Abstract
Initial reports on COVID-19 described children as largely spared from severe manifestations, with only 2–6% of children requiring intensive care treatment. However, since mid-April 2020, clusters of pediatric cases of severe systemic hyperinflammation and shock epidemiologically linked with COVID-19 have been reported. This condition was named as SARS-Cov-2-associated multisystem inflammatory syndrome in children and showed similarities to Kawasaki disease. Here, we present a narrative review of cases reported in literature and we discuss the clinical acute and follow-up management of these patients. Patients with SARS-Cov-2-associated multisystem inflammatory syndrome frequently presented with persistent fever, gastrointestinal symptoms, polymorphic rash, conjunctivitis, and mucosal changes. Elevated inflammatory markers and evidence of cytokine storm were frequently observed. A subset of these patients also presented with hypotension and shock (20–100%) from either acute myocardial dysfunction or systemic hyperinflammation/vasodilation. Coronary artery dilation or aneurysms have been described in 6–24%, and arrhythmias in 7–60%. Cardiac support, immunomodulation, and anticoagulation are the key aspects for the management of the acute phase. Long-term structured follow-up of these patients is required due to the unclear prognosis and risk of progression of cardiac manifestations.
Conclusion: Multisystem inflammatory syndrome is a novel syndrome related to SARS-CoV-2 infection. Evidence is still scarce but rapidly emerging in the literature. Cardiac manifestations are frequent, including myocardial and coronary involvement, and need to be carefully identified and monitored over time.
What is Known: • Multisystem inflammatory syndrome in children (MIS-C) has been described associated with SARS-CoV-2. What is New: • Patients with MIS-C often present with fever, gastrointestinal symptoms, and shock. • Cardiac involvement is found in a high proportion of these patients, including ventricular dysfunction, coronary artery dilation or aneurysm, and arrhythmias. • Management is based on expert consensus and includes cardiac support, immunomodulatory agents, and anticoagulation. • Long-term follow-up is required due to the unclear prognosis and risk of progression of cardiac manifestation. |
Similar content being viewed by others
Introduction
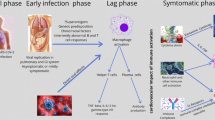
Initial reports during the early phase of the COVID-19 pandemic indicated that children were relatively spared from severe manifestations, with 2–6% of children presenting with severe illness [1,2,3]. However, since mid-April 2020, clusters of pediatric cases of severe systemic hyperinflammation and shock epidemiologically linked with COVID-19 were reported. Riphagen et al. first described a case series of 8 previously asymptomatic children presenting with hyperinflammatory shock, ventricular dysfunction, and multiorgan involvement [4]. This was followed by other reports of patients with Kawasaki disease (KD) and KD-like syndrome, frequently complicated by significant cardiac involvement [5,6,7,8,9].
The increasing number of reported cases led to a health advisory from the Royal College of Pediatrics and Child Health (RCPCH), the Centers for Disease Control and Prevention (CDC), and the World Health Organization (WHO), which identified these cases as a novel condition named multisystem inflammatory syndrome in children (MIS-C), also called pediatric multisystem inflammatory syndrome (PMIS) [10,11,12]. For the purpose of this review, the term MIS-C will be used. Here, we aim to review the published case reports and case series of patients with MIS-C, summarize the existing evidence on its cardiac manifestations in a form of narrative review, and propose a consensus-based approach for the management of MIS-C. Methods of the review process are reported as Supplemental material.
Definition of MIS-C
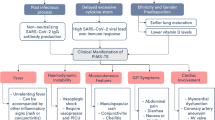
The RCPCH’s, CDC’s, and the WHO’s definitions of the novel syndrome are shown in Table 1 [10,11,12]. All three definitions include presence of fever, laboratory evidence of inflammation, and multisystem organ involvement without alternative plausible diagnoses, as well as evidence of COVID-19 infection or recent exposure to a COVID-19 case. The duration of fever, criteria for organ involvement, and need for documentation of SARS-CoV-2 infection vary between definitions.
