Abstract
Introduction
We conducted a comprehensive literature review to synthesize evidence for the relationship between corticosteroid use and mortality in patients with COVID-19.
Methods
The PUBMED, EMBASE, and Cochrane Library were searched from inception to March 13, 2021. We searched and analyzed randomized controlled trials (RCTs) and observational studies (OSs) that examined corticosteroid use in patients with COVID-19. The primary outcome was in-hospital mortality, while the secondary outcome was the need for mechanical ventilation (MV) and serious adverse events.
Results
A total of 11 RCTs and 44 OSs involving 7893 and 41,164 patients with COVID-19 were included in the study. Corticosteroid use was associated with lower COVID-19 mortality in RCTs, but was not statistically significant (OR 0.91, 95% CI 0.77–1.07; I2 = 63.4%). The subgroup analysis of pulse dose corticosteroid showed survival benefit statistically (OR 0.29, 95% CI 0.15–0.56). Moreover, the corticosteroid use may reduce the need for MV (OR 0.67, 95% CI 0.51–0.90; I2 = 7.5%) with no significant increase in serious adverse reactions (OR 0.84, 95% CI 0.30–2.37; I2 = 33.3%). In addition, the included OSs showed that the pulse dose (OR 0.66, 95% CI 0.45–0.95; I2 = 30.8%) might lower the mortality in patients with COVID-19. The pulse dose of methylprednisolone (OR 0.60, 95% CI 0.45–0.80; I2 = 0%) had a beneficial effect on survival. It was especially significant when the duration of pulse methylprednisolone use was less than 7 days (OR 0.59, 95% CI 0.43–0.80; I2 = 0%).
Conclusions
This meta-analysis indicated that corticosteroid use might cause a slight reduction in COVID-19 mortality. However, it could significantly reduce the MV requirement in patients with COVID-19 and restrict serious adverse events. Additionally, the pulse dose of methylprednisolone for less than 7 days may be a good treatment choice for patients with COVID-19.
Similar content being viewed by others
Corticosteroid use might cause a slight reduction in COVID-19 mortality. |
Corticosteroid use could reduce the Mechanical Ventiilation (MV) requirement and restrict serious adverse events in patients with COVID-19. |
The pulse dose of methylprednisolone for less than 7 days may be a good treatment choice for patients with COVID-19. |
Introduction
The rapid worldwide spread of coronavirus infections disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has threatened global health seriously [1]. The virus causing COVID-19 is a novel betacoronavirus with 96% similarity to the bat coronavirus genome [2]. It is the third most highly transmissible and pathogenic coronavirus after the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) that appeared in the twenty-first century [3, 4]. Statistically, there have been 133,552,774 confirmed infections and 2,894,295 deaths worldwide by April 10, 2021 (https://www.who.int/data#reports).
There are some drugs, such as low molecular weight heparin, remdesivir, and convalescent plasma, that attracted people’s attention during the COVID-19 epidemic. However, the results of recent large-scale, high-quality randomized controlled trials (RCTs) showed that there is no significant difference in the efficacy of convalescent plasma or remdesivir treatment between the control group and patients with COVID-19 [5, 6]. Corticosteroid administration is an important adjuvant treatment for severe viral infections because of its powerful anti-inflammatory effects [7]. During the SARS epidemic, corticosteroids were widely used in critically ill patients [8, 9]. Corticosteroid therapy is a rational option for patients with COVID-19 because it was previously used to treat patients with severe SARS [10]. Many COVID-19 RCTs have been registered to research the effect of corticosteroids on patients with COVID-19. Three recently published RCTs [11,12,13] demonstrated that corticosteroid use does not lower COVID-19 mortality; however, dexamethasone administration does have short-term survival benefits for patients with COVID-19 requiring respiratory support [14]. A prospective meta-analysis published in JAMA showed that corticosteroid use could reduce short-term all-cause mortality [15]. Corticosteroid treatment probably reduced mortality in patients with COVID-19 and non-COVID-19 acute respiratory distress syndrome (ARDS) [16]. The World Health Organization (WHO) also strongly recommended corticosteroid therapy in critically ill patients with COVID-19 recently (https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1).
