Abstract
Background
Olfactory dysfunction has shown to accompany COVID-19. There are varying data regarding the exact frequency in the various study population. The outcome of the olfactory impairment is also not clearly defined.
Objective
To find the frequency of olfactory impairment and its outcome in hospitalized patients with positive swab test for COVID-19.
Methods
This is a prospective descriptive study of 100 hospitalized COVID-19 patients, randomly sampled, from February to March 2020. Demographics, comorbidities, and laboratory findings were analyzed according to the olfactory loss or sinonasal symptoms. The olfactory impairment and sinonasal symptoms were evaluated by 9 Likert scale questions asked from the patients.
Results
Ninety-two patients completed the follow-up (means 20.1 (± 7.42) days). Twenty-two (23.91%) patients complained of olfactory loss and in 6 (6.52%) patients olfactory loss was the first symptom of the disease. The olfactory loss was reported to be completely resolved in all but one patient. Thirty-nine (42.39%) patients had notable sinonasal symptoms while rhinorrhea was the first symptom in 3 (3.26%). Fifteen patients (16.3%) had a taste impairment. Patients with sinonasal symptoms had a lower age (p = 0.01). There was no significant relation between olfactory loss and sinonasal symptoms (p = 0.07).
Conclusions
Sudden olfactory dysfunction and sinonasal symptoms have a considerable prevalence in patients with COVID-19. No significant association was noted between the sinonasal symptoms and the olfactory loss, which may suggest that other mechanisms beyond upper respiratory tract involvement are responsible for the olfactory loss.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) is an official name applied by the World Health Organization (WHO), referring to a recently emerged disease that has now become the pandemic of the twenty-first century. The responsible virus was named as “severe acute respiratory syndrome coronavirus 2” (SARS-CoV- 2). The virus causing COVID −19 is a SARS-like coronavirus with a 29,903 bp single-stranded RNA (ss-RNA) [1].
Human coronaviruses (HCoV) were identified as one of the main causes of acute upper respiratory tract infections (URTI) in the 1960s [2]. Later, various coronavirus species, including HCoV-NL63, associated with acute laryngotracheitis (croup), were identified [2, 3]. A coronavirus belonging to the 2b β-coronavirus group was detected in 2002 as the cause of severe lower respiratory tract infection (LRTI) in China, named as a severe acute respiratory syndrome (SARS) [4]. In 2012, another coronavirus belonging to the 2c β-coronavirus group caused a highly pathogenic LRTI in Saudi Arabia, named Middle East respiratory syndrome (MERS), which became epidemic mainly in the Middle East [5, 6]. In these newly emerged groups of coronaviruses, although the main site of involvement in the lower respiratory tract, mild symptoms of URTI have been reported in MERS and SARS [7, 8].
Sinonasal symptoms in an URTI are due to generalized mucosal edema that is also the reason for olfactory loss encountered during the acute phase of an URTI [9]. However, the olfactory loss may also occur following the resolution of sinonasal symptoms, the entity known as post-viral olfactory loss [10]. Although there are more than 200 subtypes of viruses associated with URTI, not all of which have been studied concerning olfactory loss, influenza or parainfluenza viruses, rhinovirus, picornavirus, human coronavirus, and Epstein-Barr virus are among those that have been mentioned as the cause of post-viral olfactory loss [11,12,13]. Olfactory loss has been identified in very limited case reports in SARS, which was not associated with sinonasal symptoms [14]. It has been suggested that the virus can infect the brain via the nasal cavity epithelium and olfactory pathway in animals infected by MERS-CoV and SARS-CoV [15].
Since the outbreak of the COVID-19 in Iran, plenty of patients with olfactory loss have been reported by the otolaryngologists. The objective of this study was to investigate the frequency of the olfactory impairment and the rate of recovery, together with any accompanying URTI (sinonasal symptoms) during the recent COVID-19 pandemic.
Methods
This prospective descriptive study was performed on hospitalized patients with COVID-19 from February to March 2020, randomly sampled from the COVID care wards of a tertiary referral hospital (Hazrat Rasool Akram Hospital) in Tehran, Iran. The protocol of the study was approved by the Institutional Review Board and ethics committee of Iran University of Medical Sciences (code number 1399.052). The study protocol was in accordance with the 1964 Helsinki declaration and its later amendments comparable ethical standards. Written informed consent was obtained from all participants.
Study participants
One hundred adult patients under the age of 80, having positive throat swab specimens in the evaluation of reverse transcriptase-polymerase chain reaction, were enrolled in the study. Patients with COVID-19 were diagnosed according to the WHO interim guideline [16, 17]. Patients with well-known neurodegenerative diseases, including Parkinson’s disease or dementia, history of olfactory dysfunction, as well as those with severe respiratory distress or under mechanical ventilation, were excluded from the study.
Data collection
The demographic characteristics (age and sex), comorbidities, previous chronic sinonasal disease or related surgery, seasonal allergy, smoking habits, time of disease onset, and the first symptom(s) at the onset, and those added later, were asked from the patients. The oxygen saturation, assessed by a pulse oximeter, was also recorded. Laboratory results, including leukocyte and lymphocyte count, c-reactive protein (CRP), erythrocyte sedimentation rate, aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), and creatinine, were recorded from the electronic medical records of the hospital. Fever was defined as an axillary temperature of ≥ 37.5 °C.