Clinical presentation
Clinical symptoms
Children with MIS-C commonly present with persistent fever, asthenia, diffuse erythematous polymorphic rash, non-purulent conjunctivitis, and prominent gastrointestinal symptoms (Table 2) [4,5,6,7,8,9, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Other commonly reported symptoms are mucosal changes and peripheral edema, which, along with the rash and conjunctivitis, resemble the clinical characteristics of KD [5,6,7,8,9, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. In contrast with adults, odynophagia and respiratory symptoms were rarely seen [4, 9, 14, 15, 22,23,24,25,26,27]. Notably, a subset of patients presents with hypotension and shock from either acute myocardial involvement or systemic hyperinflammation/vasodilation, frequently requiring intensive care admission, circulatory, and respiratory support (Tables 2 and 3) [4, 5, 8, 9, 13,14,15,16,17,18,19,20, 22,23,24,25, 27].
Factors associated with MIS-C
Although comorbidities have been associated with more severe disease in both adults and children with severe COVID-19 [2], their role in MIS-C remains unclear. While Belhadjer, Dufort and Feldstein et al. hypothesize that overweight patients may have a higher risk to present MIS-C [24, 25, 27], patients overall were reported to be previously healthy, and only occasionally had a baseline chronic condition such as asthma or autoimmune disorders (Table 2) [4, 8, 15, 19, 20, 25, 27, 28]. Interestingly, none of the reported patients had known congenital heart disease or preexisting cardiovascular disease. Finally, several case series have described a high proportion of African ethnicity or ancestry [4, 18, 20, 24, 25], as well as Hispanic subjects [23,24,25]. Future studies may help better understand the role of genetic and socioeconomic status in the pathophysiology of MIS-C.
Evidence of SARS-CoV-2 infection
While a small number of MIS-C patients have positive SARS-CoV-2 reverse-transcriptase protein chain reaction (RT-PCR) (Table 3), the majority have either known family exposures or serologic evidence of prior infection. Time from infection to onset of MIS-C symptoms varies among studies, from a few days to months [17, 18, 25, 27]. Overall, a variable percentages of subjects, from 0 [15, 30] to 100% [16] had positive RT-PCR; however, in most of the reports, SARS-CoV-2 positivity varies between 20 and 53% (Table 2) [4, 5, 14, 17, 18, 20, 22,23,24,25, 27]. Generally, a higher percentage (75–100%) had evidence of IgG antibodies (Table 2) [5, 15, 17, 18, 20,21,22,23,24,25, 27, 30] and suggest that a postinfectious immune response may be responsible for this condition [32].
Laboratory findings
Elevated inflammatory markers and evidence of hyperinflammation were widely reported and consistently found in patients with MIS-C [4,5,6,7,8,9, 13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. Supplemental Table 1 summarizes the main laboratory characteristics of the existing cases in the literature. Overall, C-reactive protein (CRP), procalcitonin (PCT), and erythrocyte sedimentation rate (ESR) are highly elevated, as well as ferritin and IL-6. A significant increase in D-dimer and fibrinogen are key features of the coagulation profile, while the hematologic aspect of the disease is characterized by leukocytosis, neutrophilia with immature forms, lymphopenia, normal or decreased red blood cell count and normal or decreased platelet count.
Cardiac involvement
Myocardial dysfunction
Left ventricular (LV) systolic dysfunction has been described in a large proportion of children diagnosed with MIS-C in both the initial reports and subsequent case series. Cardiac findings in children with MIS-C are summarized in Table 2 and Table 3. In the first MIS-C case-series reported from the UK, cardiac dysfunction was present in 6/8 patients (75%) [4]. In subsequent case series, ventricular dysfunction has been reported in 35–100% of children with MIS-C, depending on definition and inclusion criteria (Table 2) [4, 5, 8, 9, 13,14,15,16,17,18,19,20,21,22,23,24,25, 27, 30, 31].
Two of the published case series described selected cohorts of patients with myocardial dysfunction as inclusion criteria [17, 27]. Belhadjer et al. reported a selected cohort of 35 MIS-C patients who developed acute LV failure (LV ejection fraction (LVEF) < 50%) or shock, fever, and elevated inflammatory markers [27]. Management of these patients included mechanical ventilation and inotropic support in 80% of patients, and extracorporeal membrane oxygenation (ECMO) support in 28%. All patients successfully weaned off ECMO and none had died at the time of publication. [27]. Grimaud at el. reported 20 patients admitted with cardiogenic/vasoplegic shock and a median LVEF of 35% (IQR 25–55%). Nineteen out of 20 patients required inotropes/vasopressors but no ECMO support was needed. All patients had a full recovery of the LV function prior to discharge [17].