Despite there being several recent meta-analyses, the optimal corticosteroid type, dose, and duration in patients with COVID-19 remains unclear. This meta-analysis reviewed the RCT and OS literature comprehensively to establish a relationship between corticosteroid use and COVID-19 mortality. It aimed to explore the beneficial effect of corticosteroids, particularly in pulse methylprednisolone use in patients with COVID-19.
Methods
The meta-analysis (CRD42021242739) followed the PRISMA reporting guidelines [17], and was enrolled at PROSPERO (http://www.crd.york.ac.uk/PROSPERO). Supplementary Material Table S1 provides the PRISMA 2009 checklist. English articles were searched in PUBMED (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (www.embase.com), and Cochrane CENTRAL (www.cochranelibrary.com/central) databases since their inception to March 13, 2021. We used “SARS-CoV-2”, “COVID-19”, “COVID2019”, “severe acute respiratory syndrome coronavirus 2”, “adrenal cortex hormones”, “steroids”, “corticosteroid”, “glucocorticoid,” and other terms to search the database. Supplementary Material Table S2 gives the retrieval strategy in detail. EndNote X9 software was used to perform the literature screening process. Furthermore, we also looked up available references and searched the medRxiv website (https://www.medrxiv.org/) for relevant unpublished articles. The authors Yuqing Cui and Yali Sun searched the literature independently. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Eligibility Criteria
The studies were included in the meta-analysis based on the following population, intervention, comparison, outcome, and study design (PICOS) criteria: (1) adult patients with COVID-19; (2) patients with COVID-19 with or without corticosteroid therapy (low-dose corticosteroid, < 15 mg dexamethasone or equivalent per day; high-dose corticosteroid, > 15 mg dexamethasone or equivalent per day; and pulse dose corticosteroid—pulse dose was explicitly mentioned in the original studies); (3) corticosteroid- and non-corticosteroid-treated patients’ mortality, need for MV, and safety were measured; and (4) RCTs or OSs were excluded if they lacked patients’ outcome data or were animal research.
Studies Selection and Data Extraction
Available data were independently extracted on the basis of the afore mentioned eligibility criteria. The primary outcomes were the risk odds ratios (ORs) of mortality, and the secondary outcomes were the need for MV and safety of patients with COVID-19, with or without corticosteroid use. The data of each study were listed as follows: the study including first author and publication year, country, study design, gender, age, period of inclusion, sample size, corticosteroid type, daily dose and duration, disease severity, number of corticosteroid and non-corticosteroid use (deaths), follow-up, and the data of primary and secondary outcome. If ORs were missing, they were computed on the basis of original numerical values provided in the literature.
Bias Risk Assessment
Cochrane Collaboration bias risk evaluation tool [18] and Newcastle–Ottawa scale (NOS) [19] were used to assess the bias risk of outcomes in RCTs and OSs, respectively. The selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases were used to assess the risk of an RCT. If any of these items were assessed as high risk, the study was considered to have a high risk of bias. Additionally, according to the OS selection (four points at most), the comparability of OS design and analysis (two points at most), and the adequacy of outcome measures (three points at most), a maximum of nine points could be awarded; seven to nine points were considered as high quality. The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) criteria were used to estimate and summarize the quality of the RCT evidence and grade the collected data on the basis of evidence [20].
