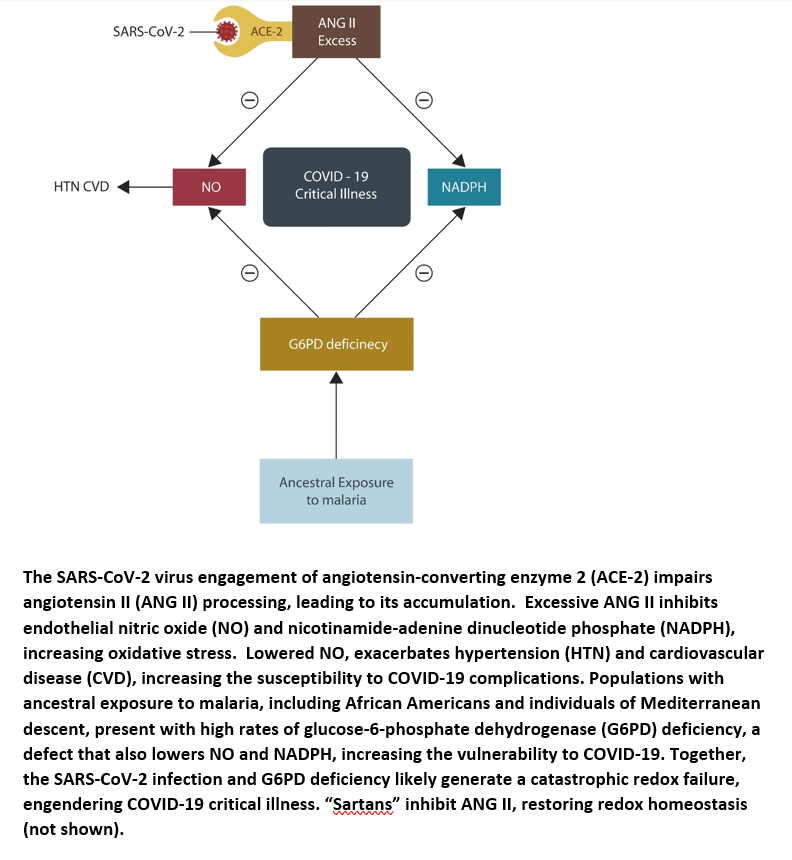
COVID-19 reveals redox vulnerabilities in two minority groups
Adonis Sfera (1), Afzaal Jafri (1), Jason Thomas (1), Carlos Manuel Zapata-Martín del Campo (4), Daniel Gehlbach (3) and Carolina Osorio (2)
- Patton State Hospital
- Loma Linda University, department of psychiatry
- University of California Riverside
- National Institute of Cardiology “Ignacio Chávez”, Mexico City, Mexico
Visual abstract
Abstract
The COVID-19 pandemic spread rapidly throughout the world, but some populations were more affected than others. For example, compared to other groups, a higher morbidity and mortality was documented in African Americans and individuals of Mediterranean descent. These populations are marked by both increased prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency, and lower utilization of angiotensin receptor blockers/angiotensin converting enzyme inhibitors in the treatment of hypertension.
In this brief report, we suggest that G6PD status should be assessed in all COVID-19 positive individuals belonging to the two ethnic groups. If detected, N-acetylcysteine should be utilized to lower the oxidative burden and “sartans” should be prescribed as first-line therapy in hypertensive individuals.
Article type: Short Communication
Received: August 10, 2020
Revised: August 30, 2020
Accepted: September 10, 2020
To cite this article:
Introduction
On March 11, 2020 the World Health Organization declared COVID-19 a pandemic. At that time, the virus had been detected in 114 countries, and 4,291 people had lost their lives. Despite its rapid spread around the globe, individuals and population groups have not been equally affected. For example, it is still unclear why older persons, African Americans and people from the Mediterranean basin have been more impacted compared to other groups. Indeed, a new study found that the SARS-CoV-2 fatality rate was 2.4 times higher in African Americans compared to Whites, Asians or Latinos, suggesting a probable racial vulnerability (1). This is further substantiated by the fact that other countries with a predominantly black population reported similar data (2). In addition, mortality rates comparable to those of African Americans have been documented in individuals from the Mediterranean basin, a region where G6PD deficiency is more common than in the rest of Europe (3).
Socioeconomic conditions likely play a major role in COVID-19 prognosis, however biological factors, including the management of hypertension and oxidative stress may be equally important. We surmise that, aside from healthcare inequalities, two modifiable risk factors may contribute to the higher COVID-19 morbidity and mortality in African Americans and Europeans of Mediterranean descent:
1) lower utilization of angiotensin receptor blockers (ARBs) and angiotensin converting enzyme inhibitors (ACEi) in the treatment of hypertension, and
2) higher oxidative stress due to increased prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency.
