Abstract
Recently, during the pandemic infection of the novel SARS-CoV-2, some cases of Miller Fisher syndrome (MFS) have been reported. We want to summarize the main features of patients with MFS and COVID-19. A PubMed search was performed on 8 October to identify references reporting cases with MFS associated with COVID-19 from the first report of COVID-19 to 8 October 2020 using the following keywords: “Miller Fisher syndrome” AND “COVID-19” OR “SARS-CoV-2”. A systematic review from the first report of coronavirus disease 2019 (COVID-19) to 8 October 2020 revealed 7 cases with Miller Fisher syndrome (MFS) associated with COVID-19. The 7 cases came from 5 countries but most of these patients were from Europe (85.7%), especially Spain. There are 5 cases of MFS diagnosed after the laboratory confirmation of SARS-CoV-2 infection. The mean onset time of MFS-associated neurological symptoms was 14.75 days after the diagnosis of COVID-19. However, the two remaining cases presented initially with MFS-associated neurological symptoms followed by the diagnosis of COVID-19. The most common symptoms of COVID-19-associated MFS were perioral paresthesias (57.1%), ataxia (57.1%), blurred vision (42.9), ophthalmoplegia (42.9), and generalized areflexia (42.9). However, more cohort and case-control studies are required to establish the epidemiological linkage.
Similar content being viewed by others
Introduction
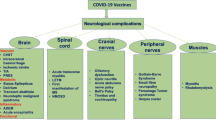
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome (SARS)-like coronavirus 2 (SARS-CoV-2), has quickly spread across the world and become as a global health emergency (Boehmer et al. 2020; Dirlikov et al. 2020; Grantz et al. 2020). The most common symptoms of COVID-19 include cough, fatigue, myalgia, sputum production, and shortness of breath, indicating that SARS-CoV-2 mainly affects the respiratory system and results in acute respiratory illness (Hauguel-Moreau et al. 2020; Munro and Faust 2020; Nie et al. 2020; Puccioni-Sohler et al. 2020). Recently, neurological symptoms of COVID-19 have been increasingly reported, with the spectrum ranging from temporary loss of smell and taste to potentially life-threatening encephalopathy and acute cerebrovascular disease (Ahmed et al. 2020; Iadecola et al. 2020; Liotta et al. 2020; Rifino et al. 2020). More recently, there have been sporadic case reports on development of Miller Fisher syndrome (MFS) in patients with COVID-19 (Fernández-Domínguez et al. 2020; Gutiérrez-Ortiz et al. 2020; Lantos et al. 2020; Manganotti et al. 2020; Ray 2020; Reyes-Bueno et al. 2020; Senel et al. 2020). Nevertheless, the epidemiological linkage between these two diseases remains unclear.
MFS is a rare variant of Guillain-Barré syndrome (GBS), an autoimmune disease of the peripheral nervous system (Al Othman et al. 2019; Gómez et al. 2019; Hsueh et al. 2020). MFS is characterized symptomatically by ophthalmoplegia, ataxia, and areflexia and biochemically by elevated cerebrospinal fluid (CSF) protein concentration and the presence of autoantibody against ganglioside GQ1b, which is abundant in the paranodal region at the nodes of Ranvier along myelinated axons (Arányi et al. 2012; Heckmann and Dütsch 2012; Teener 2012). Both axonal injury and demyelination might take part in the pathogenesis of MFS (Scelsa and Herskovitz 2000).
As of 8 October 2020, over 36 million patients have been diagnosed of COVID-19, causing more than 1,005,000 deaths worldwide. Given the heightened concern over the possible linkage between COVID-19 and MFS, the objective of the present study is to systematically review case reports on COVID-19-associated MFS, including the electrophysiological and clinical phenotypes. We also aim to identify the temporal relationship between COVID-19 and MFS so as to infer whether post-infective and/or parainfective pathogenic mechanism is at work.
