older population (Santini et al., 2020). Faced with prolonged confinement, older people may experience loneliness, pessimism, health problems, negative stereotyping, and sleep disorders (Luchetti et al., 2020). Furthermore, social isolation is an important public health issue that can lead to higher probabilities of cognitive and mental problems (Gerst-Emerson and Jayawardhana, 2015).
Recently, a feeling of “lockdown fatigue” has been associated with home confinement due to the COVID-19 pandemic. Only a few authors have investigated this particular phenomenon, without specifically targeting the older population.
For instance, Bartoszek et al. (2020) examined a sample of 471 subjects predominantly young (mean age = 25 years, SD = 2.1) and female (85.6%). The authors found that living alone and starting new therapies during the lockdown were associated with higher levels of fatigue. In a cross-sectional community-based survey, COVID-19 pandemic fatigue was experienced in about 64% of the sample; again, the over-60 population was underrepresented (Morgul et al., 2020). Labrague and Ballad (2020) analyzed the fatigue among Philippines college students during the COVID-19 lockdown in a remote cross-sectional study. Their results indicated moderate levels of fatigue, in terms of physical exhaustion, body pain, headaches, worries, and reduced motivation. Finally, in a survey of 260 subjects (mean age = 47 years), lockdown fatigue was related to worsened mood and lifestyle (Field et al., 2021).
Despite the problems highlighted in these studies, to date no longitudinal ones have analyzed the associations among lockdown fatigue, physical conditions, cognitive functions, and mood deflections in cognitively normal older adults during home confinement.
This is unfortunate, as targeted psychological interventions may have a role in primary prevention and psychological well-being, by enhancing resilience during periods of health emergency while also reducing the negative impact on physical and cognitive dysfunctions and mood deflections disorders.
To fill this gap, we investigated the relationships between pandemic lockdown fatigue, physical-cognitive functions, and mood changes in 50 older adults−60 years and older (World Health Organization, 2021)—engaged as volunteers, suffering from two or more age-related diseases (polypathology) and receiving subsequent pharmacotherapy (Masnoon et al., 2017).
The availability of pre-COVID19 baseline data (T0) of this sample allowed us to investigate the contribution of cognitive functioning and psychological state in explaining the variability of grip strength and gait speed, two crucial components of the frailty phenotypic model (Fried et al., 2001).
A longitudinal approach (from April 2019 to January 2021) was used to analyze: (a) the neuropsychogeriatric profile of the participants before and after the lockdown; (b) whether these subjects had developed lockdown pandemic fatigue; (c) whether this fatigue could be predicted by variables measured at baseline using a moderate-mediation model.
In particular, as lockdown fatigue had been previously related to depressive symptoms (Bartoszek et al., 2020; Field et al., 2021; Seiter and Curran, 2021) and a slowdown in attentional abilities (Fiorenzato et al., 2021), we expected both to be useful in predicting exhaustion due to the restrictive measures in our sample. Furthermore, as mood deflection may be influenced by cognitive dysfunction (e.g., attention deficit; Keller et al., 2019), and physical frailty (Buigues et al., 2015)—particularly gait speed (Marino et al., 2019)—we hypothesized that attentional skills and gait speed would interact in modulating depression mood changes.
Our hypothesis is that decreased psychomotor and gait speed may interact to produce a depressive state that mediates their effect on lockdown fatigue.
Materials and Methods
Participants
The creation of an innovative laboratory (LAB) on active and healthy aging for training, education, research, and development allowed neuropsychological interventions on members of the University of the Third Age in Turin (UNITRE-TO). The subjects attending the LAB activities were numerous (N = 200) and, during the academic year 2018–19, they followed specific teaching modules aimed at learning functional lifestyles in view of active and healthy aging.
Eighty-one Italian volunteer subjects (64 females, age range 60–84) out of the 200 participants (40.5%) were selected if they: (a) suffered from at least two chronic pathologies (see Supplementary Table 1) and received pharmacotherapy treatments (Smith and Bondi, 2013); (b) were aged 60 years or older in order to be classified as “older adults” (World Health Organization, 2021); and (c) had a Mini Mental State Examination (MMSE, Folstein et al., 1975) raw score ≥ 27. The latter is the best cut-off for Mild Cognitive Impairment (MCI) detection in highly educated populations (O'Bryant et al., 2008), such as the one involved in our study. Moreover, the subjects did not report subjective cognitive decline (Jessen et al., 2020) and had not undergone any medical or neurological examination for suspected MCI (Petersen and Negash, 2008).
Participants were excluded from the study if they: (1) suffered from psychiatric or neurocognitive disorders based on DSM-5 criteria (American Psychiatric Association, 2013); (2) were taking any medications that could substantially affect cognitive functioning.
All subjects were functionally independent and socially active. We performed a screening in order to investigate their cognitive, functional, and behavioral domains, and to detect the mildest neuropsychogeriatric dysfunction considering their physical health status, their cognitive functioning, and possible mood deflections in terms of anxiety and depression (T0).
During the COVID-19 pandemic, 50 out of the 81 subjects agreed to participate in LAB activities remotely in compliance with restrictive lockdown measures. They underwent semi-structured interviews (T1), neuropsychological (T2), and psychological assessment (T3) to identify any possible under cut-off values in their individual profile.
All participants gave written informed consent prior to the study, which was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the University of Turin (Prot. n. 10038 and Prot. n. 151786, before and after the pandemic, respectively).
Assessments
Assessments Measures at the Baseline (T0)
From April to October 2019, the subjects underwent a multidimensional neuropsychogeriatric assessment, which consisted of cognitive tests, functional, and behavioral scales. Three different neuropsychologists evaluated the subjects in two experimental sessions, held 1 day apart and each lasting about 90 min, in order to prevent fatigue and lack of adherence to the tasks.
Information about clinical history, symptoms, and chronic pathologies was collected using the Cumulative Illness Rating Scale (CIRS: Linn et al., 1968).