Hypertension treatment practices
European hypertension studies have demonstrated that, compared to the Northern part of the continent, ARBs and ACEi are less often prescribed in the South (4). With the same token, in the US, African Americans are less likely to receive ARBs and ACEi as first line hypertension treatment (5). This prescription practice reflects the belief that the response to these drugs is hindered by the lower renin levels, demonstrated in this population. However, numerous clinical trials have found that ARBs and ACEi are equally efficacious in Black Americans and recommended that clinicians should use these agents as first line therapy (5-6). This data is significant as both ARBs and ACEi appear to lower the COVID-19 mortality rates. For example, a novel study found that patients treated with these drugs at the time of infection with the SARS-CoV-2 virus demonstrated a lower mortality rate of 30.4% compared to 41.2 % in individuals without prior exposure to ACEi or ARBs (7).
In our previous work, we found a positive correlation between COVID-19 critical illness and excessive angiotensin II (ANG II) and stated that “sartans” should be used early in the disease course (8-9). We based this assertion on the fact that ANG II upregulates oxidative stress, while ARBs and ACEi restore the redox homeostasis by lowering ANG II.
The oxidative burden
Oxidative stress is an established hypertension risk factor and several antihypertensive drugs lower the blood pressure by acting as antioxidants (10). For example, ARBs and ACEi decrease reactive oxygen species (ROS) by upregulating both endothelial nitric oxide (NO) and the master antioxidant glutathione (GSH) (11-13). As a major product of the normal endothelium, NO acts as a vasodilator and regulator of oxygen supply by its action on the smooth muscle of blood vessels. African Americans synthesize 2 to 3 times less NO compared to the general population, placing this group at higher risk of redox dysfunction, hypertension and cardiovascular disease (14-17).
Aside from decreased NO, African Americans (even after adjustment for most variables) display lower GSH plasma levels compared to other groups (18). Indeed, decreased GSH in this population was also associated with a higher incidence of prostate cancer, suggesting that ARBs and ACEi may be protective as they lower oxidative stress (19-20)(11-13).
Taken together, the SARS-CoV-2 virus alters the body redox systems by inhibiting ANG II hydrolysis, increasing oxidative stress. This may prove catastrophic in individuals or populations with preexistent redox defects, such as those described in malaria-exposed groups (see the next section). In addition, underutilization of ARBs and ACEi as antihypertensive treatments in this population, further disrupts the redox homeostasis, increasing COVID-19 morbidity and mortality.
Ancestral malaria and oxidative stress
Malaria is an old enemy of mankind that throughout many centuries exacted a heavy mortality toll on the population of Africa and the surrounding regions, including the Mediterranean basin. In response, the residents of these areas gradually developed plasmodium-resistant red blood cell phenotypes, including glucose-6-phosphate dehydrogenase (G6PD) deficiency, α+ thalassemia, and hemoglobin C (21). Although protective against malaria, these genetic variants increased oxidative stress, contributing to other pathologies, such as cancer, cardiovascular disease and neuropsychiatric disorders (22-26). Novel data links G6PD deficiency to COVID-19 complications, suggesting that populations with ancestral malaria exposure are at increased risk of critical illness. Indeed, the US Army statistics estimate that 12.2% of African American males and 4.1% of females present with G6PD deficiency, connecting the oxidative stress in this group to unfavorable COVID-19 prognosis (27).
G6PD is the rate-limiting enzyme that catalyzes the conversion of NADP to NADPH, preventing GSH depletion (28). Therefore, deficient G6PD contributes to ROS accumulation, a pathology described in severely ill COVID-19 patients (29-30). In addition, G6PD deficiency is also associated with chronically depleted NO, predisposing to hypertension, cardiovascular disease and coagulopathies, conditions prevalent in many African Americans (31). For this reason, G6PD status should be assessed in all COVID-19 positive individuals, especially those belonging to the two ethnic groups. Moreover, as N-acetylcysteine (NAC) is an FDA approved drug and an established GSH enhancer, it should be routinely utilized in African Americans and southern Europeans with COVID-19 (32). Indeed, NAC is currently in clinical trials for COVID-19, emphasizing further the importance of redox dysfunctions in this viral infection (NCT04792021).
Future directions
Older individuals are more susceptible to COVID-19 complications and respond less well to vaccines, therefore the development of new treatments for SARS-CoV-2 critical illness should never be abandoned (34).
Further studies are needed to determine whether COVID-19 morbidity and mortality can be lowered by the routine assessment of G6PD status and utilization of ARBs and ACEi as first line therapy for hypertension in susceptible populations.
Conclusion
African Americans and people of Mediterranean descent present with a higher prevalence of hypertension and cardiovascular disease, along with an increased risk of COVID-19 critical illness.