Methods
A PubMed search was performed on 8 October to identify references reporting cases with MFS associated with COVID-19 from the first report of COVID-19 to 8 October 2020 using the following keywords: “Miller Fisher syndrome” AND “COVID-19” OR “SARS-CoV-2”. Full-text references in English were collated and analyzed and detailed information of each patient were collected. Data were extracted from each report according to a pre-defined template. Clinical characteristics were retrieved as the number of patients in whom the variable was present as the numerator and the total number of reported cases as the denominator: n/N (%). If clinical features were reported at multiple time points, data representing the full disease course were presented. Continuous variables (age, time between the onset of infectious and neuropathic symptoms) were expressed as medians. Certainty of GBS and MFS diagnosis was assessed, on the basis of the reported findings, by the Brighton Collaboration GBS Working Group criteria. A level 1 diagnosis based on Brighton criteria indicates the highest degree of diagnostic certainty supported by nerve conduction studies and the presence of albuminocytological dissociation in CSF. A level 2 diagnosis was supported by either a CSF white-cell count of less than 50 cells/μl (with or without an elevated protein level) or nerve conduction studies consistent with the polyneuropathy patterns described for GBS and MFS if the CSF is unavailable. A level 3 diagnosis is based on clinical features without support from nerve conduction or CSF studies. A diagnostic classification was also employed to categorize the different GBS and MFS presentations. This systematic review was conducted in accordance with, wherever applicable, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement.
Results
A total of 7 case studies reporting on 7 individual patients with COVID-19-associated MFS were identified. The clinical, imaging, and laboratory findings and demographic data of these 7 cases are summarized in Table 1 and Table 2. The majority of cases were men (57.1%) with a median age of 55 years. The first case, a man who came from Madrid, Spain, was published online on 17 Apr 2020. Overall, cases were from 5 countries but most of these patients were from Europe (85.7%) and especially from Spain (42.9%).
Five (71.4%) cases presented to the hospital due to the COVID-19 symptoms and then developed neurological symptoms consistent with MFS. The two remaining cases (28.6%) were presented to the hospital due to neurological symptoms. The diagnosis of COVID-19 was made by quantitative RT-PCR for SARS-CoV-2 in nasopharyngeal swab in 6 cases (85.7%) and by serology positive for antibodies against SARS-CoV-2 in 1 case (14.3%). The diagnosis of COVID-19 was made before the onset of MFS in 5 cases (71.4%) and during hospitalization for MFS in the two remaining cases (28.6%).
The most common symptoms of COVID-19 in these 7 patients were fever (71.4%) and cough (42.9%). Other symptoms of COVID-19 included headache, bilateral pneumonia, taste alteration, chills, myalgia, heavy night sweat, weight loss, and diarrhea. The temporal relationship between the onset of MFS and COVID-19 symptoms in 3 cases (42.9%) was not reported. The median onset time of the neurological symptoms related to MFS was 14.75 days after the diagnosis of COVID-19 in the remaining 4 cases. The symptoms of COVID-19 clinically resolved before onset of MFS in 2 cases (28.6%).
The most common symptoms of COVID-19-assocaited MFS were perioral paresthesias (57.1%), ataxia (57.1%), blurred vision (42.9), ophthalmoplegia (42.9), generalized areflexia (42.9), and other neurological features. Some of these patients (42.9%) have electrodiagnostic features of F-wave delay. The examination of CSF was done in 6 (85.7%) cases and showed an albuminocytological dissociation in 5 out of 6 (83.3%) patients at which SARS-CoV-2 RNA could not be detected in 3 patients. Antiganglioside antibodies were detected in 5 cases (71.4%) and specific anti-GD1b IgG in 1 case (20%). MRI of the head was carried out in 3 cases (42.9%), among which one patient showed high-resolution imaging of the orbits and retro-orbital region with hyperintense signal of the left cranial nerve III. All cases were given intravenous immunoglobulins and eventually recovered.