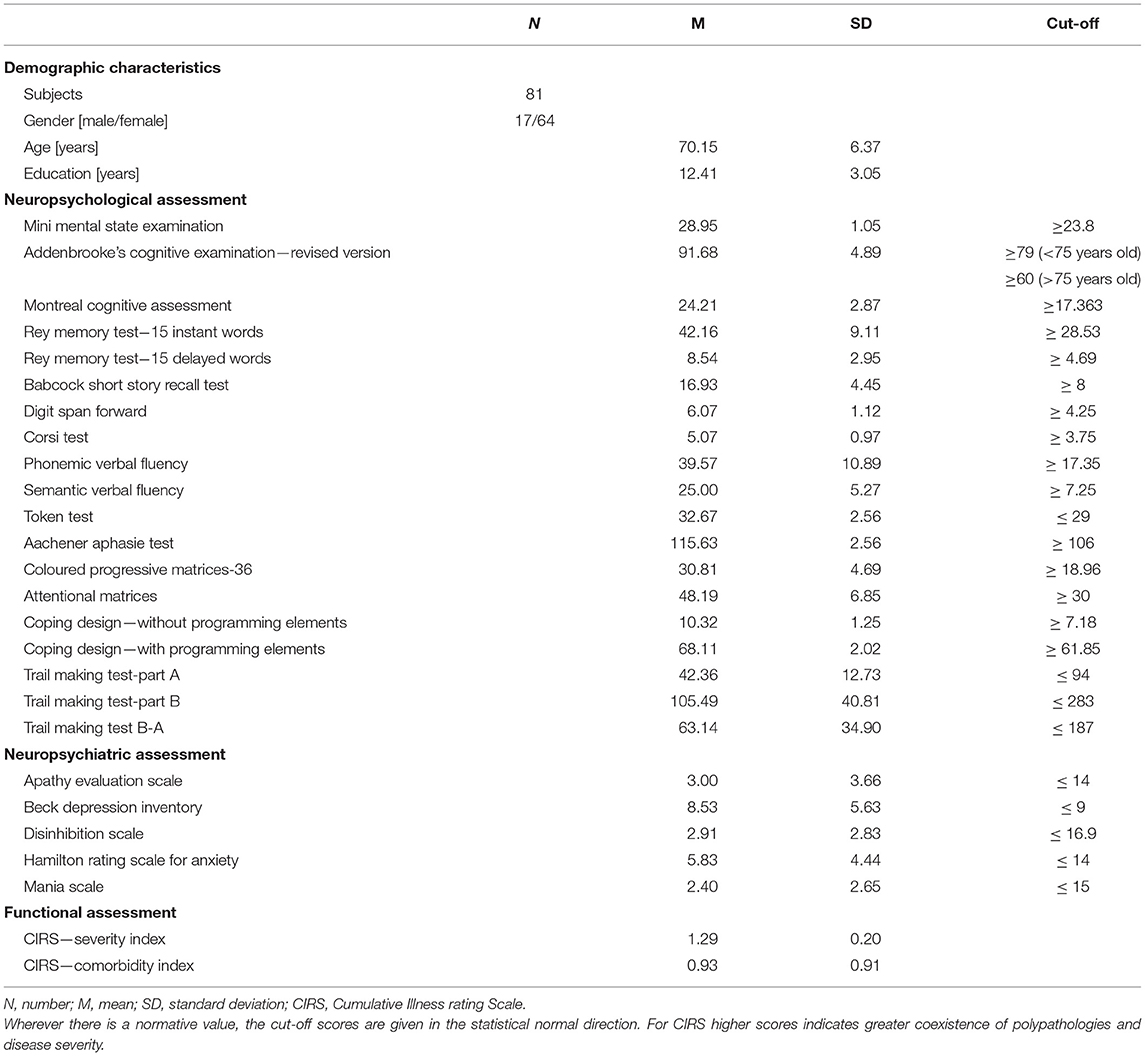
Global cognitive functioning was assessed by using the Addenbrooke's Cognitive Examination—Revised version (ACE-R: Mioshi et al., 2006) and the Montreal Cognitive Assessment (MoCA: Conti et al., 2015). Then, a detailed evaluation was carried out considering several cognitive domains: episodic long-term memory (Rey Memory Test and Short Story Recall: Spinnler and Tognoni, 1987), short-term memory (Digit Span and Corsi Test: Spinnler and Tognoni, 1987), attention (attentional matrices: Spinnler and Tognoni, 1987), language and fluencies (Token Test—TT: De Renzi and Vignolo, 1962; Phonemic and Semantic Fluency: Spinnler and Tognoni, 1987; Naming subtest of Aachner Aphasia Test—AAT: De Bleser et al., 1986), visuo-constructive abilities (Copy Design: Carlesimo et al., 1996), problem-solving (Raven's Coloured Progressive Matrices—CPM-36: Spinnler and Tognoni, 1987), and executive functions (Trail Making Test—TMT: Giovagnoli et al., 1996).
Psychiatric rating scales were used to assess: depression (Beck Depression Inventory—BDI: Beck et al., 1988), apathy (Apathy Evaluation Scale—AES: Marin, 1996), anxiety (Hamilton Rating Scale for Anxiety—HAM-A: Hamilton, 1959), disinhibition (Disinhibition Scale—DIS-S: Starkstein et al., 2004), and hypomania (Mania Scale—MAS: Bech et al., 1978).
The possible presence of a physical frailty status was assessed by adopting the phenotypic model (Fried et al., 2001). According to Fried's criteria, frailty is a pathophysiological syndrome characterized by 5 determinants: (1) unintentional weight loss; (2) limitation of physical activity; (3) asthenia; (4) handgrip strength reduction; and (5) slowing in walking speed. The presence of three out of five criteria indicates a state of frailty, while one or two criteria identify a pre-frailty status.
Semi-Structured Interviews at T1
From April to May 2020, during the lockdown period, all 81 subjects assessed at T0 were contacted by phone, text message, or email in order to ask for their availability to participate in the study. Fifty of them agreed.
At first, a semi-structured interview was carried out to collect information about housing status, health conditions, measures to avoid contagion, and sources of information considered as reliable. In addition, they were asked for any changes in their habits in terms of diet, physical activity, sleep, smoking, use of drugs and alcohol, and cognitively stimulating activities (Supplementary Table 2).
At the end of the interview, the participants were asked to schedule a second appointment for a neuropsychological evaluation.
Neuropsychological Assessment at T2
This third phase was carried out remotely via video-call from May to July 2020, during the so-called “phase 2” of the Italian quarantine, when restrictive measures were eased.
The choice of neuropsychological tests was as close as possible to T0. Cognitive functioning was assessed by ACE-R, and MMSE. The test subsections involving visuo-spatial skills were presented in screen-sharing mode. We also used the MoCA-Blind test (Wittich et al., 2010). This version was originally designed for people with visual impairment. In our research, we used such instrument in order to overcome the issues related to the performance of some of the visual tasks of the original test (e.g., TMT-B, short version) through the screen-sharing mode. The blind version is scored out of 22 but, as suggested on www.mocatest.org (Nasreddine, 2020), the official MoCA website, it needs to be converted back to 30 as the original test.
Mood changes were evaluated using BDI, AES, HAM-A, DIS-S, and MAS. The socioeconomic characteristics of the sample were analyzed using the Four Factor Index of Social Status (Hollingshead index, HI: Hollingshead, 1975, 2011). This index is based on the educational level and the type of employment of family members (for more details on scoring, please see Hollingshead, 1975, 2011). In this case, since our group consisted of retired people, their last job was taken into account.
Neuropsychological Assessment at T3
Remote phone calls were used to carry out this last phase, which occurred during another period of very restrictive measures in Italy (December 2020–January 2021).
In order to achieve our aims, we chose to test the subjects only on BDI and HAM-A. These scales are very suitable for a remote assessment and, furthermore, depression and anxiety are two of the most frequently reported and studied psychological aspects in the literature concerning the COVID-19 pandemic (Salari et al., 2020) and subsequent home confinement (Bartoszek et al., 2020; Morgul et al., 2020; Field et al., 2021).
In addition, we investigated the subjects' fatigue related to restrictive measures using the Lockdown Fatigue Scale (LFS: Labrague and Ballad, 2020), which has been recently designed to assess exhaustion during quarantine due to the COVID-19 pandemic. The LFS consists of 10 items concerning the psychological effects of lockdown, such as mood deflection, attentional deficits, and possible somatization (i.e., weakness, headache). Subjects are asked how often they experience those effects during home confinement. Each item is scored on a Likert scale that ranges from 1 (never) to 5 (always). This instrument allows the detection of four different levels of fatigue: low (1–12), mild (13–25), moderate (26–37), and severe (38–50).