Aside from the healthcare disparities, biological factors likely predispose these populations to oxidative stress and SARS-CoV-2 complications. Indeed, increased prevalence of G6PD deficiency and the less frequent use of ARBs and ACEi in the treatment of hypertension can explain the higher mortality rates demonstrated in African Americans and southern Europeans. As these are modifiable risk factors, screening for G6PD deficiency and adjusting hypertension treatments could save lives.
Clinicians should also be mindful that individuals with G6PD deficiency may be more prone to COVID-19-related thromboembolic events and that several commonly used therapeutics, including aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), quinolones, nitrofurantoin and hydroxychloroquine can exacerbate this pathology (33). For this reason, the above drugs should probably be avoided in G6PD deficient patients with COVID-19.
References:
- Doumas M, Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Karagiannis A. COVID19 and increased mortality in African Americans: socioeconomic differences or does the renin angiotensin system also contribute? [published online ahead of print, 2020 Jul 15]. J Hum Hypertens. 2020;1-4. doi:10.1038/s41371-020-0380-y
- Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi:10.1001/jama.2020.6548
- Vick DJ. Glucose-6-Phosphate Dehydrogenase Deficiency and COVID-19 Infection. Mayo Clin Proc. 2020;95(8):1803-1804. doi:10.1016/j.mayocp.2020.05.035
- Ouwerkerk W, Voors AA, Anker SD, Cleland JG, Dickstein K, Filippatos G, van der Harst P, Hillege HL, Lang CC, Ter Maaten JM, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zannad F, Metra M, Zwinderman AH. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J. 2017 Jun 21;38(24):1883-1890. doi: 10.1093/eurheartj/ehx026. PMID: 28329163.
- Williams SF, Nicholas SB, Vaziri ND, Norris KC. African Americans, hypertension and the renin angiotensin system. World J Cardiol. 2014;6(9):878-889. doi:10.4330/wjc.v6.i9.878
- Flack JM, Mensah GA, Ferrario CM. Using angiotensin converting enzyme inhibitors in African-American hypertensives: a new approach to treating hypertension and preventing target-organ damage. Curr Med Res Opin. 2000;16(2):66-79. PMID: 10893650.
- Negreira-Caamaño M, Piqueras-Flores J, Martínez-DelRio J, et al. Impact of Treatment with Renin-Angiotensin System Inhibitors on Clinical Outcomes in Hypertensive Patients Hospitalized with COVID-19 [published online ahead of print, 2020 Sep 19]. High Blood Press Cardiovasc Prev. 2020;1-8. doi:10.1007/s40292-020-00409-7
- Sfera A, Osorio C, Jafri N, Diaz EL, Campo Maldonado JE. Intoxication With Endogenous Angiotensin II: A COVID-19 Hypothesis. Front Immunol. 2020;11:1472. Published 2020 Jun 19. doi:10.3389/fimmu.2020.01472
- Sfera A. Are “Sartans” The Common Treatment For COVID-19 And Parkinson’s Disease? Journal of Alzheimers & Neurodegenerative Diseases (2020) DOI:10.24966/AND-9608/100048
- Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, Pawliszak W, Kedziora J. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol. 2016 Jun;67(3):331-7. PMID: 27511994.
- de Cavanagh EM, Ferder L, Carrasquedo F, Scrivo D, Wassermann A, Fraga CG, Inserra F. Higher levels of antioxidant defenses in enalapril-treated versus non-enalapril-treated hemodialysis patients. Am J Kidney Dis. 1999 Sep;34(3):445-55. doi: 10.1016/s0272-6386(99)70071-5. PMID: 10469854.
- Chrysant SG, Chrysant GS. The pleiotropic effects of angiotensin receptor blockers. J Clin Hypertens (Greenwich). 2006 Apr;8(4):261-8. doi: 10.1111/j.1524-6175.2005.05264.x. PMID: 16596029.
- Mason RP, Jacob RF, Kubant R, et al. Effects of angiotensin receptor blockers on endothelial nitric oxide release: the role of eNOS variants. Br J Clin Pharmacol. 2012;74(1):141-146. doi:10.1111/j.1365-2125.2012.04189.x
- Mata-Greenwood E, Chen DB. Racial differences in nitric oxide-dependent vasorelaxation. Reprod Sci. 2008;15(1):9-25. doi:10.1177/1933719107312160
- Feairheller DL, Park JY, Sturgeon KM, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4(1):32-37. doi:10.1111/j.1752-8062.2011.00264.x
- Mason RP, Jacob RF, Kubant R, et al. Effects of angiotensin receptor blockers on endothelial nitric oxide release: the role of eNOS variants. Br J Clin Pharmacol. 2012;74(1):141-146. doi:10.1111/j.1365-2125.2012.04189.x
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation. 2004 Jun 1;109(21):2511-7. doi: 10.1161/01.CIR.0000129087.81352.7A. Epub 2004 May 24. PMID: 15159296.