Discussion
Recently, the neurological symptoms of COVID-19 were increasingly reported (Edén et al. 2020; Guadarrama-Ortiz et al. 2020). There are multiple case studies reporting on the association between COVID-19 and MFS. In this systematic review, based on these case reports, we learned about the clinical characteristic of COVID-19 patients developing. However, whether COVID-19 is indeed epidemiologically linked to MFS awaits confirmation by large cohort studies. Given that the COVID-19 is still spreading quickly, more research would be needed to investigate how COVID-19 could impact on the nervous system. Moreover, if COVID-19 really increases the risk for MFS, it is crucial to understand the underlying mechanism. MFS is a rare neurological disorder that is considered to be a variant of GBS (Abu-Rumeileh et al. 2020; Mayer et al. 2020; Verboon et al. 2019). The incidence of GBS is about 1–2 per 1,000, 000 of adults and about 0.4–1.4 per 100,000 of children (Melone et al. 2020; Stojanov et al. 2020). To this end, several infectious diseases, including infection with Zika virus, cytomegalovirus, human immunodeficiency virus, Epstein–Barr virus, and Campylobacter jejuni, have shown epidemiological linkage to GBS (Brito Ferreira et al. 2020; De Sanctis et al. 2020; Dyachenko et al. 2018; Korinthenberg and Sejvar 2020; Leung et al. 2020). Until now, no child with COVID-19-associated MFS has been reported.
Until now, there are only 7 reported patients with COVID-19-associated MFS. It is therefore impossible to draw a conclusion as to whether post-infective and/or parainfective pathogenic mechanism is at work. Pathologically, it is plausible that SARS-CoV-2 might directly induce neuropathogenic effect due to the widespread expression of ACE2 (host receptor for SARS-CoV-2) in the nervous system. Alternatively, deregulated immune response upon SARS-CoV-2 infection might underlie COVID-19-associated MFS. Particularly, increasing amount of evidence has illustrated that SARS-CoV-2 can induce severe immune and inflammatory reaction that leads to tissue damage. Thus, targeting the inflammatory cascade, for example, with corticosteroids, might be effective against COVID-19-associated MFS.
Conclusion
There are sporadic reports on patients with concurrent diagnosis of COVID-19 and MFS, suggesting a possible link between these two diseases. However, more cohort and case-control studies are required to confirm the epidemiological linkage. Nevertheless, it is important for physicians to pay more attention to the neurological manifestations of COVID-19.
References
Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M (2020) Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. https://doi.org/10.1007/s00415-020-10124-x
Ahmed M et al. (2020) Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review Frontiers in neurology 11:518 https://doi.org/10.3389/fneur.2020.00518
Al Othman B, Raabe J, Kini A, Lee A (2019) Update: the Miller Fisher variants of Guillain-Barré syndrome Current opinion in ophthalmology 30:462-466 doi:https://doi.org/10.1097/icu.0000000000000611
Arányi Z, Kovács T, Sipos I, Bereczki D (2012) Miller Fisher syndrome: brief overview and update with a focus on electrophysiological findings Eur J Neurol 19:15-20, e11-13 doi:https://doi.org/10.1111/j.1468-1331.2011.03445.x
Boehmer T et al. (2020) Changing Age Distribution of the COVID-19 Pandemic - United States, May-August 2020 MMWR Morbidity and mortality weekly report 69:1404-1409 https://doi.org/10.15585/mmwr.mm6939e1
Brito Ferreira M et al (2020) Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study. Lancet Neurol 19:826–839. https://doi.org/10.1016/s1474-4422(20)30232-5