Data Analyses
Principal Component Analyses
T0 data were examined in order to investigate whether handgrip strength and walking speed could be predicted by a combination of variables concerning cognitive performance, psychological status, and physical comorbidity (Supplementary Table 1). In our analyses, only grip strength and walking speed were taken into consideration because: (a) their values presented more variance, not being dichotomous (presence/absence); and (b) they are more related to cognitive functioning—particularly concerning executive control (Hooghiemstra et al., 2017)—and mood changes (Gordon et al., 2019; Brooks et al., 2020).
To this purpose, in a preliminary data-reduction stage, two Principal Component Analyses (PCA) were used to unveil superordinate factors transcending: (a) the single scores of cognitive functioning; and (b) the single scales of psychological status. The first PCA was carried out on cognitive tasks, while the second included the scales used to assess possible mood changes in terms of depression (BDI), apathy (AES), anxiety (HAM-A), disinhibition (DIS-S), and hypomania (MAS). We retained the components with eigenvalue >0.70 (Jolliffe, 1972), using an orthogonal rotation (Varimax) to facilitate their interpretation. Subsequently, two multiple regression models were used to estimate the contribution of the resulting “cognitive” and “psychological” factors (independent variables), in explaining variability in grip strength and gait speed (adjusted for gender/BMI and for gender/height, respectively). Then, the “cognitive” and “psychological” factors associated with a significant effect were modeled in additional multiple regressions including grip strength and gait speed as dependent variables, and comorbidity, age, and education as predictors.
Based on the outcome of these analyses, we assessed a mediation-moderate model to investigate the direction of the relationship among the factors predicting lockdown pandemic fatigue. Specifically, a moderate mediation, also known as conditional indirect effects, occurs when the effect of the independent variable “TMT-A” on the outcome variable “LFS” via the mediating variable “BDI” differs according to the levels of the moderating variable “gait speed.”
Moderate-Mediation Model
We tested a moderate-mediation model to assess the hypothesis that: (a) depression at T0 mediates the negative relationship between attentional/executive resources (as tracked by TMT-A performance) at T0 and lockdown-fatigue scale at T3; and (b) this indirect effect is conditional on a moderating variable represented by frailty (as tracked by walking speed). Age, gender, and educational level were modeled as nuisance variables. We used the PROCESS macro (v.3.5) for SPSS (IBM, v.23) to test Hayes's (2017) model 7 (moderated mediation), after checking for the assumptions concerning the lack of outliers (based on Mahalanobis distance), the normal distribution of the residuals (Lilliefors, p > 0.05), multicollinearity (maximum variance inflation factor = 1.31; minimum tolerance = 0.763), independence of residuals (Durbin-Watson = 2.184), homoscedasticity (Breusch-Pagan test, p > 0.05). Our hypothesis concerning the moderation of mediated effects was tested through conditional process analysis based on Ordinary Least Squares (OLS) regression, using bootstrapping resampling (50,000 samples) to generate confidence intervals for direct and mediated effects, as well as for an index of moderated-mediation. Interaction variables were centered (to a mean of 0) before entering the analyses, and the Johnson and Neyman's (1936) approach was used to compute the range of significance and simple slopes for the interaction analyses, which were assessed 1 standard deviation below and above the mean. The statistical threshold was set at p < 0.05 (two-tailed).
Results
T0 Main Results
The mean scores obtained at T0 on global cognitive tests and behavioral scales are reported in Table 1. Even if the MMSE scores did not suggest the presence of MCI (i.e., O'Bryant et al., 2008) and the participants did not report subjective cognitive decline, their performance was below the reference cut-off value on some tests. In particular, on: MoCA (2.5%), Attentional matrices (1%), Copy design with programming elements (1%), Rey memory test instant recall (1%), Delayed recall (4%); Short story recall (1%), Digit span (4%); Corsi test (1%), and the Phonetic fluency test (4%). Although some deficits were found on neuropsychological tests, the percentages of under cut-off scores are consistent with the margin of error on tests in the normative population.
Moreover, considering the behavioral scales, the subjects presented mood changes in terms of depression (BDI = 39%) and states of anxiety (HAM-A = 4%).
According to Fried criteria, 63% of the sample was robust, 34.5% pre-frail, and only 2.5% frail. Significantly, these subjects presented a decrease in handgrip strength and walking speed (29.6 and 6.1%, respectively).
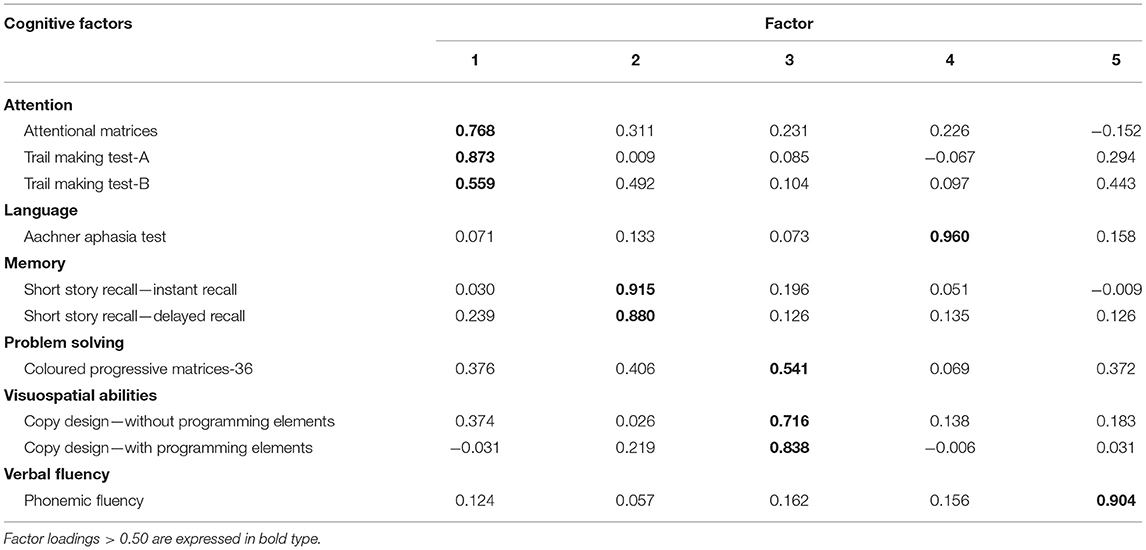
As detailed above, a PCA led to reduce the initial dataset of cognitive scores (10 cognitive tests) to 5 factors explaining 82.8% of their variance (Table 2): (1) Attention/Executive, which refers to attention and executive functions, in term of cognitive set-shifting, assessed by the attentional matrices, and TMT-A, TMT-B; (2) Memory, represented by Short Story Recall (immediate and delayed recall); (3) Visual-Constructive, which refers to visuo-constructive abilities and abstract-non-verbal reasoning, assessed by Design Copying (with and without programming elements) and CPM-36; (4) Language, represented by AAT; (5) Fluency, represented by the Phonemic Verbal Fluency.