A high proportion of patients also had elevated troponin level or B-type natriuretic peptide (BNP)/pro-BNP values (Table 2), which may be a useful marker for myocardial involvement. Most patients had recovery of ventricular function, but 6–14% of patients had persistent dysfunction at discharge (Table 3).
The mechanism underlying myocardial dysfunction in MIS-C has not been yet fully elucidated. Possible causes of myocardial injury in adults with COVID-19 include acute myocarditis, hypoxic injury, ischemic injury caused by cardiac microvascular damage or coronary artery disease, right heart strain (acute cor pulmonale), stress cardiomyopathy (Takotsubo), and systemic inflammatory response syndrome [3, 34,35,36,37]. The variable timing and modality of presentation with ventricular dysfunction suggests that different pathophysiological mechanisms may be responsible: while the acute infection may explain the occurrence of acute myocardial damage, a second phase characterized by a post-viral immunological reaction and systemic hyperinflammation may explain the occurrence of myocardial inflammation and dysfunction in predisposed subjects. In this second phase, a combination of cardiogenic and distributive shock may be observed. Advanced cardiac imaging in patients with MIS-C and ventricular dysfunction may help us better understand the underlying mechanism of injury, and presence of long-term scar or myocardial damage.
Coronary involvement
Coronary artery dilation or aneurysms have been described in 6–24% of patients (Table 2) [4, 5, 14, 15, 18,19,20,21,22,23,24,25, 27]. Most cases described mild coronary artery dilation with z-scores 2–2.5. As coronary artery z-scores are based on healthy, non-febrile children, some of the findings in the acute phase may be related to coronary vasodilation in the setting of fever and inflammation. However, there have also been reports of large and giant coronary artery aneurysms [4, 20], and progression of coronary aneurysm following discharge raising concerns for coronary artery intimal disruption [4, 5, 19, 20]. The late development of coronary artery aneurysm highlights the need for ongoing follow-up of those patients.
Arrhythmia
Studies focusing on arrhythmic manifestations have described 7–60% of patients having rhythm abnormalities of variable severity (Table 2). The most frequently reported electrocardiogram (ECG) anomalies were non-specific and included ST segment changes, QTc prolongation, and premature atrial or ventricular beats. First- and second-degree atrioventricular blocks were reported in one series, while atrial fibrillation was described in two reports [9, 20]. However, there have also been reported cases of sustained arrhythmias leading to hemodynamic collapse and need for ECMO support [4, 20].
Hyperinflammatory state and resemblance to Kawasaki disease
MIS-C overlaps with many features of KD [5, 20, 32]. KD is an acute pediatric vasculitis involving medium-sized vessels typically affecting children < 5 years of age [38, 39]. The etiology of KD is still unknown, but it has been considered an inflammatory syndrome likely resulting from an infectious or other environmental trigger in a genetically susceptible host. While no specific infectious trigger has been confirmed, several viruses have been implicated, including coronaviruses [40, 41]. Clinical diagnostic criteria include a persistent fever (> 5 days) and at least 4 of 5 clinical symptoms including mucocutaneous involvement, non-purulent conjunctivitis, polymorphous rash, unilateral lymphadenopathy, and palmar/plantar erythema and desquamation. An incomplete form of KD is defined by persistent fever and presence of < 4 of classical symptoms with suggestive laboratory data and/or echo findings [38, 39]. In the acute phase of the disease, about 7% of patients manifest hemodynamic instability, a condition known as KD shock syndrome (KDSS) [39, 42]. Compared with KD patients without any signs of shock, KDSS patients were more frequently female, had a larger proportion of bands, higher CRP, and lower hemoglobin and platelet counts [42].
The small case series from Bergamo, Italy, reported a 30-fold increase in the incidence of KD or KD-like illness during the height of COVID-19 outbreak in the region (uncorrected for seasonal incidence), with many patients testing positive for IgG antibody and negative RT-PCR [5]. When these cases were compared with 19 classical KD, COVID-19-associated cases were found to be older (7.5 ± 3.5 vs 3.0 ± 2.5 years), more likely to present in shock (50% vs 0%), to have more cardiac involvement (abnormal echocardiogram in 60% vs 10%), and more likely to have elevation in troponin or BNP. Similarly, Whittaker et al. compared patients meeting the MIS-C definition with classical KD or KDSS patients [20], reporting that patients with MIS-C were generally older, had higher white blood cell count, neutrophil count, CRP, fibrinogen levels and higher troponin, as well as more profound lymphopenia, anemia, and lower platelet counts.