De Sanctis P, Doneddu P, Viganò L, Selmi C, Nobile-Orazio E (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection. A systematic review. Eur J Neurol 27:2361–2370. https://doi.org/10.1111/ene.14462
Dirlikov E et al. (2020) CDC Deployments to State, Tribal, Local, and Territorial Health Departments for COVID-19 Emergency Public Health Response - United States, January 21-July 25, 2020 MMWR Morbidity and mortality weekly report 69:1398-1403 https://doi.org/10.15585/mmwr.mm6939a3
Dyachenko P, Smiianova O, Kurhanskaya V, Oleshko A, Dyachenko A (2018) Epstein-barr virus-associated encephalitis in a case-series of more than 40 patients Wiadomosci lekarskie (Warsaw, Poland : 1960) 71:1224-1230
Edén A et al (2020) CSF biomarkers in patients with COVID-19 and neurological symptoms: A case series Neurology. https://doi.org/10.1212/wnl.0000000000010977
Fernández-Domínguez J, Ameijide-Sanluis E, García-Cabo C, García-Rodríguez R, Mateos V (2020) Miller-Fisher-like syndrome related to SARS-CoV-2 infection (COVID 19) Journal of neurology 267:2495-2496. https://doi.org/10.1007/s00415-020-09912-2
Gómez Á, Díaz A, Carrión-Penagos J, Reyes J, Reyes S (2019) Clinical and electrophysiological characteristics of Guillain-Barré syndrome in Colombia Journal of the peripheral nervous system : JPNS 24:268-271 doi:https://doi.org/10.1111/jns.12340
Grantz K et al (2020) The use of mobile phone data to inform analysis of COVID-19 pandemic epidemiology. Nat Commun 11:4961. https://doi.org/10.1038/s41467-020-18190-5
Guadarrama-Ortiz P, Choreño-Parra J, Sánchez-Martínez C, Pacheco-Sánchez F, Rodríguez-Nava A, García-Quintero G (2020) Neurological Aspects of SARS-CoV-2 Infection: Mechanisms and Manifestations Frontiers in neurology 11:1039 https://doi.org/10.3389/fneur.2020.01039
Gutiérrez-Ortiz C et al. (2020) Miller Fisher syndrome and polyneuritis cranialis in COVID-19 Neurology 95:e601-e605 https://doi.org/10.1212/wnl.0000000000009619
Hauguel-Moreau M et al (2020) Occurrence of pulmonary embolism related to COVID-19 Journal of thrombosis and thrombolysis. https://doi.org/10.1007/s11239-020-02292-4
Heckmann J, Dütsch M (2012) Recurrent Miller Fisher syndrome: clinical and laboratory features. Eur J Neurol 19:944–954. https://doi.org/10.1111/j.1468-1331.2011.03584.x
Hsueh H, Chang K, Chao C, Hsieh S (2020) A Pilot Study on Serial Nerve Ultrasound in Miller Fisher Syndrome. Front Neurol 11:865. https://doi.org/10.3389/fneur.2020.00865
Iadecola C, Anrather J, Kamel H (2020) Effects of COVID-19 on the Nervous System Cell. https://doi.org/10.1016/j.cell.2020.08.028
Korinthenberg R, Sejvar J (2020) The Brighton Collaboration case definition: Comparison in a retrospective and prospective cohort of children with Guillain-Barré syndrome Journal of the peripheral nervous system : JPNS. https://doi.org/10.1111/jns.12411
Lantos J, Strauss S, Lin E (2020) COVID-19-Associated Miller Fisher Syndrome: MRI Findings AJNR American journal of neuroradiology 41:1184-1186 https://doi.org/10.3174/ajnr.A6609
Leung J, Sejvar J, Soares J, Lanzieri T (2020) Guillain-Barré syndrome and antecedent cytomegalovirus infection, USA 2009-2015. Neurol Sci 41:885–891. https://doi.org/10.1007/s10072-019-04156-z
Liotta E, Batra A, Clark J, Shlobin N, Hoffman S, Orban Z, Koralnik I (2020) Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol. https://doi.org/10.1002/acn3.51210
Manganotti P et al (2020) Miller Fisher syndrome diagnosis and treatment in a patient with SARS-CoV-2. J Neurovirol 26:605–606. https://doi.org/10.1007/s13365-020-00858-9
