Clinical Characteristics, Care Trajectories and Mortality Rate of SARS-CoV-2 Infected Cancer Patients: A Multicenter Cohort Study
Abstract
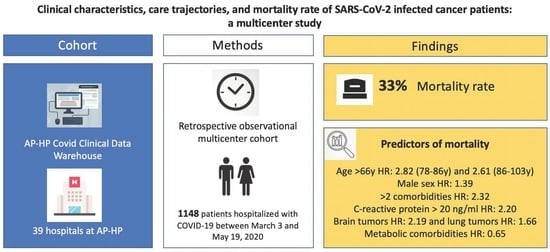
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Study Population
2.4. Procedures
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Factors Associated with All-Cause Mortality
3.3. Care Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ouyang, W.; Chua, M.L.K.; Xie, C. SARS-CoV-2 Transmission in Patients with Cancer at a Tertiary Care Hospital in Wuhan, China. 2020. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7097836/ (accessed on 21 May 2020).
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- The Lancet Oncology. COVID-19: Global Consequences for Oncology. Lancet Oncol. 2020, 21, 467. Available online: http://www.sciencedirect.com/science/article/pii/S1470204520301753 (accessed on 21 May 2020). [CrossRef]
- Brat, G.A.; Weber, G.M.; Gehlenborg, N.; Avillach, P.; Palmer, N.P.; Chiovato, L.; Cimino, J.; Waitman, L.R.; Omenn, G.S.; Malovini, A.; et al. International electronic health record-derived COVID-19 clinical course profiles: The 4CE consortium. NPJ Digit. Med. 2020, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, E.I.; Smeeth, L.; Guttmann, A.; Harron, K.; Moher, D.; Petersen, I.; Sørensen, H.T.; von Elm, E.; Langan, S.M.; RECORD Working Committee. The reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med. 2015, 12, e1001885. [Google Scholar] [CrossRef]
- Kuderer, N.M.; Choueiri, T.K.; Shah, D.P.; Shyr, Y.; Rubinstein, S.M.; Rivera, D.R.; Shete, S.; Hsu, C.-Y.; Desai, A.; Lopes, G.D.L.; et al. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet 2020, 395, 1907–1918. [Google Scholar] [CrossRef]
- Lièvre, A.; Turpin, A.; Ray-Coquard, I.; Le Malicot, K.; Thariat, J.; Ahle, G.; Neuzillet, C.; Paoletti, X.; Bouché, O.; Aldabbagh, K.; et al. Risk factors for Coronavirus Disease 2019 (COVID-19) severity and mortality among solid cancer patients and impact of the disease on anticancer treatment: A French nationwide cohort study (GCO-002 CACOVID-19). Eur. J. Cancer 2020, 141, 62–81. [Google Scholar] [CrossRef]
- Yang, K.; Sheng, Y.; Huang, C.; Jin, Y.; Xiong, N.; Jiang, K.; Lu, H.; Liu, J.; Yang, J.; Dong, Y.; et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 904–913. [Google Scholar] [CrossRef]
- Tian, J.; Yuan, X.; Xiao, J.; Zhong, Q.; Yang, C.; Liu, B.; Cai, Y.; Lu, Z.; Wang, J.; Wang, Y.; et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: A multicentre, retrospective, cohort study. Lancet Oncol. 2020, 21, 893–903. [Google Scholar] [CrossRef]
- Lee, L.Y.; Cazier, J.B.; Starkey, T.; Turnbull, C.D.; Team, U.C.C.M.P.; Kerr, R.; Middleton, G. COVID-19 mortality in patients with cancer on chem-otherapy or other anticancer treatments: A prospective cohort study. Lancet 2020, 395, 1919–1926. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, T.L.; Hallas, J.; Friis, S.; Herrstedt, J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br. J. Cancer 2012, 106, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, S.A.E.; MacMahon, S.; Woodward, M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: Comparisons with influenza/pneumonia and coronary heart disease. Diabetes Obes. Metab. 2021, 23, 258–262. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Smati, S.; Tramunt, B.; Wargny, M.; Caussy, C.; Gaborit, B.; Vatier, C.; Vergès, B.; Ancelle, D.; Amadou, C.; Bachir, L.A.; et al. Relationship between obesity and severe COVID -19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes Obes. Metab. 2021, 23, 391–403. [Google Scholar] [CrossRef]