While generally self-limited, KD can have a number of long-term sequelae, the most important of which are cardiovascular. In addition to ventricular and valvular dysfunction, patients with KD can develop persistent coronary aneurysms, occurring in 20–25% of untreated children [38, 43, 44]. Coronary dilation or aneurysms have been reported in up to 25% of MIS-C patients, suggesting a pathophysiologic similarity with KD. Even if MIS-C patients have different clinical characteristics and laboratory findings compared with classical KD, the similarity in clinical features and the development of coronary artery aneurysms in both disorders may represent a key point for the future understanding of underlying pathophysiologic mechanisms [32]. Further studies will be needed to deeply understand the pathophysiology of this disorder.
Management
Management of patients with MIS-C is reported in Table 3. Overall, admission to the intensive care unit (ICU) for management of shock was described in 20–100% of the patients, most often for inotropic support (Table 3) [4, 5, 8, 9, 13,14,15,16,17,18,19,20,21,22,23,24,25, 27, 30, 31]. More rarely, patients required V-A ECMO support (0–28%) [4, 9, 20, 23,24,25, 27]. Most patients received immunomodulatory treatment with intravenous immunoglobulin. The use of corticosteroids was less consistent and ranged from low-dose treatment to high-dose methylprednisolone pulses [4, 5, 8, 13, 14, 16,17,18,19,20,21,22,23,24,25, 27, 30, 31]. The use of anti-inflammatory dosages of aspirin has been also occasionally reported [5, 6, 21, 22]. Not infrequently, cytokine blockers have been added as a supplemental therapy, with a preference for IL-6 inhibitors (tocilizumab), but also IL-1 or tumor-necrosis-factor (TNF)-αinhibitors (anakinra, infliximab) [4, 14,15,16,17, 19, 20, 22, 23, 25, 27, 28, 31, 33]. Antiplatelet treatment with aspirin was frequently adopted, especially in patients with KD-like clinical presentations, or in those with evidence of coronary involvement. A therapeutic or prophylactic anticoagulation approach was less frequently used, except for a few case series [16, 19, 23, 25, 27, 31].
Due to the scarce knowledge and the small number of reported cases so far, the management of patients with MIS-C has been largely based on expert opinion and extrapolated from KD treatment, adult experience with COVID-19, and other systemic inflammatory disorders in children. Here, we describe a consensus-based approach for the acute and medium-term management of children with MIS-C, as well as a follow-up algorithm, developed within our Institution. However, it is necessary to emphasize that there are currently no approved therapies for MIS-C patients, and data from higher-evidence studies may quickly lead to changes in clinical practice.
Proposed clinical approach
A multidisciplinary team should be involved in the management of patients with MIS-C, including cardiology, rheumatology, intensive care, and infectious disease specialists. Given the lack of established treatment, possibility of harm, and limited drug supply, treatment is currently not recommended for (a) prevention or postexposure prophylaxis or (b) non-hospitalized patients.
Cardiac support
As described above, a high proportion of patients will present with shock and require acute resuscitation. Pediatric resuscitation guidelines should be followed [45]. In patients with suspicion or evidence of ventricular dysfunction, smaller fluid boluses (10 mg/kg) should be administered with careful reassessment for signs of fluid overload between each. Extracorporeal membrane oxygenation should be considered if medical support fails.
Immunomodulatory therapy
There may be a benefit of immunomodulatory therapy in patients with MIS-C, severe disease, and evidence of cytokine storm syndrome and/or those with cardiac involvement. Due to recent emergence of MIS-C, no randomized trials or comparative effectiveness studies have evaluated treatment strategies, but the benefits of immunomodulatory therapy are well established in KD [39], and they are often used for the treatment of infective myocarditis [46,47,48] and other systemic inflammatory diseases [49, 50]. Therefore, based on the experience in similar conditions, it appears reasonable to suggest an immunomodulation approach based on intravenous immunoglobulins (IVIGs). Slower IVIG administration should be considered in patients with myocardial dysfunction to decrease the risk of fluid overload. Low-dose corticosteroids should be considered in sicker patients, in patients with known baseline conditions which can benefit from steroid treatment, or based on clinical judgment. The use of biologic drugs (tocilizumab, anakinra, infliximab) could be considered in patients with severe or critical illness, especially if they did not respond to first-line treatments.