The radiological assessment of all the patients was carried out using chest computed tomographic (CT) scans. The patients were interviewed regarding sinonasal symptoms. Seven prominent symptoms including needing to blow the nose, sneeze, rhinorrhea, postnasal discharge, thick nasal discharge, facial pain over sinuses, and nasal congestion/obstruction were evaluated using 7 Likert scale questions (five-point scale; 0: no complaint, 5: extreme severe problem). The group with sinonasal symptoms was defined considering the sum of the Likert score when the change in each one was higher than 1 (very mild). The olfaction and taste were also evaluated using 2 Likert scale questions (five-point scale; 0: no change, 5: complete loss). The same questions were applied when the patients were called for follow-up. The patients were asked to score according to the most severe symptoms they had since the disease onset (and not the severity sensed at the time of the interview). The highest score was adopted for each patient. The severity of COVID-19 was defined for the patients according to the international guidelines for community-acquired pneumonia [18]. The throat swab specimens of patients were analyzed using the SARS-CoV-2 nucleic acid detection kit. (LightMix® Sarbeco V E-gene plus EAV control 530/660).
Statistical analysis
Characteristics were summarized as mean ± standard deviation (SD) for quantitative variables and as a percentage for qualitative variables, respectively. Inter-group comparison of categorical and quantitative variables was performed with Fisher’s exact test and t test, respectively. SPSS (version 22.0, IBM Corp., Armonk, NY, USA) was used to analyze the statistical variables. P values less than 0.05 were set as statistically significant levels.
Results
One hundred hospitalized patients who had SARS-CoV2 infection were eligible to be included in the study. Ninety-two patients completed the follow-up and hence were analyzed (3 patients were not interested with follow-up, 2 patients did not answer the phone after several attempts, one had a wrong telephone number registered in the medical record, one patient deceased after admission to another hospital, and one patient had a cerebrovascular accident a few days after being discharged from the hospital). The mean follow-up duration was 20.10 ± 7.42 days, and 71 (77.2%) patients had more than 2-week follow-up.
Clinical, radiological, and laboratory findings
According to the international guidelines for community-acquired pneumonia, 72 (78.3%) patients had mild and 20 patients (21.7%) had moderate pneumonia. In the patients with mild pneumonia, the mean leukocyte and lymphocyte count were 6095.07 ± 3669/μl and 1066.76 ± 542/μl, respectively. In the patients with moderate pneumonia, the mean leukocyte count and lymphocyte count were 5940 ± 2880/μl and 939.17 ± 441/μl, respectively. The mean age in mild group was 50.75 ± 12.31 while in the moderate group it was 60.85 ± 13.82 years. There was a significant difference between the severity of pneumonia and age (p = 0.002). The severity of pneumonia and mean leukocyte and lymphocyte count had no association with sinonasal symptoms or olfactory loss. The details of demographic data, comorbidities, and laboratory findings of the patients are all presented in Table 1. In 95.7% of our patients, CRP was higher than the normal level, which was not significantly different in patients with or without sinonasal symptoms or olfactory loss. The CT chest scans of patients were compatible with ground-glass opacity in 71.2%, consolidation in 21.9%, and crazy paving pattern in 6.8%. The mean of oxygen blood saturation was 91.81% ± 4.34.
Sinonasal symptoms
Sinonasal symptoms were found in 39 (42.39%) patients. Although the most common presenting symptoms were myalgia and fever, each in more than one-third of the patients, rhinorrhea was among the first symptom in 3 (3.26%) of the cases. Considering the severity, 12 patients (13.04%) had sinonasal symptoms with a Likert score of 4 or more. The patients’ manifestations, including sinonasal symptoms and olfaction, are presented in Table 2. Patients with sinonasal symptoms had a lower age (p = 0.01) patients with diabetes mellitus had a lesser chance of presenting with sinonasal symptoms (p = 0.03). However, patients with diabetes mellitus (DM) were significantly younger (59.86 ± 12.23 vs. 50.77 ± 12.89, p = 0.004). There was no significant difference in the other demographic data, comorbidities, and laboratory findings between those who had sinonasal symptoms comparing to others.
Olfactory loss
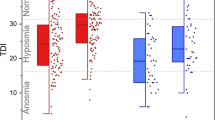
The olfactory loss was reported by 22 (23.91%) patients, of whom 6 (6.52%) patients reported it as the first symptom of their disease. Anosmia was reported by 9 (40.9%) and hyposmia by 13 (59.1%), respectively. Two patients reported hyperosmia. Fifteen patients (16.3%) reported simultaneous taste impairment. The frequency and severity of sinonasal symptoms and the olfactory and taste impairment (based on the Likert scale) are shown in Table 3. There was no significant difference in the demographic data, comorbidities, and laboratory findings between those who had olfactory loss comparing to others. In the 22 patients with olfactory loss, the median (mean ± SD) time of the onset of olfactory was 3 days (3.41 ± 2.46) after the onset of COVID-19 symptoms. The olfactory loss lasted for a median of 8 (10.73 ± 8.26) days and completely resolved in 21 (95.45%) patients (mean follow-up for 22 patients with olfactory impairment was 20.63 ± 5.95, ranging from 10 to 30 days). One patient with anosmia remained symptomatic 30 days after the onset of the symptom. However, he had shown some degree of improvement at that time (Likert score = 2).