- Martinez-Tapia, C.; Diot, T.; Oubaya, N.; Paillaud, E.; Poisson, J.; Gisselbrecht, M.; Morisset, L.; Caillet, P.; Baudin, A.; Pamoukdjian, F.; et al. The obesity paradox for mid- and long-term mortality in older cancer patients: A prospective multicenter cohort study. Am. J. Clin. Nutr. 2021, 113, 129–141. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Wu, C.; Wei, M.; Xu, J.; Chao, Y.-C.; Song, J.; Hou, D.; Zhang, Y.; Du, C.; et al. Coagulopathy is a major extrapulmonary risk factor for mortality in hospitalized patients with COVID-19 with type 2 diabetes. BMJ Open Diabetes Res. Care 2020, 8, e001851. [Google Scholar] [CrossRef]
- McPadden, J.; Warner, F.; Young, H.P.; Hurley, N.C.; Pulk, R.A.; Singh, A.; Durant, T.J.; Gong, G.; Desai, N.; Haimovich, A.; et al. Clinical characteristics and outcomes for 7995 patients with SARS-CoV-2 infection. PLoS ONE 2021, 16, e0243291. [Google Scholar] [CrossRef]
- Feng, Q.; Wei, W.Q.; Chaugai, S.; Leon, B.G.C.; Mosley, J.D.; Leon, D.A.C.; Jiang, L.; Ihegword, A.; Shaffer, C.M.; Linton, M.F.; et al. Association Between Low-Density Lipoprotein Cho-lesterol Levels and Risk for Sepsis Among Patients Admitted to the Hospital with Infection. JAMA Netw. Open 2019, 2, e187223. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Qin, J.-J.; Cheng, X.; Shen, L.; Zhao, Y.-C.; Yuan, Y.; Lei, F.; Chen, M.-M.; Yang, H.; Bai, L.; et al. In-Hospital Use of Statins Is Associated with a Reduced Risk of Mortality among Individuals with COVID-19. Cell Metab. 2020, 32, 176–187.e4. [Google Scholar] [CrossRef]
- Sun, K.; Chen, S.; Xu, J.; Li, G.; He, Y. The prognostic significance of the prognostic nutritional index in cancer: A systematic review and meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1537–1549. [Google Scholar] [CrossRef]
- Drucker, D.J. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: The end of the beginning. Cell Metab. 2021, 33, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Saini, K.S.; de Azambuja, E.; Punie, K.; Westphalen, C.B.; Morgan, G.; Pronzato, P.; et al. Mortality in adult patients with solid or hematological malignancies and SARS-CoV-2 infection with a specific focus on lung and breast malignancies: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 163, 103365. [Google Scholar] [CrossRef]
- Provencio, M.; Gallego, J.M.M.; Calles, A.; Antoñanzas, M.; Pangua, C.; Rubio, X.M.; Nadal, E.; Castro, R.L.; López-Martín, A.; del Barco, E.; et al. Lung cancer patients with COVID-19 in Spain: GRAVID study. Lung Cancer 2021, 157, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Ismael, J.; Losco, F.; Quildrian, S.; Sanchez, P.; Pincemin, I.; Lastiri, J.; Bella, S.; Chinellato, A.; Dellamea, G.; Ahualli, A.; et al. Multidisciplinary approach to COVID-19 and cancer: Consensus from scientific societies in Argentina. Ecancermedicalscience 2020, 14, 1044. [Google Scholar] [CrossRef] [PubMed]