Statistical Analysis
Statistical analyses were conducted with STATA 14.0 (College Station, Texas, 77845, USA, Serial number 401406267051) and Review Manager (RevMan), version 5.3 (Cochrane Collaboration). Inverse variance random-effects meta-analyses were used for the included studies, and the pooled effect of each outcome was measured. The OR and 95% CI from each included study were either calculated or directly extracted from the data. I2 estimated the heterogeneity of the included studies, where heterogeneity, not sampling error, resulted in variability. The heterogeneity was recorded as moderate when I2 equaled 51–74% and was recorded as high when I2 was more than 74%. Subgroup analyses were conducted on the basis of corticosteroid type and its dose and severity in patients with COVID-19. The stability of outcomes was verified by sensitivity analysis. Funnel plots and Begg’s linear regression were performed to evaluate the publication bias.
Results
Study Selection
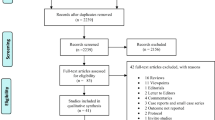
In total, we identified 6717 articles from the three databases. Three RCT records [DEXA-COVID 19 (The efficacy of dexamethasone treatment for patients with ARDS caused by COVID-19; NCT04325061); COVID STEROID (The hydrocortisone for COVID-19 and severe hypoxia; NCT04348305); Steroids-SARI (Glucocorticoid therapy for COVID-19 critically ill patients with severe acute respiratory failure; NCT04244591)] were searched by related meta-analysis [15] to obtain the prospective data; the other two unpublished records [21, 22] were identified by searching the medRxiv website. There were 4508 records left after removing the duplicates. Furthermore, we identified 106 studies after a preliminary screening by title or abstract. Finally, our meta-analysis included 11 RCTs and 44 OSs enrolling 7893 and 41,164 patients (Fig. 1).
Study Characteristics
There were 11 RCTs [11,12,13,14, 23,24,25,26] (DEXA-COVID 19, NCT04325061; COVID STEROID, NCT04348305; Steroids-SARI, NCT04244591) and 44 OSs [21, 22, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] that reported an association between mortality and corticosteroid therapy in patients with COVID-19. There were only one RCT [23] and eight OSs [33, 34, 46, 49, 55,56,57,58] that used pulse dose of corticosteroid. The OSs were included to further explore the corticosteroid type and pulse dose duration that were beneficial to patients with COVID-19. Table 1 and Supplementary Material Table S3 present the characteristics of literature included.
Risk of Bias Assessment
Supplementary Material Fig. S1 presents the RCTs of the Cochrane Collaboration bias risk evaluation tool. Four RCTs [12, 14, 23, 26] were at high risk of bias because of performance bias. Three RCTs [11, 13, 25] were at low risk of bias, and one trial [24] had unclear risk bias. According to the NOS, all 44 eligible OSs [21, 22, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] scored greater than or equal to seven points, indicating a low risk of bias. Supplementary Material Table S4 reports the specific contents of risk bias in the included OSs.
Effects of Corticosteroids on Outcomes
Figure 2 shows the preliminary results of RCTs. The results showed that the corticosteroid use did not reduce the in-hospital mortality significantly (OR 0.91, 95% CI 0.77–1.07; I2 = 63.4%; evidence rank, moderate). The association between corticosteroid and COVID-19 mortality was also not statistically significant in OSs (OR 0.89, 95% CI 0.74–1.08; I2 = 83.3%) (Supplementary Material Fig. S2). Supplementary Material Figs. S3 and S4 show the secondary outcomes of the RCTs. Corticosteroid administration did reduce the need for MV (OR 0.67, 95% CI 0.51–0.90; I2 = 7.5%; evidence rank, moderate) and did not statistically increase the serious adverse events (OR 0.84, 95% CI 0.30–2.37; I2 = 33.3%; evidence rank, moderate) among patients with COVID-19.