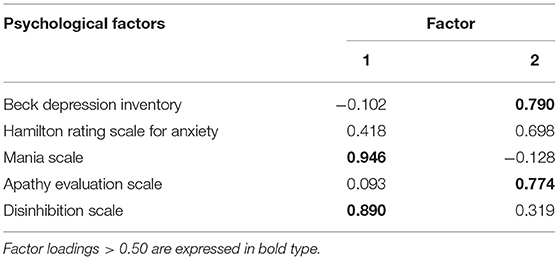
We used the same approach to reduce the 5 scales of psychological status to 2 factors explaining 74.3% of their variance (Table 3): (1) Mania-disinhibition, measured with MAS, DIS-S; and (2) Depression-apathy-anxiety, assessed by BDI, HAM-A, AES (Table 2).
Subsequently, the contribution of these components was estimated in order to explain the variability of the crucial determinants of Fried's phenotypic model. When testing the predictors of grip strength, a significant model (p = 0.0051) showed that “cognitive” and “psychological” status explained 13% of the variance. In particular, increased grip strength reflected: (a) an increase of the Attention/Executive performance FACTOR 1-on PCA 1 (p = 0.0228); and (b) a decrease of “depression-apathy-anxiety,” FACTOR 2-on PCA 2 (p = 0.01006). There was no multicollinearity among explanatory variables (maximum variance inflation factor: VIF = 1.004), and the residuals were normally distributed (Kolmogorov–Smirnov test: K-S = 0.7795, p > 0.2). Moreover, 15% of the variance in gait speed (p = 0.0015) was also explained by Attention/Executive performance FACTOR 1-on PCA 1 (p = 0.0046), and depression-apathy-anxiety FACTOR 2-on PCA 2 (p = 0.0100). There was no multicollinearity among explanatory variables (maximum VIF = 1.004), and the residuals were normally distributed (K-S = 0.05775, p > 0.2). None of the other cognitive or psychological factors was significantly associated with handgrip strength or walking speed. To refine this finding, the contribution of Attention/Executive and depression-apathy-anxiety factors to grip strength and gait speed was assessed while taking into account age, education, and comorbidity (in terms of CIRS). In line with the above results, a significant model showed that 10% of the variance in grip strength (r2 = 0.10395, p = 0.0051) was explained by the Attention/Executive performance FACTOR 1-on PCA 1 (p = 0.0229), and depression-apathy-anxiety FACTOR 2-on PCA 2 (p = 0.0100). There was no multicollinearity among explanatory variables (maximum VIF = 1.37), and the residuals were normally distributed (K-S = 0.07795, p > 0.2). Moreover, a significant model showed that 13% of the variance in gait speed (r2 = 0.1315, p = 0.0015) was explained by the same factors: Attention/Executive performance FACTOR 1-on PCA 1 (p = 0.0046) and depression-apathy-anxiety FACTOR 2-on PCA 2 (p = 0.0100). There was no multicollinearity among explanatory variables (maximum VIF = 1.37), and the residuals were normally distributed (K-S = 0.05774, p > 0.2).
T1 Main Results
The subjects' socio-demographic characteristics, most reliable sources of information, percentages on the use of protective devices, and changes in daily life habits are shown in Supplementary Table 2.
T2 Main Results
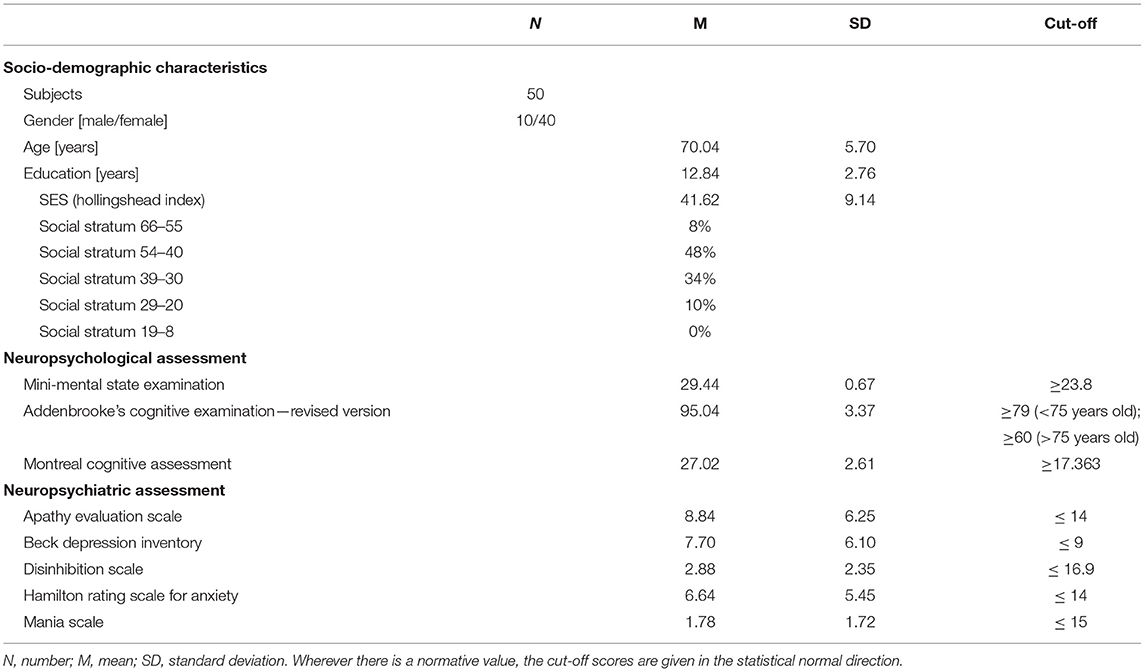
Table 4 reports the subjects' mean scores on the global cognitive tasks and their socioeconomic status (SES). Specifically, they performed well on all cognitive tests, without any scores under the cut-off, on ACE-R, MMSE, and MoCA.
On mood assessment scales, they obtained normal scores on DIS-S and MAS; on the other hand, there were under cut-off values on BDI (28%), AES (20%), and HAM-A (10%).
With regard to their SES, according to the Hollingshead Index (HI) 8% of the subjects fell into the highest social stratum, 48% in the second, 34% in the third, 10% in the fourth, and none of them in the lowest one.
T3 Main Results
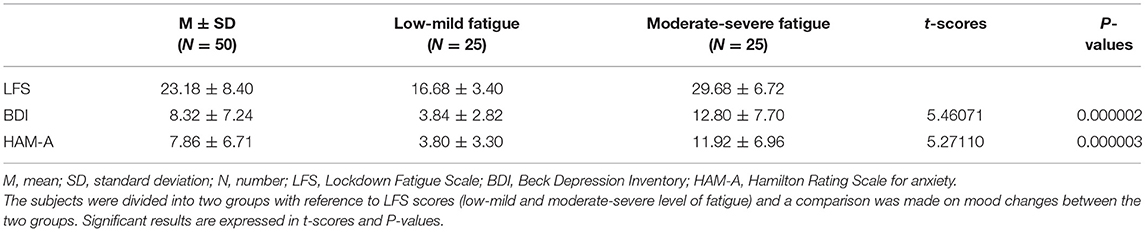
The scores obtained at T3 on LFS, BDI, and HAM-A are reported in Table 5.