| Patients’ Characteristics | All Patients (N = 1148) | Patients Who Survived (N = 765) | Patients Who Died (N = 383) | p Value * |
|---|---|---|---|---|
| Sex (%) | ||||
| Female | 466 (40.6) | 337 (44.1) | 129 (33.7) | <0.001 |
| Age, years | ||||
| Median (range) | 75.5 (4–103) | 74.0 (8–103) | 79.0 (4–100) | <0.001 |
| <66 | 327 (28.5) | 246 (32.2) | 81 (21.1) | |
| 66–75 | 277 (24.1) | 192 (25.1) | 85 (22.2) | |
| 76–85 | 282 (24.6) | 162 (21.2) | 120 (31.3) | |
| ≥86 | 262 (22.8) | 165 (21.6) | 97 (25.3) | |
| Comorbidities (%) | ||||
| Hypertension | 575 (50.1) | 371 (48.5) | 204 (53.3) | 0.144 |
| Coronary heart disease or congestive heart failure | 385 (33.5) | 224 (29.3) | 161 (42) | <0.0001 |
| Cardiac arrythmia | 294 (25.6) | 170 (22.2) | 124 (32.4) | <0.0001 |
| Diabetes mellitus | 351 (30.6) | 231 (30.2) | 120 (31.3) | 0.745 |
| Hyperlipidemia | 172 (15.0) | 125 (16.3) | 47 (12.3) | 0.083 |
| Obesity | 96 (8.4) | 72 (9.4) | 24 (6.3) | 0.089 |
| Metabolic comorbidities ** | 465 (40.5) | 318 (41.6) | 147 (38.4) | 0.33 |
| Chronic obstructive pulmonary disease | 251 (21.9) | 148 (19.3) | 103 (26.9) | 0.004 |
| Smoker or history of smoking | 122 (10.7) | 76 (10.1) | 46 (12.1) | 0.352 |
| Chronic kidney disease | 314 (27.4) | 190 (24.8) | 124 (32.4) | 0.008 |
| Thrombosis | 195 (17.0) | 127 (16.6) | 68 (17.8) | 0.684 |
| Number of comorbidities (%) | ||||
| 0 | 256 (22.3) | 181 (23.7) | 75 (19.6) | 0.003 |
| 1 | 216 (18.8) | 163 (21.3) | 53 (13.8) | |
| 2 | 212 (18.5) | 137 (17.9) | 75 (19.6) | |
| ≥3 | 464 (40.4) | 284 (37.1) | 180 (47) | 0.002 |
| Laboratory findings (%) | ||||
| Leukocytes | ||||
| <10 × 109/L | 689 (60) | 481(62.9) | 208 (54.3) | <0.001 |
| ≥10 × 109/L | 219 (19.1) | 123 (16.1) | 96 (25.1) | |
| Missing data | 240 (20.9) | 161 (21) | 79 (20.6) | |
| Lymphocytes | ||||
| <1.5 × 109/L | 705 (61.4) | 468 (61.2) | 237 (61.9) | 0.965 |
| ≥1.5 × 109/L | 184 (16) | 124 (16.2) | 60 (15.6) | |
| Missing data | 259 (22.6) | 173 (22.6) | 86 (22.5) | |
| Platelets | ||||
| <150 × 109/L | 250 (21.8) | 165 (21.6) | 85 (22.2) | 0.966 |
| ≥150 × 109/L | 658 (57.3) | 439 (57.4) | 219 (57.2) | |
| Missing data | 240 (20.9) | 161 (21) | 79 (20.6) | |
| C-reactive protein | ||||
| <20 mg/L | 417 (36.3) | 321 (41.9) | 96 (25) | <0.001 |
| ≥20 mg/L | 519 (45.2) | 302 (39.5) | 217 (56.7) | |
| Missing data | 212 (18.5) | 142 (18.6) | 70 (18.3) | |
| Cancers’ Characteristics | All Patients (N = 1148) | Patients Who Survived (N = 765) | Patients Who Died (N = 383) | p Value * |
|---|---|---|---|---|
| Tumor stage | ||||
| Metastatic | 318 (27.7) | 204 (26.7) | 114 (29.8) | 0.3 |
| Tumor type (%) | ||||
| Hematologic | 264 (23) | 171 (22.4) | 93 (24.3) | 0.094 |
| Digestive | 129 (11.2) | 89 (11.6) | 40 (10.4) | |
| Urologic | 191 (16.6) | 117 (15.3) | 74 (19.3) | |
| Gynecologic | 37 (3.2) | 24 (3.1) | 13 (3.4) | |
| Pulmonary | 85 (7.4) | 48 (6.3) | 37 (9.7) | |
| Breast | 99 (8.6) | 75 (9.8) | 24 (6.3) | |
| Primary Central Nervous System | 29 (2.5) | 18 (2.4) | 11 (2.9) | |
| Head and neck | 15 (1.3) | 9 (1.2) | 6 (1.6) | |
| Melanoma of the skin | 75 (6.5) | 52 (6.8) | 23 (6) | |
| Other solid tumors | 107 (9.3) | 81 (10.6) | 26 (6.8) | |