Mayer J, McNamara C, Mayer J (2020) Miller Fisher syndrome and Guillain-Barré syndrome: dual intervention rehabilitation of a complex patient case Physiotherapy theory and practice:1-10 https://doi.org/10.1080/09593985.2020.1736221
Melone M et al (2020) Early mechanical ventilation in patients with Guillain-Barré syndrome at high risk of respiratory failure: a randomized trial. Ann Intensive Care 10:128. https://doi.org/10.1186/s13613-020-00742-z
Munro A, Faust S (2020) COVID-19 in children: current evidence and key questions Current opinion in infectious diseases. https://doi.org/10.1097/qco.0000000000000690
Nie K, Yang Y, Deng M, Wang X (2020) Gastrointestinal insights during the COVID-19 epidemic World journal of clinical cases 8:3934-3941 https://doi.org/10.12998/wjcc.v8.i18.3934
Puccioni-Sohler M, Poton A, Franklin M, Silva S, Brindeiro R, Tanuri A (2020) Current evidence of neurological features, diagnosis, and neuropathogenesis associated with COVID-19 Revista da Sociedade Brasileira de Medicina Tropical 53:e20200477. https://doi.org/10.1590/0037-8682-0477-2020
Ray A (2020) Miller Fisher syndrome and COVID-19: is there a link? BMJ case reports 13:e236419. https://doi.org/10.1136/bcr-2020-236419
Reyes-Bueno J, García-Trujillo L, Urbaneja P, Ciano-Petersen N, Postigo-Pozo M, Martínez-Tomás C, Serrano-Castro P (2020) Miller-Fisher syndrome after SARS-CoV-2 infection European journal of neurology. https://doi.org/10.1111/ene.14383
Rifino N, Censori B, Agazzi E, Alimonti D, Bonito V, Camera G, Conti MZ, Foresti C, Frigeni B, Gerevini S, Grimoldi M, la Gioia S, Partziguian T, Quadri S, Riva R, Servalli MC, Sgarzi M, Storti B, Vedovello M, Venturelli E, Viganò M, Callegaro A, Arosio M, Sessa M (2020) Neurologic manifestations in 1760 COVID-19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. https://doi.org/10.1007/s00415-020-10251-5
Scelsa S, Herskovitz S (2000) Miller Fisher syndrome: axonal, demyelinating or both? Electromyogr Clin Neurophysiol 40:497–502
Senel M, Abu-Rumeileh S, Michel D, Garibashvili T, Althaus K, Kassubek J, Otto M (2020) Miller-Fisher syndrome after COVID-19: neurochemical markers as an early sign of nervous system involvement. Eur J Neurol. https://doi.org/10.1111/ene.14473
Stojanov A et al (2020) Incidence and mortality rates of Guillain-Barré syndrome in Serbia Journal of the peripheral nervous system : JPNS. https://doi.org/10.1111/jns.12412
Teener J (2012) Miller Fisher's syndrome Semin Neurol 32:512-516. https://doi.org/10.1055/s-0033-1334470
Verboon C et al (2019) Current treatment practice of Guillain-Barré syndrome Neurology 93:e59-e76. https://doi.org/10.1212/wnl.0000000000007719
Author information
Authors and Affiliations
Contributions
Conceptualization: all authors; methodology, formal analysis, and investigation: Zheng Li, Xingye Li, Jianxiong Shen; writing—original draft preparation: Zheng Li and Xingye Li; writing—review and editing: Matthew T.V. Chan, William Ka Kei Wu
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no confict of interest related to the content of this article.
Ethical standard
For the present study, no authorization to an Ethics Committee was asked, because the original reports, nor this work, provided any personal information of the patients.
Additional information
Responsible editor: Lotfi Aleya
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, Z., Li, X., Shen, J. et al. Miller Fisher syndrome associated with COVID-19: an up-to-date systematic review. Environ Sci Pollut Res 28, 20939–20944 (2021). https://doi.org/10.1007/s11356-021-13233-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-021-13233-w