Although results on psychological scales indicated mean scores in the normal range, 28% of subjects showed mood deflections in terms of depression (BDI) and 18% in terms of anxiety (HAM-A).
Regarding LFS scores, 10% of the sample fell into low level of fatigue, 56% into mild level, 30% presented moderate fatigue, while 4% reported severe lockdown fatigue.
In order to test for possible differences concerning the degree of lockdown fatigue, the subjects were divided into two groups according to the median scores: low-mild level of fatigue (17 females and 8 males) and moderate-severe level of fatigue (23 females and 2 males). Interestingly, 62.5% of the pre-frail subjects fell into the moderate-severe fatigue group.
Considering these two groups, the t-test showed a significant difference in terms of mood changes. Specifically, subjects feeling more fatigued due to the lockdown presented higher levels of anxiety, in terms of HAM-A scores (t-value = 5.27110; p = 0.000003), and depression as assessed by BDI (t-value = 5.46071; p = 0.000002).
On the basis of these findings, we assessed our hypotheses regarding the role of specific cognitive, physical, and psychological aspects in the emergence of lockdown fatigue.
A significant moderated-mediation model (see Supplementary Figure 1) highlighted the interacting effects of psychomotor speed, gait speed, and depression at T0 on lockdown fatigue scale at T3 (see Table 6). Indeed, a conditional process analysis showed that the effect of TMT-A performance on lockdown fatigue at T3 was mediated by depression, but this mediation was moderated by gait speed. Namely, a significant interaction between low psychomotor speed (TMT-A) and low gait speed (i.e., moderation) was associated with stronger mood deflection. Therefore, only at low gait speed the strength of depressive state mediated the enhancing effect of low psychomotor speed on lockdown fatigue.
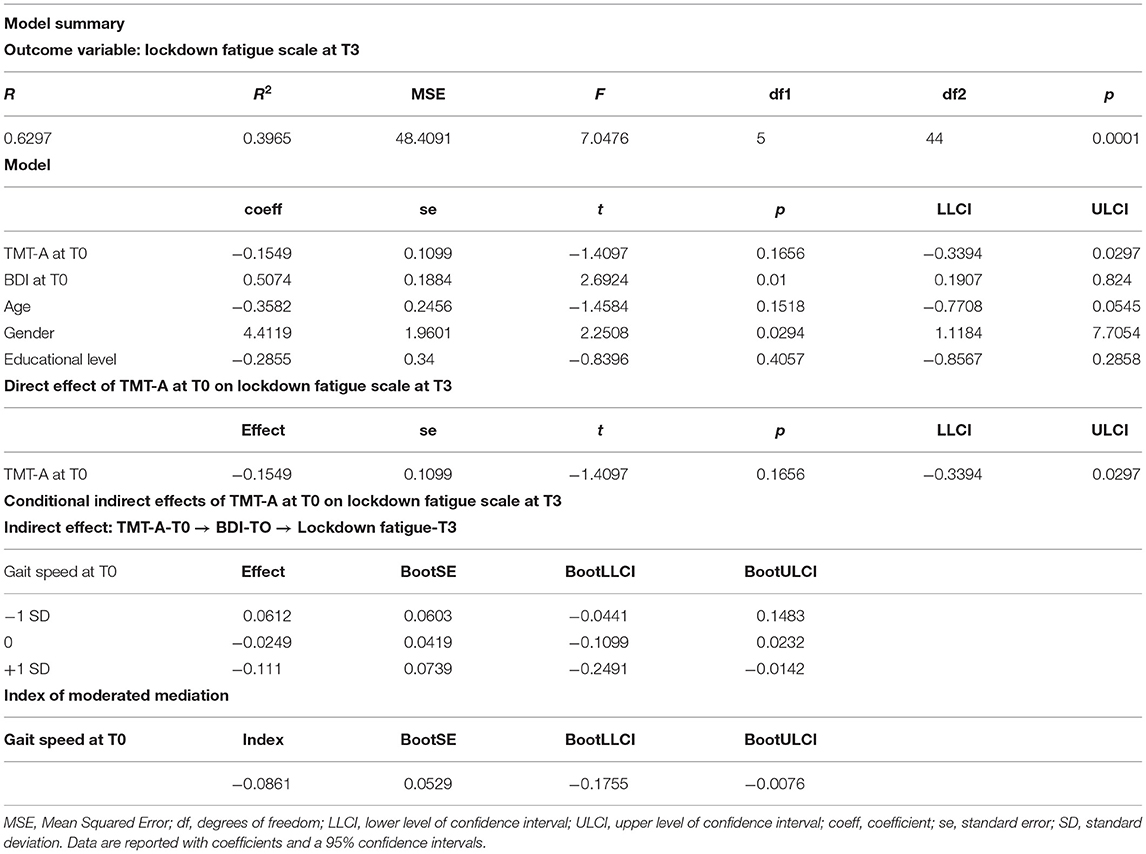
Table 6. Moderated-mediation analysis for predicting lockdown fatigue scale at T3 based on TMT-A, BDI, and gait speed at T0.
Discussion
To the best of our knowledge, this is the first study in the literature monitoring healthy cognitive aging subjects before and after very restrictive COVID-19 containment measures. We analyzed the impact of the lockdown fatigue in relation to pre-existing aspects of physical frailty, cognitive functioning, and mood deflections. Indeed, T0 data allowed us to better study the associations between neuropsychological variables assessed before the pandemic and the subsequent home confinement.
Although most of the participants were robust at T0, some of them were pre-frail or frail. Only a few minor neuropsychological deficits were observed in cognitive functioning. However, mood changes, particularly in terms of depression, were present in about 40% of the population.
We considered the association among the main characteristics of frailty and cognitive and behavioral aspects in a socially active population with a medium-high SES.
In the first place, the contribution of “cognitive” and “psychological” factors was estimated, resulting from PCAs, in order to explain variability in grip strength and gait speed. The choice of these frailty determinants, associated with cognitive changes, was also supported by studies on community-dwelling people. In particular, Robertson et al. (2014) showed that individuals with slow gait and weak grip had lower scores on executive functions tests. Slow gait speed also showed an important effect on attention and psychomotor speed. Asthenia was associated with global cognition, while low physical activity and unintentional weight loss were not independently associated with cognitive domains. Moreover, a small number of studies on patients with functional and/or cognitive impairment, due to major neurocognitive disorder, highlighted a relationship between a reduction in gait speed or grip strength and impaired attention and executive dysfunction (i.e., Hooghiemstra et al., 2017). Nevertheless, executive-attentional functions mediated by the prefrontal cortex were also related to gait speed in physical frailty (Amboni et al., 2013).
Mood changes—particularly depression and anxiety—are not only common in older people but they are also significant risk factors for frailty. For instance, in a cross-sectional study on community-dwelling older people, Ní Mhaoláin et al. (2012) found that higher levels of depression and anxiety were more prevalent in pre-frail and frail subjects than in robust ones. In addition, they showed a significantly higher probability of anxiety and depressive symptoms. Some authors reported how depression could be considered not only a consequence but also a risk factor for frailty (i.e., Robertson et al., 2013). Particularly, a decrease in handgrip strength was associated with an increased depressive symptomatology (Brooks et al., 2020), which seemed also to be a predictor of slow walking speed (Staples et al., 2020). Another study showed that higher levels of depression and anxiety were associated with a slowdown in gait speed in subjects aged 65 and older with atrial fibrillation (Marino et al., 2019). On the other hand, anxiety seemed to be inversely related to grip strength in the older population (Gordon et al., 2019).