Antiplatelet treatment and anticoagulation
Children with MIS-C are at risk of thrombotic complications from multiple causes, including hypercoagulable state, possible endothelial injury, stasis from immobilization, ventricular dysfunction, and coronary artery aneurysm. For these reasons, antiplatelet and/or anticoagulation treatment is recommended. Decisions about anticoagulation should be based on coagulation tests, viscoelastic testing [51, 52], and clinical presentation. Patients with evidence of myocardial involvement or coronary artery dilation may benefit from antiplatelet therapy and prophylactic anticoagulation. In addition, therapeutic anticoagulation may be considered in patients with very abnormal coagulation profile (i.e., D-dimer ≥ 3 mg/mL), documented thrombosis, arrhythmia, ventricular dysfunction greater than moderate, or giant coronary artery aneurysm. However, it should be emphasized that this is based on experts’ opinion, with no evidence to support recommendations.
Antiviral therapy
The role of antiviral therapies (e.g., remdesivir) in the management of children with MIS-C is uncertain [53, 54]. Evidence suggests that MIS-C represents a postinfectious complication rather than an active infection. Although we did not include antiviral therapies as an established step in our algorithm, antiviral therapy may be considered in patients with severe manifestations and concerns for ongoing infection with positive RT-PCR. A consultation with specialists in infectious disease is highly recommended in this case.
Outpatient follow-up
Cardiac manifestations often improve and/or normalize prior to hospital discharge, but some patients have shown residual cardiac lesions. Additionally, some series reported progression of coronary artery aneurysm following discharge, highlighting our limited knowledge of this disease and the potential for long-term complications. Therefore, it is essential to guarantee an adequate medium and long term follow-up to these patients.
At this point of knowledge, we recommend follow-up for at least a year after initial diagnosis (Fig. 1). At initial visits, laboratory testing should be obtained to document normalization of inflammatory markers and resolution of hematologic anomalies. Laboratory testing may also guide weaning of corticosteroids if used in the acute phase. Echocardiograms should be obtained at regular intervals for evaluation of ventricular function and coronary artery dimensions. ECGs should also be obtained due to reports of arrhythmias including atrioventricular block, which may progress after initial diagnosis. If anomalies are identified on ECG, Holter monitors may be useful as further investigation. In patients with a history of ventricular dysfunction, cardiac magnetic resonance imaging (MRI) may be considered 2–6 months after initial diagnosis for evaluation of ventricular function, edema, diffuse fibrosis, and scar.
While the prothrombotic risk is greatest in the acute phase, the optimal duration of antiplatelet and anticoagulation remains unclear. Patients with documented thrombosis should be continued on anticoagulation for at least 3 months after discharge. Coagulation, D-dimer, and viscoelastic testing may help guide discontinuation of anticoagulation. Moreover, patients with persistent ventricular dysfunction and/or coronary artery aneurysm may also benefit from long-term antiplatelet and/or anticoagulation depending on the severity of cardiac involvement.
Exercise restriction
Due to the high prevalence of myocardial involvement with MIS-C, the safety to return to physical activity and exercise after discharge is unanswered. While the etiology of the myocardial involvement remains unknown, it is clear that there are similarities to acute myocarditis. Thus, one can argue that guidelines for return to sport participation after myocarditis should be followed in those patients [48, 55]. After acute myocarditis, restriction from physical activity for at least 6 months following diagnosis is recommended. Preparticipation evaluation with echocardiograms and exercise testing may be beneficial to document the safety of exercise participation.
Conclusion
MIS-C is a novel syndrome related to SARS-CoV-2 infection characterized by fever, signs of inflammation, and organ dysfunction. Evidence is still scarce but rapidly emerging from literature. Myocardial involvement, due to either acute myocarditis or secondary hyperinflammation, is frequent in children with MIS-C. Coronary dilation or aneurysm and arrhythmias may develop and evolve over time. Cardiac support, immunomodulation, and antiplatelet/anticoagulation treatments are part of the management of acute MIS-C. Finally, follow-up of MIS-C patients is essential to better understand the evolution and prognosis of this disease. Future studies are needed to define evidence-based management of this novel condition.