| Cancer treatment in the 3 months preceding COVID-19 diagnosis (%) | ||||
| Chemotherapy Missing data | 216 (18.8) 95 (8.3) | 142 (18.6) 60 (7.8) | 74 (19.3) 35 (9.1) | 0.69 |
| Hormone therapy Missing data | 67 (5.8) 97 (8.4) | 45 (5.9) 62 (8.1) | 22 (5.7) 35 (9.1) | 0.837 |
| Immunotherapy Missing data | 85 (7.4) 97 (8.4) | 50 (6.5) 62 (8.1) | 35 (9.1) 35 (9.1) | 0.217 |
| Radiotherapy Missing data | 26 (2.3) 98 (8.5) | 13 (1.7) 62 (8.1) | 13 (3.4) 36 (9.4) | 0.136 |
| Surgery Missing data | 66 (5.7) 144 (12.5) | 54 (7.1) 87 (11.4) | 12 (3.1) 57 (14.9) | 0.009 |
| Targeted treatment Missing data | 81 (7.1) 96 (8.4) | 53 (6.9) 61 (8) | 28 (7.3) 35 (9.1) | 0.764 |
| Study | Lee et al. [12] | Kuderer et al. [8] | Yang et al. [10] | Lievre et al. [9] | Benderra et al. |
|---|---|---|---|---|---|
| Cancer population, n | 800 | 928 | 205 | 1289 | 1148 |
| Region | UK, 55 centers | US, Canada, Spain | China (Hubei), 9 centers | France, 153 centers | France (metropolitan Paris area), 39 centers |
| COVID-19 definition | RT-PCR | RT-PCR | RT-PCR | RT-PCR or imaging features | RT-PCR |
| Age, years | 69 (59–76) | 66 (57–76) | 63 (56–70) | 67 | 76 (65–86) |
| Female gender | 349 (44) | 459 (49) | 109 (53) | 113 (49) | 466 (41) |
| Comorbidities—no. (%, on available data) | |||||
| Hypertension | 247 (31) | NA | 67 (33) | 529 (46) | 575 (50) |
| Cardiovascular disease | 109 (14) | NA | 16 (8) | 194 (16) | 385 (34) |
| COPD | 61 (8) | NA | 5 (2) | 124 (12) | 251 (22) |
| Diabetes | 131 (16) | NA | 22 (11) | 241 (21) | 351 (31) |
| Cancer type—no. (%, on available data) | |||||
| GI | 150 (19) | 108 (12) | 40 (20) | 470 (36) | 129 (11) |
| Pulmonary | 90 (11) | 91 (10) | 24 (12) | 311 (24) | 85 (7) |
| Breast | 102 (13) | 191 (21) | 40 (20) | 173 (13) | 99 (9) |
| Hematological malignancies | 169 (22) | 204 (22) | 22 (11) | 0 | 264 (23) |
| Metastatic cancer—no. (%) | 347 (43) | NA | NA | 758 (59) | 318 (28) |
| ICU admission—no. (%, on available data) | 53 (7) | 132 (14) | 30 (15) | 110 (10) | 217 (19) |
| Mortality—no. (%) | 226 (28) | 121 (13) | 40 (20) | 370 (29) | 383 (33) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benderra, M.-A.; Aparicio, A.; Leblanc, J.; Wassermann, D.; Kempf, E.; Galula, G.; Bernaux, M.; Canellas, A.; Moreau, T.; Bellamine, A.; et al. Clinical Characteristics, Care Trajectories and Mortality Rate of SARS-CoV-2 Infected Cancer Patients: A Multicenter Cohort Study. Cancers 2021, 13, 4749. https://doi.org/10.3390/cancers13194749
Benderra M-A, Aparicio A, Leblanc J, Wassermann D, Kempf E, Galula G, Bernaux M, Canellas A, Moreau T, Bellamine A, et al. Clinical Characteristics, Care Trajectories and Mortality Rate of SARS-CoV-2 Infected Cancer Patients: A Multicenter Cohort Study. Cancers. 2021; 13(19):4749. https://doi.org/10.3390/cancers13194749
Chicago/Turabian StyleBenderra, Marc-Antoine, Ainhoa Aparicio, Judith Leblanc, Demian Wassermann, Emmanuelle Kempf, Gilles Galula, Mélodie Bernaux, Anthony Canellas, Thomas Moreau, Ali Bellamine, and et al. 2021. "Clinical Characteristics, Care Trajectories and Mortality Rate of SARS-CoV-2 Infected Cancer Patients: A Multicenter Cohort Study" Cancers 13, no. 19: 4749. https://doi.org/10.3390/cancers13194749