- Morris AA, Zhao L, Patel RS, et al. Differences in systemic oxidative stress based on race and the metabolic syndrome: the Morehouse and Emory Team up to Eliminate Health Disparities (META-Health) study. Metab Syndr Relat Disord. 2012;10(4):252-259. doi:10.1089/met.2011.0117
- Medeiros R, Vasconcelos A, Costa S, Pinto D, Ferreira P, Lobo F, Morais A, Oliveira J, Lopes C. Metabolic susceptibility genes and prostate cancer risk in a southern European population: the role of glutathione S-transferases GSTM1, GSTM3, and GSTT1 genetic polymorphisms. Prostate. 2004 Mar 1;58(4):414-20. doi: 10.1002/pros.10348. PMID: 14968442.
- Lavender, N.A., Benford, M.L., VanCleave, T.T. et al. Examination of polymorphic glutathione S-transferase (GST) genes, tobacco smoking and prostate cancer risk among Men of African Descent: A case-control study. BMC Cancer 9, 397 (2009). https://doi.org/10.1186/1471-2407-9-397
- Kwiatkowski DP. How malaria has affected the human genome and what human genetics can teach us about malaria. Am J Hum Genet. 2005;77(2):171-192. doi:10.1086/432519
- Buinitskaya Y, Gurinovich R, Clifford G. Wlodaver CG, Kastsiuchenka S. Centrality of G6PD in COVID-19: The Biochemical Rationale and Clinical Implications. Front. Med., 22 October 2020 | https://doi.org/10.3389/fmed.2020.584112
- Tiwari M. Glucose 6 phosphatase dehydrogenase (G6PD) and neurodegenerative disorders: Mapping diagnostic and therapeutic opportunities. Genes Dis. 2017;4(4):196-203. Published 2017 Sep 23. doi:10.1016/j.gendis.2017.09.001
- Bocchetta A. Psychotic mania in glucose-6-phosphate-dehydrogenase-deficient subjects. Ann Gen Hosp Psychiatry. 2003;2(1):6. Published 2003 Jun 13. doi:10.1186/1475-2832-2-6
- Manjurano A, Sepulveda N, Nadjm B, et al. African glucose-6-phosphate dehydrogenase alleles associated with protection from severe malaria in heterozygous females in Tanzania. PLoS Genet. 2015;11(2):e1004960. Published 2015 Feb 11. doi:10.1371/journal.pgen.1004960
- Parsanathan, R., Jain, S.K. Glucose-6-phosphate dehydrogenase (G6PD) deficiency is linked with cardiovascular disease. Hypertens Res 43, 582–584 (2020). https://doi.org/10.1038/s41440-020-0402-8
- Chinevere TD, Murray CK, Grant E Jr, Johnson GA, Duelm F, Hospenthal DR. Prevalence of glucose-6-phosphate dehydrogenase deficiency in U.S. Army personnel. Mil Med. 2006 Sep;171(9):905-7. doi: 10.7205/milmed.171.9.905. PMID: 17036616.
- Jain SK, Parsanathan R, Levine SN, Bocchini JA, Holick MF, Vanchiere JA. The potential link between inherited G6PD deficiency, oxidative stress, and vitamin D deficiency and the racial inequities in mortality associated with COVID-19 [published online ahead of print, 2020 Oct 7]. Free Radic Biol Med. 2020;161:84-91. doi:10.1016/j.freeradbiomed.2020.10.002
- Miripour ZS, Sarrami-Forooshani R, Sanati H, et al. Real-time diagnosis of reactive oxygen species (ROS) in fresh sputum by electrochemical tracing; correlation between COVID-19 and viral-induced ROS in lung/respiratory epithelium during this pandemic. Biosens Bioelectron. 2020;165:112435. doi:10.1016/j.bios.2020.112435
- Feairheller DL, Park JY, Sturgeon KM, et al. Racial differences in oxidative stress and inflammation: in vitro and in vivo. Clin Transl Sci. 2011;4(1):32-37. doi:10.1111/j.1752-8062.2011.00264.x
- Hecker PA, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Impact of glucose-6-phosphate dehydrogenase deficiency on the pathophysiology of cardiovascular disease. Am J Physiol Heart Circ Physiol. 2013;304(4):H491-H500. doi:10.1152/ajpheart.00721.2012
- Ibrahim H, Perl A, Smith D, et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. doi:10.1016/j.clim.2020.108544
- Bubp J, Jen M, Matuszewski K. Caring for Glucose-6-Phosphate Dehydrogenase (G6PD)-Deficient Patients: Implications for Pharmacy. P T. 2015;40(9):572-574.
- Lord JM. The effect of ageing of the immune system on vaccination responses. Hum Vaccin Immunother. 2013;9(6):1364-1367. doi:10.4161/hv.24696