Abbreviations
- BNP:
-
B-type natriuretic peptide
- BMI:
-
Body mass index
- CDC:
-
Centers for Disease Control and Prevention
- COVID-19:
-
Coronavirus disease 2019
- CRP:
-
C-reactive protein
- ECG:
-
Electrocardiogram
- ECMO:
-
Extracorporeal membrane oxygenation
- ESR:
-
Erythrocyte sedimentation rate
- ICU:
-
Intensive care unit
- IL:
-
Interleukin
- KD:
-
Kawasaki disease
- KDSS:
-
Kawasaki disease shock syndrome
- LV:
-
Left ventricular
- LVEF:
-
LV ejection fraction
- MIS-C:
-
Multisystem inflammatory syndrome in children
- MRI:
-
Magnetic resonance imaging
- PCT:
-
Procalcitonin
- RCPCH:
-
Royal College of Pediatrics and Child Health
- RT-PCR:
-
Reverse-transcriptase protein chain reaction
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- TNF:
-
Tumor necrosis factor
- WHO:
-
World Health Organization
References
Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, Tong S (2020) Epidemiology of COVID-19 among children in China. Pediatrics 145:1–10. https://doi.org/10.1542/peds.2020-0702
Liguoro I, Pilotto C, Bonanni M et al (2020) SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. https://doi.org/10.1007/s00431-020-03684-7
Sanna G, Serrau G, Bassareo PP et al (2020) Children’s heart and COVID-19: up-to-date evidence in the form of a systematic review. Eur J Pediatr. https://doi.org/10.1007/s00431-020-03699-0
Riphagen S, Gomez X, Gonzalez-martinez C et al (2020) Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 395:1607–1608. https://doi.org/10.1016/S0140-6736(20)31094-1
Verdoni L, Mazza A, Gervasoni A et al (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 6736:1–8. https://doi.org/10.1016/S0140-6736(20)31103-X
Jones VG, Mills M, Suarez D, Hogan CA, Yeh D, Segal JB, Nguyen EL, Barsh GR, Maskatia S, Mathew R (2020) COVID-19 and Kawasaki disease : novel virus and novel case. Hosp Pediatr 10:537–540. https://doi.org/10.1542/hpeds.2020-0123
Rivera-Figueroa EI, Santos R, Simpron S, Garg P (2020) Incomplete Kawasaki disease in a child with Covid-19. Indian Pediatr 19:1–4
Licciardi F, Pruccoli G, Denina M, Parodi E (2020) SARS-CoV-2 – induced Kawasaki-like hyperinflammatory syndrome : a novel COVID phenotype in children. Pediatrics. https://doi.org/10.1542/peds.2020-1711
Deza Leon MP, Redzepi A, McGrath E et al (2020) COVID-19–associated pediatric multisystem inflammatory syndrome. J Pediatric Infect Dis Soc 1-2. https://doi.org/10.1002/jmv.25807
Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-19. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19
Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. https://emergency.cdc.gov/han/2020/han00432.asp
World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Published May 15, 2020
Rauf A, Vijayan A, John S et al (2020) Multisystem inflammatory syndrome with features of atypical Kawasaki disease during COVID-19 pandemic. Indian J Pediatr 2-4. https://doi.org/10.1007/s12098-020-03357-1
Chiotos K, Bassiri H, Behrens EM et al (2020) Multisystem inflammatory syndrome in children during the COVID-19 pandemic: a case series. J Pediatric Infect Dis Soc. https://doi.org/10.1093/jpids/piaa069
Waltuch T, Gill P, Zinns LE, Whitney R, Tokarski J, Tsung JW, Sanders JE (2020) Features of COVID-19 post-infectious cytokine release syndrome in children presenting to the emergency department. Am J Emerg Med. https://doi.org/10.1016/j.ajem.2020.05.058
Wolfler A, Mannarino S, Giacomet V et al (2020) Acute myocardial injury: a novel clinical pattern in children with COVID-19. Lancet child Adolesc Heal 4642:1016–1017. https://doi.org/10.1016/S2352-4642(20)30168-1
Grimaud M, Starck J, Levy M et al (2020) Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care 10:1–5. https://doi.org/10.1186/s13613-020-00690-8
Toubiana J, Poirault C, Corsia A et al (2020) Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. Br Med J 1-7. https://doi.org/10.1136/bmj.m2094
Cheung EW, Zachariah P, Gorelik M et al (2020) Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York city. JAMA J Am Med Assoc 8–10. https://doi.org/10.1001/jama.2020.10374