Forest plot showing the association between corticosteroid dose and COVID-19 mortality in RCTs using the random-effects model. Low, using low dose of corticosteroid; high, using high dose of corticosteroid; pulse, using pulse dose of corticosteroid; using afixed dose or bshock-dependent dose of corticosteroid
Subgroup Analysis and Effect on Mortality
We analyzed subgroups to explore the sources of high heterogeneity, according to corticosteroid type and dose. We found that the pulse dose corticosteroid treatment improved survival in one RCT (pulse: OR 0.29, 95% CI 0.15–0.56; low: OR 0.93, 95% CI 0.81–1.07; high: OR 1.34, 95% CI 0.66–2.69) (Fig. 2) and OSs (pulse: OR 0.66, 95% CI 0.45–0.95; I2 = 30.8%; low: OR 0.86, 95% CI 0.69–1.06; high: OR 1.03, 95% CI 0.66–1.61) (Fig. 3). Furthermore, we found that the pulse dose of methylprednisolone (pulse Me: OR 0.60, 95% CI 0.45–0.80; Hy: OR 3.28, 95% CI 0.98–10.99) significantly lowered the hospital mortality in patients with COVID-19 (Fig. 4), particularly in patients with a duration less than 7 days (pulse methylprednisolone less than 7 days: OR 0.59, 95% CI 0.43–0.80; more than 7 days: OR 1.08, 95% CI 0.27–4.40) (Fig. 5).
Forest plot showing the association between corticosteroid dose and COVID-19 mortality in OSs using the random-effects model. Low, using low dose of corticosteroid; high, using high dose of corticosteroid; pulse, using pulse dose of corticosteroid; Me, methylprednisolone; De, dexamethasone; early, early use of corticosteroid; delay, delayed use of corticosteroid; severe, using corticosteroid in severe cases; critical, using corticosteroid in critical cases; out-of-week-2-MP, receiving pulse dose of methylprednisolone at week 1 or 3; week-2-MP, receiving pulse dose of methylprednisolone during week 2; non-pulse, receiving non-pulse dose of methylprednisolone. aReceiving steroids within 48 h of ICU admission; breceiving steroids between 48 h and 7 days from admission; creceiving their first dose of steroids more than 7 days after ICU admission
Forest plot showing the association between pulse corticosteroid type and COVID-19 mortality in OSs using the random-effects model. Pulse, using pulse dose of corticosteroid; Me, methylprednisolone; Hy, hydrocortisone; out-of-week-2-MP, receiving pulse dose of methylprednisolone at week 1 or 3; week-2-MP, receiving pulse dose of methylprednisolone during week 2
Forest plot showing the association between duration of pulse methylprednisolone use and COVID-19 mortality in OSs using the random-effects model. Pulse, using pulse dose of corticosteroid; out-of-week-2-MP, receiving pulse dose of methylprednisolone at week 1 or 3; week-2-MP, receiving pulse dose of methylprednisolone during week 2
Sensitivity Analyses
As a result of the high heterogeneity of our results, we conducted a sensitivity analysis to evaluate the impact of any single study on the pooled OR and 95% CI by omitting one study at a time. We found that the results of these RCTs and OSs were robust and reliable (Supplementary Material Figs. S5 A and B).
Publication Bias Assessment
We generated funnel plots (Supplementary Material Figs. S6 A and B) and performed Begg’s regression tests to examine the publication bias of the included studies. There was no significant publication bias in RCTs (P = 0.732) and OSs (P = 0.659).
Discussion
The meta-analysis identified 11 RCTs (7893 patients) and 44 OSs (41,164 patients) based on corticosteroid and COVID-19. The results of RCTs demonstrated that corticosteroid use had survival benefits, especially the pulse corticosteroid use. Furthermore, its use lowered the need for MV without significantly increasing the serious adverse events. Additionally, the OS analysis showed that the pulse dose of methylprednisolone for less than 7 days leads to a significant decrease in mortality.