Our results showed how increased grip strength reflects better Attention/Executive performances and lower mood changes, in terms of depression, apathy, and anxiety. In the same direction, a variance in gait speed was also explained by Attention/Executive performance and depression-apathy-anxiety mood changes. Significantly, the contribution of these factors was not explained by age, comorbidity, or educational level.
T1 results showed that almost all subjects took the precautions recommended by the Italian Ministry of Health (Ministero della Salute - Italian Ministry of Health, 2020) to prevent SARS-CoV-2 infection. It is noteworthy that the majority of our sample (90%) belongs to the middle-high social class, according to HI. Combined with a high level of education, such aspect highlights the peculiarity of this population, characterized by social resources, which allowed them to take the necessary precautions to avoid the risk of contagion.
Our T2 results showed no cognitive decline nor mood deflection. However, some under cut-off scores were found in terms of depression, apathy, and anxiety. These aspects align with previous studies on the general population during the COVID-19 pandemic (Prati and Mancini, 2021).
With regard to the subsequent lockdown (T3), which occurred from December 2020 to January 2021, subjects complaining of higher lockdown fatigue exhibited increased levels of depression and anxiety than those with lower fatigue.
These results are consistent with another study on COVID-19 lockdown fatigue (Field et al., 2021), in which the fatigue was related to depression and anxiety but also to sleep disturbance, increased posttraumatic stress symptoms, and a worsened lifestyle (e.g., decreased physical activity, fewer experiences of recreational activities, fewer interactions with others, and more time spent gaming and chatting about the virus). In particular, psychological factors, such as depression, sleep disturbance, and anxiety, explained 51% of the variance of lockdown fatigue (Field et al., 2021).
The mechanism related to physical, behavioral and cognitive factors, and LFS is still unknown. Therefore, we used a moderate-mediation model to explore the complex pathways leading to the onset of lockdown pandemic fatigue. Specifically, we examined the potential mediating effect of depression and attention, and the moderated effect of gait speed in this well-established association. We found a two-way interaction (moderation) between TMT-A and gait speed in influencing the mediator BDI; thus, when walking speed is below the mean, limited psychomotor speed resources are associated with greater depression. Consequently, for above-average slow walk values, the depressive state mediates the effect of limited psychomotor speed resources on subsequent fatigue. Some studies have highlighted how not only attention performances are impaired in normal aging (e.g., Periáñez et al., 2007), but also how usual walking speed is associated with slower test performance on TMT-A in MCI patients (Knapstad et al., 2019). The association between depression and psychomotor speed is already well-known. Patients with major depressive disorders aged 60 years and older showed worse attention abilities, especially when frail (Potter et al., 2016).
Concerning the lockdown pandemic fatigue, higher scores of depression and everyday fatigue were found in the Polish population during COVID-19 home confinement (Bartoszek et al., 2020). Moreover, a previous study on social distancing issues related to the COVID-19 pandemic in the general population (aged 18 years and older) showed an association between exhaustion and depressive symptoms (Seiter and Curran, 2021). Indeed, increased stress levels due to pandemic uncertainty and restrictions could trigger depressive symptoms and greater fatigue burden (Schrack et al., 2020).
A recent study investigated the psychological effects of lockdown on cognitive functions showing general deterioration, in particular regarding concentration and attention (Fiorenzato et al., 2021). Difficulties in keeping focused and concentrated could lead to mental fatigue and, consequently, to greater feelings of lockdown pandemic fatigue in older adults.
Conclusion
To our knowledge, this is the first study examining the association among specific cognitive, physical, behavioral, and functional characteristics before, during, and after restrictive lockdown measures in cognitively normal aging subjects. It is important to underline how UNITRE participants, with mild neuropsychological and physical alterations, represent an optimal reference sample to implement possible primary prevention pathways on older adults.
Our results highlight that: (a) physical functions, executive attention, and mood changes can play an important role in the current COVID-19 pandemic; (b) the subjects showing moderate-severe fatigue reported more depressive and anxiety issues than subjects with low-mild fatigue; (c) cognitive functioning, in terms of psychomotor speed, seems also to play an important role in the perception of fatigue due to COVID-19 restrictive measures.
Since lockdown fatigue is related to mood deflections, such as depression and anxiety, it would be useful to study this aspect more in depth, as well as other COVID-19 related psychological issues (Field et al., 2021).
Limitations Section
Although this novel study was carefully designed and achieved its aims, the sample size, set at 50 participants, may be considered a critical aspect.
Furthermore, because of the COVID-19 containment measures in place, it was not possible to perform a proper face-to-face neuropsychogeriatric assessment, as in T0. The restrictions led us to select the most suitable neuropsychological tests for remote administration in line with our aims. To address any potential critical issue related to the remote administration of neuropsychological tests, we adopted ad hoc tools for screen sharing in video calls (e.g., MOCA-Blind test).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University of Turin. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MA conceptualized and designed the study, wrote the draft of the manuscript, reviewed it, supervised the project and was responsible for funding acquisition. NC analyzed study data, wrote the draft of the manuscript, and reviewed it. MB and GEC took part in the writing process, edited the manuscript, and collected data. SP collected data and took part in the writing process. SC reviewed the manuscript and supervised the project. All authors listed have made a substantial contribution to the conception, development of methodological approach and interpretation of results, and approved it for publication.
Funding
This work was supported by the Fondazione CRT (Cassa di Risparmio di Torino, IT) under different Grants: (a) 2019 GAIA-MENTE 4 (ROL ID: 66346); (b) 2019 GAIA-MENTE 3 (ROL ID: 65063); (c) 2017 GAIA-MENTE 2 (ROL ID: 61450); (d) 2017 GAIA-MENTE (ROL ID: 58266).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Marcella Aimo for her valuable help in proofreading the article.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyg.2021.685180/full#supplementary-material
Supplementary Figure 1. The moderate-mediation model, adapted from Hayes' model 7 (page 588). BDI, Beck Depression Inventory; TMT-A, Trail Making Test- Part A; LFS, Lockdown Fatigue Scale.
References
Amboni, M., Barone, P., and Hausdorff, J. M. (2013). Cognitive contributions to gait and falls: evidence and implications. Move. Disord. 28, 1520–1533. doi: 10.1002/mds.25674
American Psychiatric Association. (2013). Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. Washington, DC: American Psychiatric Association Publishing.