Whittaker E, Bamford A, Kenny J et al (2020) Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA - J Am Med Assoc 1-11. https://doi.org/10.1001/jama.2020.10369
Ramcharan T, Nolan O, Lai CY et al (2020) Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol 2. https://doi.org/10.1007/s00246-020-02391-2
Pouletty M, Borocco C, Ouldali N et al (2020) Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 999–1006. https://doi.org/10.1136/annrheumdis-2020-217960
Kaushik S, Aydin SI, Derespina KR et al (2020) Multisystem inflammatory syndrome in children (MIS-C) associated with SARS-CoV-2 infection: a multi-institutional study from New York City. J Pediatr 2-7. https://doi.org/10.1016/j.jpeds.2020.06.045
Dufort EM, Koumans EH, Chow EJ et al (2020) Multisystem inflammatory syndrome in children in New York state. N Engl J Med 1-12. https://doi.org/10.1056/NEJMoa2021756
Feldstein LR, Rose EB, Horwitz SM et al (2020) Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 1-13. https://doi.org/10.1056/NEJMoa2021680
Balasubramanian SK, Tiruvoipati R, Amin M, Aabideen KK, Peek GJ, Sosnowski AW, Firmin RK (2007) Factors influencing the outcome of paediatric cardiac surgical patients during extracorporeal circulatory support. J Cardiothorac Surg 2:1–9. https://doi.org/10.1186/1749-8090-2-4
Belhadjer Z, Méot M, Bajolle F et al (2020) Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 33. https://doi.org/10.1161/CIRCULATIONAHA.120.048360
Dolinger MT, Person H, Smith R et al (2019) Pediatric Crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. https://doi.org/10.1097/MPG.0000000000002809
Labé P, Ly A, Sin C et al (1827) Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J Eur Acad Dermatol Venereol 0-1. https://doi.org/10.1111/jdv.16666
Blondiaux E, Parison P, Redheuil A et al (2012) Cardiac MRI of children with multisystem inflammatory syndrome (MIS-C) associated with COVID-19: case series Eléonore. Radiology 78:1–15
Greene AG, Saleh M, Roseman E, Sinert R (2020) Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med 1207:5–6. https://doi.org/10.1016/j.ajem.2020.05.117
Mccrindle BW, Manlhiot C (2020) SARS-CoV-2-related inflammatory multisystem syndrome in children: different or shared etiology and pathophysiology as Kawasaki disease? JAMA - J Am Med Assoc 8–10. https://doi.org/10.1038/ng.2007.59
Balasubramanian S, Nagendran T, Ramachandran B, Ramanan A (2020) Hyper-inflammatory syndrome in a child With COVID-19 treated successfully with intravenous immunoglobulin and tocilizumab. Indian Pediatr 1–5. https://doi.org/10.1007/s13312-020-1901-z
Shi S, Qin M, Shen B et al (2020) Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 1-8. https://doi.org/10.1001/jamacardio.2020.0950
Creel-Bulos C, Hockstein M, Amin N et al (2020) Acute cor pulmonale in critically ill patients. N Engl J Med 89-92. https://doi.org/10.1056/NEJMc2010459
Zheng YY, Ma YT, Zhang JY, Xie X (2020) COVID-19 and the cardiovascular system. Nat Rev Cardiol 17:259–260. https://doi.org/10.1038/s41569-020-0360-5
Tersalvi G, Vicenzi M, Calabretta D, Biasco L, Pedrazzini G, Winterton D (2020) Elevated troponin in patients with coronavirus disease 2019: possible mechanisms. J Card Fail 00:1–6. https://doi.org/10.1016/j.cardfail.2020.04.009
Son MBF, Newburger JW (2018) Kawasaki disease. Pediatr Rev 39:78–90. https://doi.org/10.1542/pir.2016-0182
McCrindle BW, Rowley AH, Newburger JW et al (2017) Diagnosis, treatment, and long-term management of Kawasaki disease. Circulation 135:927–999. https://doi.org/10.1161/CIR.0000000000000484
Esper F, Shapiro ED, Weibel C, Ferguson D, Landry ML, Kahn JS (2005) Association between a novel human coronavirus and Kawasaki disease. J Infect Dis 191:499–502
Turnier JL, Anderson MS, Heizer HR, Jone PN, Glode MP, Dominguez SR (2020) Concurrent respiratory viruses and Kawasaki disease. Pediatrics 136:e609–e614. https://doi.org/10.1542/peds.2015-0950