The pathophysiology of COVID-19 includes host-mediated excessive inflammation and cytokine storm that causes severe endothelial and alveolar damage [69]. COVID-19-related death is mainly due to excessive inflammation and uncontrolled immune response [70]. Corticosteroids are well tolerated and widely used worldwide. They could reduce cytokine storm risk and inflammation in COVID-19 [71]. Moreover, they also could regulate inflammation-mediated lung injury, thereby reducing the progression of respiratory failure and death [14, 72]. Earlier studies on the efficacy and safety of corticosteroid therapy in severe pneumonia [73] indicated a significant association between corticosteroid use and reduction in ARDS risk as well as hospital and ICU length of stay [74]. Three recent high-quality meta-analyses [15, 75, 76] included RCTs and have shown a significant mortality advantage in corticosteroid-treated patients with COVID-19, in particular severely ill patients with COVID-19. The latest guidelines strongly recommend the short application of systemic corticosteroids in patients with MV, no matter whether complicated with ARDS or not [77]. Corticosteroids alter inflammatory pathways at the genomic level or through rapid non-genomic pathways [78]. The genomic mechanism includes activation of cytosolic glucocorticoid receptors, thereby activating or inhibiting protein synthesis, including cytokines, chemokines, and adhesion molecules, which has a direct inhibitory effect on inflammatory cells [79]. Non-genomic mechanisms may play other roles in pulse therapy [80]. At present, the existing evidence supports the use of corticosteroid in patients with COVID-19, but the type, dosage, starting time, and duration need more research. Compared with dexamethasone, methylprednisolone had short half-life and high affinity for glucocorticoid receptor [81]. Pulse methylprednisolone use could quickly reach glucocorticoid receptor saturation to perform genomic and non-genomic functions [82]. Additionally, short-term corticosteroid treatment could minimize severe adverse effects, such as reduced excessive inflammation and exposure time, which may have obvious therapeutic effects.
This meta-analysis had several advantages. The study included the largest number of published RCTs and OSs, including manually searched meta-analysis and unpublished literature, available to date. Therefore, our study had the most comprehensive inclusion of articles. The GRADE and NOS were performed to assess the evidence quality and bias risk. The sensitivity analysis validated the study results to be robust and reliable. The starting time of corticosteroid administration is inconsistent in included studies, and there are few further studies on the dosage and duration of corticosteroid use. In our meta-analysis, we used as comprehensive literature as possible to explore suitable corticosteroids types, dosage, and duration in patients with COVID-19.
The study had a few limitations. Only one RCT on pulse dose, even though we searched all relevant literature; however, eight OSs were included and also confirmed the protective effect of pulse dose corticosteroid in patients with COVID-19. Our result was limited by high heterogeneity, which may come from differences in the study population, medical conditions, disease severity, type of corticosteroid used, dose, duration, and so on. It suggested that patients with COVID-19 with individual differences and different genotypes may need different glucocorticoid treatment strategies, such as different dosage or duration of use, but further larger-sample clinical trials are needed to explore this. However, the GRADE assessment showed our conclusions to be convincing.
Conclusions
The meta-analysis indicated that corticosteroid administration might be safe for COVID-19 treatments and showed a statistically significant difference in reducing the need for MV. Moreover, pulse methylprednisolone administration might have a beneficial effect on the survival of patients with COVID-19, especially with a duration less than 7 days.
References
Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–207.
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9(1):221–36.
Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–54.
Liu J, Xie W, Wang Y, et al. A comparative overview of COVID-19, MERS and SARS: review article. Int J Surg. 2020;81:1–8.
Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2021;384(7):619–29.
Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. 2020;324(11):1048–57.
Sibila O, Agustí C, Torres A. Corticosteroids in severe pneumonia. Eur Respir J. 2008;32(2):259–64.
So LK, Lau AC, Yam LY, et al. Development of a standard treatment protocol for severe acute respiratory syndrome. Lancet. 2003;361(9369):1615–7.
Chen RC, Tang XP, Tan SY, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. 2006;129(6):1441–52.
Atzrodt CL, Maknojia I, McCarthy RDP, et al. A guide to COVID-19: a global pandemic caused by the novel coronavirus SARS-CoV-2. FEBS J. 2020;287(17):3633–50.
Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–29.
Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–16.
Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: a randomized clinical trial. JAMA. 2020;324(13):1298–306.
Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704.
Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324(13):1330–41.
Chaudhuri D, Sasaki K, Karkar A, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med. 2021;47(5):521–37.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Chou R, Fu R, Carson S, Saha S, Helfand M. Methodological shortcomings predicted lower harm estimates in one of two sets of studies of clinical interventions. J Clin Epidemiol. 2007;60(1):18–28.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Brozek JL, Akl EA, Alonso-Coello P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–77.
Laure G, Viet-Thi T, Elodie P, et al. Corticosteroids are associated with increased survival in elderly presenting severe SARS-Cov2 infection. medRxiv. 2020:11.10.20226886. https://doi.org/10.1101/2020.11.10.20226886.
Rahman O, Trigonis RA, Craft MK, et al. Corticosteroid use in severely hypoxemic COVID-19 patients: an observational cohort analysis of dosing patterns and outcomes in the early phase of the pandemic. medRxiv. 2020:07.29.20164277. https://doi.org/10.1101/2020.07.29.20164277.
Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020;56(6):2002808.
Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021;897:173947.
Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2021;72(9):e373–e381.
Tang X, Feng YM, Ni JX, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–26.
Albani F, Fusina F, Granato E, et al. Corticosteroid treatment has no effect on hospital mortality in COVID-19 patients. Sci Rep. 2021;11(1):1015.
Almas T, Ehtesham M, Khan AW, et al. Safety and efficacy of low-dose corticosteroids in patients with non-severe coronavirus disease 2019: a retrospective cohort study. Cureus. 2021;13(1):e12544.
Bahl A, Johnson S, Chen NW. Timing of corticosteroids impacts mortality in hospitalized COVID-19 patients. Intern Emerg Med. 2021. https://doi.org/10.1007/s11739-021-02655-6.
Bartoletti M, Marconi L, Scudeller L, et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect. 2021;27(1):105–11.
Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):748–55.
Chen H, Xie J, Su N, et al. Corticosteroid therapy is associated with improved outcome in critically ill patients with COVID-19 with hyperinflammatory phenotype. Chest. 2021;159(5):1793–1802.
Cusacovich I, Aparisi Á, Marcos M, et al. Corticosteroid pulses for hospitalized patients with COVID-19: effects on mortality. Mediators Inflamm. 2021;2021:6637227.
Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. 2020;64(9):e01168–20. https://doi.org/10.1128/AAC.01168-20.
Gutiérrez-Abejón E, Tamayo E, Martín-García D, Álvarez FJ, Herrera-Gómez F. Clinical profile, treatment and predictors during the first COVID-19 wave: a population-based registry analysis from Castile and Leon Hospitals. Int J Environm Res Public Health. 2020;17(24):9360. https://doi.org/10.3390/ijerph17249360
Hoertel N, Sánchez-Rico M, Vernet R, et al. Dexamethasone use and mortality in hospitalized patients with coronavirus disease 2019: a multicentre retrospective observational study. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.14784.
Jiao X, Wang Y, Liu D, et al. A real-world study of glucocorticoid treatment in COVID-19 patients with different disease severities. Clin Transl Med. 2020;10(8):e235.
Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020;15(8):489–93.
Ko JJ, Wu C, Mehta N, Wald-Dickler N, Yang W, Qiao R. A comparison of methylprednisolone and dexamethasone in intensive care patients with COVID-19. J Intensive Care Med. 2021:36(6):673–80. https://doi.org/10.1177/0885066621994057.
Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110–8.
Li Y, Li J, Ke J, et al. Adverse outcomes associated with corticosteroid use in critical COVID-19: a retrospective multicenter cohort study. Front Med. 2021;8:604263.
Li Y, Meng Q, Rao X, et al. Corticosteroid therapy in critically ill patients with COVID-19: a multicenter, retrospective study. Crit Care. 2020;24(1):698.
Liang MY, Chen P, He M, et al. Corticosteroids treatment of patients with coronavirus disease 2019: a propensity score matching study. Curr Med Sci. 2021;41(1):24–30.
Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Investig. 2020;130(12):6417–28.
Liu Z, Li X, Fan G, et al. Low-to-moderate dose corticosteroids treatment in hospitalized adults with COVID-19. Clin Microbiol Infect. 2021;27(1):112–7.
Lu X, Chen T, Wang Y, Wang J, Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit Care. 2020;24(1):241.
Ma Q, Qi D, Deng XY, et al. Corticosteroid therapy for patients with severe novel coronavirus disease 2019. Eur Rev Med Pharmacol Sci. 2020;24(15):8194–201.
Majmundar M, Kansara T, Lenik JM, et al. Efficacy of corticosteroids in non-intensive care unit patients with COVID-19 pneumonia from the New York Metropolitan region. PLoS ONE. 2020;15(9):e0238827.
Masiá M, Fernández-González M, García JA, Padilla S, Gutiérrez F. Lack of detrimental effect of corticosteroids on antibody responses to SARS-CoV-2 and viral clearance in patients hospitalized with COVID-19. J Infect. 2021;82(3):414–51.
Maulin L, Martinez S. Corticosteroids in patients hospitalised for COVID-19 pneumonia who require oxygen: observational comparative study using routine care data. Clin Microbiol Infect. 2020;27(4):603–10.
Monedero P, Gea A, Castro P, et al. Early corticosteroids are associated with lower mortality in critically ill patients with COVID-19: a cohort study. Crit Care. 2021;25(1):2.
Mongardon N, Piagnerelli M, Grimaldi D, Perrot B, Lascarrou JB. Impact of late administration of corticosteroids in COVID-19 ARDS. Intensive Care Med. 2021;47(1):110–2.
Nelson BC, Laracy J, Shoucri S, et al. Clinical outcomes associated with methylprednisolone in mechanically ventilated patients with COVID-19. Clin Infect Dis. 2021;72(9):e367–e372. https://doi.org/10.1093/cid/ciaa1163.
Papamanoli A, Yoo J, Grewal P, et al. High-dose methylprednisolone in nonintubated patients with severe COVID-19 pneumonia. Eur J Clin Investig. 2021;51(2):e13458.
Pascual Pareja JF, García-Caballero R, Soler Rangel L, et al. Effectiveness of glucocorticoids in patients hospitalized for severe SARS-CoV-2 pneumonia. Med Clin (Barc). 2021;156(5):221–8.
Piniella-Ruiz E, Bellver-Álvarez MT, Mestre-Gómez B, et al. Impact of systemic corticosteroids on mortality in older adults with critical COVID-19 pneumonia. J Gerontol Ser A Biol Sci Med Sci. 2021;76(8):e127–e132. https://doi.org/10.1093/gerona/glab074.
Rodríguez-Baño J, Pachón J, Carratalà J, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19). Clin Microbiol Infect. 2021;27(2):244–52.
Ruiz-Irastorza G, Pijoan JI, Bereciartua E, et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS ONE. 2020;15(9):e0239401.
Saggi SJ, Nath S, Culas R, et al. Early experience with methylprednisolone on SARS-CoV-2 infection in the African American population, a retrospective analysis. Clin Med Insights Circ Respir Pulm Med. 2020;14:1179548420980699.
Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020;7(10):ofaa421.
Sheshah E, Sabico S, Albakr RM, et al. Prevalence of diabetes, management and outcomes among Covid-19 adult patients admitted in a specialized tertiary hospital in Riyadh, Saudi Arabia. Diabetes Res Clin Pract. 2021;172:108538.
Tomasoni D, Inciardi RM, Lombardi CM, et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur J Heart Fail. 2020;22(12):2238–47.
Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43.
Wu C, Hou D, Du C, et al. Corticosteroid therapy for coronavirus disease 2019-related acute respiratory distress syndrome: a cohort study with propensity score analysis. Crit Care. 2020;24(1):643.