Bartoszek, A., Walkowiak, D., Bartoszek, A., and Kardas, G. (2020). Mental well-being (depression, loneliness, insomnia, daily life fatigue) during COVID-19 related home-confinement-a study from Poland. Int. J. Environ. Res. Public Health 17:7417. doi: 10.3390/ijerph17207417
Bech, P., Rafaelsen, O. J., Kramp, P., and Bolwig, T. G. (1978). The mania rating scale: Scale construction and inter-observer agreement. Neuropharmacology 17, 430–431. doi: 10.1016/0028-3908(78)90022-9
Beck, A. T., Steer, R. A., and Carbin, M. G. (1988). Psychometric properties of the beck depression inventory: twenty-five years of evaluation. Clin. Psychol. Rev. 8, 77–100. doi: 10.1016/0272-7358(88)90050-5
Brooks, S. K., Webster, R. K., Smith, L. E., Woodland, L., Wessely, S., Greenberg, N., et al. (2020). The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 395, 912–920. doi: 10.1016/S0140-6736(20)30460-8
Buigues, C., Padilla-Sánchez, C., Garrido, J. F., Navarro-Martínez, R., Ruiz-Ros, V., and Cauli, O. (2015). The relationship between depression and frailty syndrome: a systematic review. Aging Mental Health 19, 762–772. doi: 10.1080/13607863.2014.967174
Carlesimo, G. A., Caltagirone, C., Gainotti, G., Fadda, L., Gallassi, R., Lorusso, S., et al. (1996). The mental deterioration battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 36, 378–384. doi: 10.1159/000117297
Conti, S., Bonazzi, S., Laiacona, M., Masina, M., and Coralli, M. V. (2015). Montreal Cognitive Assessment (MoCA)-Italian version: regression based norms and equivalent scores. Neurol. Sci. 36, 209–214. doi: 10.1007/s10072-014-1921-3
De Bleser, R., Denes, G. F., Luzzati, C., and Mazzucchi, A. (1986). L'Aachener Aphasie Test (AAT): I. Problemi e soluzioni per una versione italiana del Test e per uno studio crosslinguistico dei disturbi afasici [The Aachen Aphasia Test: I. Problems and solutions for an Italian version of the test and for a cross-linguistic study of aphasic disturbances]. Arch. Psicol. Neurol. Psichiatr. 47, 209–237.
De Renzi, E., and Vignolo, L. A. (1962). The token test: a sensitive test to detect receptive disturbances in aphasics. Brain J Neurol. 85, 665–678. doi: 10.1093/brain/85.4.665
Field, T., Mines, S., Poling, S., Diego, M., Bendell, D., and Veazey, C. (2021). COVID-19 lockdown fatigue. Am. J. Psychiatr. Res. Rev. 4, 27–27. doi: 10.28933/ajprr-2020-12-0906
Fiorenzato, E., Zabberoni, S., Costa, A., and Cona, G. (2021). Cognitive and mental health changes and their vulnerability factors related to COVID-19 lockdown in Italy. PLoS ONE 16:e0246204. doi: 10.1371/journal.pone.0246204
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). "Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Fried, L. P., Tangen, C. M., Walston, J., Newman, A. B., Hirsch, C., Gottdiener, J., et al. (2001). Frailty in older adults: evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 56, M146–M156. doi: 10.1093/gerona/56.3.m146
Gavazzi, G., and Krause, K. H. (2002). Ageing and infection. Lancet Infect. Dis. 2, 659–666. doi: 10.1016/s1473-3099(02)00437-1
Gerst-Emerson, K., and Jayawardhana, J. (2015). Loneliness as a public health issue: the impact of loneliness on health care utilization among older adults. Am. J. Public Health 105, 1013–1019. doi: 10.2105/AJPH.2014.302427
Giovagnoli, A. R., Del Pesce, M., Mascheroni, S., Simoncelli, M., Laiacona, M., and Capitani, E. (1996). Trail making test: normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 17, 305–309. doi: 10.1007/BF01997792
Gómez-Salgado, J., Bernabeu-Wittel, M., Aguilera-González, C., Goicoechea-Salazar, J. A., Larrocha, D., Nieto-Martín, M. D., et al. (2019). Concordance between the clinical definition of polypathological patient versus automated detection by means of combined identification through ICD-9-CM codes. J. Clin. Med. 8:613. doi: 10.3390/jcm8050613
Gordon, B. R., McDowell, C. P., Lyons, M., and Herring, M. P. (2019). Associations between grip strength and generalized anxiety disorder in older adults: results from the Irish longitudinal study on ageing. J. Affect. Disord. 255, 136–141. doi: 10.1016/j.jad.2019.05.043
Hamilton, M. (1959). The assessment of anxiety states by rating. Br. J. Med. Psychol. 32, 50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x
Hayes, A. F. (2017). Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY: Second Edition. Guilford publications.
Hollingshead, A. B. (1975). Four Factor Index of Social Status. Unpublished manuscript, Yale University, New Haven, CT, United States.
Hooghiemstra, A. M., Ramakers, I., Sistermans, N., Pijnenburg, Y., Aalten, P., Hamel, R., et al. (2017). Gait speed and grip strength reflect cognitive impairment and are modestly related to incident cognitive decline in memory clinic patients with subjective cognitive decline and mild cognitive impairment: findings from the 4c study. J. Gerontol. A Biol. Sci. Med. Sci. 72, 846–854. doi: 10.1093/gerona/glx003
Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., et al. (2020). The characterisation of subjective cognitive decline. Lancet Neurol. 19, 271–278. doi: 10.1016/S1474-4422(19)30368-0
Johnson, P. O., and Neyman, J. (1936). Tests of certain linear hypotheses and their application to some educational problems. Statist. Res. Memoirs 1, 57–93.
Jolliffe, I. T. (1972). Discarding variables in a principal component analysis. I: artificial data. J. R. Statist. Soc. 21, 160–173. doi: 10.2307/2346488
Keller, A. S., Leikauf, J. E., Holt-Gosselin, B., Staveland, B. R., and Williams, L. M. (2019). Paying attention to attention in depression. Transl. Psychiatry 9:279. doi: 10.1038/s41398-019-0616-1
Knapstad, M. K., Steihaug, O. M., Aaslund, M. K., Nakling, A., Naterstad, I. F., Fladby, T., et al. (2019). Reduced walking speed in subjective and mild cognitive impairment: a cross-sectional study. J. Geriatr. Phys. Therapy 42, E122–E128. doi: 10.1519/JPT.0000000000000157
Labrague, L., and Ballad, C. A. (2020). Lockdown fatigue among college students during the COVID-19 pandemic: predictive role of personal resilience, coping behaviors, and health. MedRxiv. doi: 10.1101/2020.10.18.20213942. [Epub ahead of print].
Linn, B. S., Linn, M. W., and Gurel, L. (1968). Cumulative illness rating scale. J. Am. Geriatr. Soc. 16, 622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x
Luchetti, M., Lee, J. H., Aschwanden, D., Sesker, A., Strickhouser, J. E., Terracciano, A., et al. (2020). The trajectory of loneliness in response to COVID-19. Am. Psychol. 75, 897–908. doi: 10.1037/amp0000690
Marin, R. S. (1996). Apathy: concept, syndrome, neural mechanisms, and treatment. Semin. Clin. Neuropsychiatry 1, 304–314. doi: 10.1053/SCNP00100304
Marino, F. R., Lessard, D. M., Saczynski, J. S., McManus, D. D., Silverman-Lloyd, L. G., Benson, C. M., et al. (2019). Gait speed and mood, cognition, and quality of life in older adults with atrial fibrillation. J. Am. Heart Assoc. 8:e013212. doi: 10.1161/JAHA.119.013212
Masnoon, N., Shakib, S., Kalisch-Ellett, L., and Caughey, G. E. (2017). What is polypharmacy? A systematic review of definitions. BMC Geriatr. 17:230. doi: 10.1186/s12877-017-0621-2
Ministero della Salute - Italian Ministry of Health (2020). Recommendations to Contain the Spread of Coronavirus. Available online at: http://www.salute.gov.it/imgs/C_17_opuscoliPoster_443_3_alleg.pdf (accessed March 27, 2020).