Kanegaye JT, Wilder MS, Molkara D, Frazer JR, Pancheri J, Tremoulet AH, Watson VE, Best BM, Burns JC (2009) Recognition of a Kawasaki disease shock syndrome. Pediatrics 123:783–789. https://doi.org/10.1542/peds.2008-1871
Friedman KG, Gauvreau K, Hamaoka-okamoto A et al (2016) Coronary artery aneurysms in Kawasaki disease : risk factors for progressive disease and adverse cardiac events in the US population. J Am Hear Assoc 5:e003289. https://doi.org/10.1161/JAHA.116.003289
Liu L, Luo C, Hua Y, Wu M, Shao S, Liu X, Zhou K, Wang C (2020) Risk factors associated with progression and persistence of small- and medium-sized coronary artery aneurysms in Kawasaki disease: a prospective cohort study. Eur J Pediatr 179:891–900. https://doi.org/10.1007/s00431-019-03492-8
Edelson DP, Sasson C, Chan PS et al (2020) Interim guidance for basic and advanced life support in adults, children, and neonates with suspected or confirmed COVID-19: from the Emergency Cardiovascular Care Committee and Get With the Guidelines-Resuscitation Adult and Pediatric Task Forces of the American Heart Association. Circulation 1–12. https://doi.org/10.1161/CIRCULATIONAHA.120.047463
Drucker NA, Colan SD, Lewis AB, Beiser AS, Wessel DL, Takahashi M, Baker AL, Perez-Atayde AR, Newburger JW (1994) Y-globulin treatment of acute myocarditis in the pediatric population. Circulation 89:252–257
Heidendael JF, Den Boer SL, Wildenbeest JG et al (2020) Intravenous immunoglobulins in children with new onset dilated cardiomyopathy. Cardiol Young 46–54. https://doi.org/10.1017/S1047951117001561
Canter CE, Simpson KE (2014) Diagnosis and treatment of myocarditis in children in the current era. Circulation 129:115–128. https://doi.org/10.1161/CIRCULATIONAHA.113.001372
Bayry J, Negi VS, Kaveri SV (2011) Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol 7:349–359. https://doi.org/10.1038/nrrheum.2011.61
de Chambrun MP, Luyt C-E, Beloncle F et al (2017) The clinical picture of severe systemic capillary-leak syndrome episodes requiring ICU admission. Crit Care Med 45:1216–1223. https://doi.org/10.1097/CCM.0000000000002496
Wright FL, Vogler TO, Moore EE et al (2020) Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg:1–11. https://doi.org/10.1016/j.jamcollsurg.2020.05.007
Raval JS, Burnett AE, Rollins-Raval MA et al (2020) Viscoelastic testing in COVID-19: a possible screening tool for severe disease? Transfusion 4-5. https://doi.org/10.1111/trf.15847
Beigel JH, Tomashek KM, Dodd LE et al (2020) Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med 1-12. https://doi.org/10.1056/NEJMoa2007764
Goldman JD, Lye DCB, Hui DS et al (2020) Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med 1-11. https://doi.org/10.1056/NEJMoa2015301
Caforio ALP, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM, European Society of Cardiology Working Group on Myocardial and Pericardial Diseases (2013) Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 34:2636–2648. https://doi.org/10.1093/eurheartj/eht210
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
F.S. and A.D conceptualized the review, systematically reviewed the literature, evaluated articles for eligibility, extracted relevant data, interpreted the results, and drafted the manuscript. K.F., J.N., M.B.S., and C.J.V. critically revised the first draft of the manuscript and contributed with important intellectual content. All the authors approved the final version of the manuscript to be published and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This is a review article. No ethical approval is required.
Additional information
Communicated by Gregorio Paolo Milani
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 24 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sperotto, F., Friedman, K.G., Son, M.B.F. et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr 180, 307–322 (2021). https://doi.org/10.1007/s00431-020-03766-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-020-03766-6