Wu J, Huang J, Zhu G, et al. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J Clin Endocrinol Metab. 2020;105(12):e4230–e4239. https://doi.org/10.1210/clinem/dgaa627.
Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81.
Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62.
Zhu HM, Li Y, Li BY, et al. Effect of methylprednisolone in severe and critical COVID-19: analysis of 102 cases. World J Clin Cases. 2020;8(23):5952–61.
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93.
Rowaiye AB, Okpalefe OA, Onuh Adejoke O, et al. Attenuating the effects of novel COVID-19 (SARS-CoV-2) infection-induced cytokine storm and the implications. J Inflamm Res. 2021;14:1487–510.
Bhaskar S, Sinha A, Banach M, et al. Cytokine storm in COVID-19-immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648.
Feng Y, Ling Y, Bai T, et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–8.
Wan YD, Sun TW, Liu ZQ, Zhang SG, Wang LX, Kan QC. Efficacy and safety of corticosteroids for community-acquired pneumonia: a systematic review and meta-analysis. Chest. 2016;149(1):209–19.
Lu X, Han W, Gao YX, et al. Efficacy and safety of corticosteroids in immunocompetent patients with septic shock. World J Emerg Med. 2021;12(2):124–30.
van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(1):696.
Cano EJ, Fonseca Fuentes X, Corsini Campioli C, et al. Impact of corticosteroids in coronavirus disease 2019 outcomes: systematic review and meta-analysis. Chest. 2021;159(3):1019–40.
Alhazzani W, Evans L, Alshamsi F, et al. Surviving Sepsis Campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–34.
Ayyar VS, Jusko WJ. Transitioning from basic toward systems pharmacodynamic models: lessons from corticosteroids. Pharmacol Rev. 2020;72(2):414–38.
Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet. 2005;44(1):61–98.
Panettieri RA, Schaafsma D, Amrani Y, Koziol-White C, Ostrom R, Tliba O. Non-genomic effects of glucocorticoids: an updated view. Trends Pharmacol Sci. 2019;40(1):38–49.
Li S, Miller D, Yates C. Evaluation of AP-1 and NF-kB inhibitory potency for oral glucocorticoids. PharmSci. 2003;5(S1):Abstract R6173.
Croxtall JD, van Hal PT, Choudhury Q, Gilroy DW, Flower RJ. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br J Pharmacol. 2002;135(2):511–9.
Acknowledgements
We would like to thank the Chinese Evidence Based Medicine Center, West China Hospital, Sichuan University, for providing the Stata 14.0 statistical software.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author Contributions
All the authors contributed substantially to the work presented in this article. TWS conceived of the study. YQC, YLS and JYS contributed to the data interpretation. HYL, XFD, XYS and DW contributed to the study protocol. TWS revised the article. All authors have approved the final and submitted version of the manuscript.
Data Availability
The datasets used and/or analysed in the present study are available from the corresponding author on reasonable request.
Disclosures
Yuqing Cui, Yali Sun, Junyi Sun, Huoyan Liang, Xianfei Ding, Xueyi Sun, Dong Wang and Tongwen Sun have nothing to disclose.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Funding
This study was supported by the United Fund of National Natural Science Foundation of China (Grant No. U2004110); The special fund for young and middle-aged medical research of China International Medical Exchange Foundation (Grant No. Z-2018–35); The integrated thinking research foundation of the China foundation for International Medical Exchange (Grant No. Z-2016-23-2001); The study of mechanism of gabexate mesilate in the treatment of sepsis and septic shock (Grant No. 2019-hx-45); the 2021 youth talent promotion project in Henan Province (Grant No. 2021HYTP053).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Cui, Y., Sun, Y., Sun, J. et al. Efficacy and Safety of Corticosteroid Use in Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis. Infect Dis Ther 10, 2447–2463 (2021). https://doi.org/10.1007/s40121-021-00518-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-021-00518-3