Mioshi, E., Dawson, K., Mitchell, J., Arnold, R., and Hodges, J. R. (2006). The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int. J. Geriatr. Psychiatry 21, 1078–1085. doi: 10.1002/gps.1610
Morgul, E., Bener, A., Atak, M., Akyel, S., Aktaş, S., Bhugra, D., et al. (2020). COVID-19 pandemic and psychological fatigue in Turkey. Int. J. Soc. Psychiatr. doi: 10.1177/0020764020941889. [Epub ahead of print].
Ní Mhaoláin, A. M., Fan, C. W., Romero-Ortuno, R., Cogan, L., Cunningham, C., Kenny, R. A., et al. (2012). Frailty, depression, and anxiety in later life. Int. Psychogeriatr. 24, 1265–1274. doi: 10.1017/S1041610211002110
O'Bryant, S. E., Humphreys, J. D., Smith, G. E., Ivnik, R. J., Graff-Radford, N. R., Petersen, R. C., et al. (2008). Detecting dementia with the mini-mental state examination in highly educated individuals. Arch. Neurol. 65, 963–967. doi: 10.1001/archneur.65.7.963
Onder, G., Rezza, G., and Brusaferro, S. (2020). Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323, 1775–1776. doi: 10.1001/jama.2020.4683
Periáñez, J. A., Ríos-Lago, M., Rodríguez-Sánchez, J. M., Adrover-Roig, D., Sánchez-Cubillo, I., Crespo-Facorro, B., et al. (2007). Trail making test in traumatic brain injury, schizophrenia, and normal ageing: sample comparisons and normative data. Arch. Clin. Neuropsychol. 22, 433–447. doi: 10.1016/j.acn.2007.01.022
Petersen, R., and Negash, S. (2008). Mild cognitive impairment: an overview. CNS Spectr. 13, 45–53. doi: 10.1017/S1092852900016151
Potter, G. G., McQuoid, D. R., Whitson, H. E., and Steffens, D. C. (2016). Physical frailty in late-life depression is associated with deficits in speed-dependent executive functions. Int. J. Geriatr. Psychiatry 31, 466–474. doi: 10.1002/gps.4351
Prati, G., and Mancini, A. D. (2021). The psychological impact of COVID-19 pandemic lockdowns: a review and meta-analysis of longitudinal studies and natural experiments. Psychol. Med. 51, 201–211. doi: 10.1017/S0033291721000015
Robertson, D., Savva, G., and Kenny, R. (2013). Frailty and cognitive impairment-a review of the evidence and causal mechanisms. Ageing Res. Rev. 12, 840–851. doi: 10.1016/j.arr.2013.06.004
Robertson, D. A., Savva, G., Coen, R. F., and Kenny, R. A. (2014). Cognitive function in the prefrailty and frailty syndrome. J. Am. Geriatr. Soc. 62, 2118–2124. doi: 10.1111/jgs.13111
Salari, N., Hosseinian-Far, A., Jalali, R., Vaisi-Raygani, A., Rasoulpoor, S., Mohammadi, M., et al. (2020). Prevalence of stress, anxiety, depression among the general population during the COVID-19 pandemic: a systematic review and meta-analysis. Globalization Health 16:57. doi: 10.1186/s12992-020-00589-w
Santini, Z. I., Jose, P. E., York Cornwell, E., Koyanagi, A., Nielsen, L., Hinrichsen, C., et al. (2020). Social disconnectedness, perceived isolation, and symptoms of depression and anxiety among older Americans (NSHAP): a longitudinal mediation analysis. Lancet Public Health 5, e62–e70. doi: 10.1016/S2468-2667(19)30230-0
Schrack, J. A., Wanigatunga, A. A., and Juraschek, S. P. (2020). After the COVID-19 pandemic: the next wave of health challenges for older adults. J. Gerontol. A Biol. Sci. Med. Sci. 75, e121–e122. doi: 10.1093/gerona/glaa102
Seiter, J. S., and Curran, T. (2021). Social-distancing fatigue during the COVID-19 pandemic: a mediation analysis of cognitive flexibility, fatigue, depression, and adherence to CDC guidelines. Commun. Res. Rep. 38, 68–78. doi: 10.1080/08824096.2021.1880385
Smith, G. E., and Bondi, M. W. (2013). Mild Cognitive Impairment and Dementia: Definitions, Diagnosis, and Treatment. New York, NY: Oxford University Press.
Spinnler, H., and Tognoni, G. (1987). Standardizzazione e taratura italiana di test neuropsicologici: Gruppo italiano per lo studio neuropsicologico dell'invecchiamento. Italian J. Neurol. Sci. 6(Suppl. 8), 8–120.
Staples, W. H., Kays, A., and Richman, R. (2020). Examination of the correlation between physical and psychological measures in community-dwelling older adults. Clin Interv. Aging 15, 293–300. doi: 10.2147/CIA.S239053
Starkstein, S. E., Garau, M. L., and Cao, A. (2004). Prevalence and clinical correlates of disinhibition in dementia. Cogn. Behav. Neurol. 17, 139–147. doi: 10.1097/01.wnn.0000119241.65522.90
Wittich, W., Phillips, N., Nasreddine, Z. S., and Chertkow, H. (2010). Sensitivity and specificity of the montreal cognitive assessment modified for individuals who are visually impaired. J. Visual Impairment Blindness, 104, 360–368. doi: 10.1177/0145482X1010400606
World Health Organization (2021). Health Topics, Ageing. Available online at: https://www.who.int/health-topics/ageing#tab=tab_1 (accessed January 23, 2021).
Keywords: normal aging, executive functions, mood deflections, gait speed, handgrip strength, lockdown pandemic fatigue
Citation: Amanzio M, Canessa N, Bartoli M, Cipriani GE, Palermo S and Cappa SF (2021) Lockdown Effects on Healthy Cognitive Aging During the COVID-19 Pandemic: A Longitudinal Study. Front. Psychol. 12:685180. doi: 10.3389/fpsyg.2021.685180
Received: 24 March 2021; Accepted: 27 April 2021;
Published: 24 May 2021.
Edited by:
Daniela Smirni, University of Palermo, ItalyReviewed by:
Silvia Zabberoni, Santa Lucia Foundation (IRCCS), ItalyFabrizio Stasolla, Giustino Fortunato University, Italy
Copyright © 2021 Amanzio, Canessa, Bartoli, Cipriani, Palermo and Cappa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Bartoli, massimo.bartoli@unito.it
†These authors have contributed equally to this work and share first authorship
 Martina Amanzio
Martina Amanzio Nicola Canessa
Nicola Canessa Massimo Bartoli
Massimo Bartoli Giuseppina Elena Cipriani
Giuseppina Elena Cipriani Sara Palermo
Sara Palermo Stefano F. Cappa
Stefano F. Cappa