Among 22 patients with olfactory loss, 9 patients had no sinonasal symptoms. Likewise, among 39 patients with sinonasal symptoms, 26 patients did not have any olfactory complain. There was no significant relation between olfactory loss and upper respiratory tract involvement (p = 0.07).
Discussion
The present study followed 92 hospitalized COVID-19 patients for around 3 weeks after the start of their disease symptoms. The olfactory loss was reported by almost 24% of the patients. URTI symptoms were present in 42% of the patients (severe form only in around 13% of the patients), which did not correlate with the olfactory loss. Patients with sinonasal symptoms were significantly younger. The same finding was observed by Krajewska et al. [19]. Until April 3, 2020, there have been 3,917,366 and 106,220 laboratory-confirmed cases of COVID-19 globally and in Iran, respectively [20]. There is accumulating evidence from all around the world, revealing the association of olfactory loss with the COVID-19 pandemic. The reported frequency of olfactory impairment in recent peer-reviewed or not peer-reviewed studies varies greatly [21, 22]. This wide range may be due to various factors including study design (i.e., retrospective [22], prospective [23], online survey [24, 25] study population, subdivided in hospitalized patients [21, 22, 26], out-patient setting [27], and mixed [23, 28]; regional differences in population (racial diversity) [23] and use of olfactory tests and the test being used [21, 28]. For instance, Giacomelli et al. [26] found that when being asked, 33.9% of hospitalized patients with COVID-19 reported olfactory and/or taste disorder. While Mao et al. [22], collecting the data from medical electronic records, reported 5.1% of the hospitalized patients had a complaint of hyposmia.
Olfactory clefts are narrow upper parts of nasal cavities containing olfactory epithelium with olfactory receptor neurons necessary for olfaction [29]. Various pathologies, including common viral URTI, can cause prominent mucosal edema and subsequent obstruction in the middle and the lower parts of the nasal cavities, hindering the odors to reach the olfactory cleft [29]. There was no relation between the upper respiratory tract involvement and olfactory loss in our patients. This may suggest that the olfactory loss is not due to generalized mucosal edema and nasal obstruction, which occurs during a common upper respiratory infection [9].
Post-viral olfactory dysfunction is the cause of 18% to 42% of the patients with olfactory loss [30]. Suzuki et al. [31] were able to detect rhinovirus, coronavirus, parainfluenza virus, and Epstein-Barr virus in the nasal discharge of patients with post-viral olfactory dysfunction for the first time. They suggested that other mechanisms, apart from nasal obstruction, can be responsible for olfactory loss in this setting [31]. The exact mechanism of post-viral olfactory loss is not completely understood. Direct damage of the olfactory pathway by the virus, viral involvement of the olfactory bulb, and indirect damage caused by the subsequent inflammation of olfactory neurons and supporting cells are among the suggested mechanisms (as was noted in influenza infection) [9, 32]. There was no significant relationship between sinonasal symptoms and olfactory loss in our study, which may support this proposed mechanism. However, the post-viral olfactory loss is usually noted a few weeks after the resolution of sinonasal symptoms, while our patients reported the olfactory loss during the first weeks of their disease. There are few studies regarding the recovery rate of olfactory dysfunction in COVID-19. Lechien et al. [23] reported a 44.0% short-term (2 weeks) olfaction recovery rate in COVID-19 patients, while Vaira et al. [28] reported 66% complete recovery of olfactory dysfunction in their COVID-19 patients when being asked (mean of days from symptom onset = 19.3). After mean follow-up of 20 days (10–30 days), 95.5% of these patients reported complete improvement of the olfactory impairment which is quite higher than the recovery rate of common post-viral olfactory loss [28, 33].
Trotier et al. [29] showed that in some patients with idiopathic olfactory loss, the inflammatory obstruction can be found only in the olfactory clefts, and not in the rest of the nasal cavities and sinuses. Hoffmann et al. [34] showed that the SARS-CoV-2 infects cells through interactions between its spike (S) protein and the ACE2 protein on target cells. This interaction requires cleavage of the S protein by the cell surface protease TMPRSS. Base on analyzing the RNA-sequencing datasets, Brann et al. [33] found that both ACE2 and TMPRSS are expressed by olfactory epithelial supporting cell and stem cells, and not olfactory sensory neurons, per se. Accordingly, they hypothesized that the infection of these cells is the cause of olfactory dysfunction in patients with COVID-19 [34]. We know that the infected cells secret pro-inflammatory cytokines and chemokines, resulting in an influx of inflammatory cells [9]. This reaction can also lead to localized mucosal edema in the narrow olfactory cleft, hindering the odors pass to the olfactory mucosa (Fig. 1) [35]. This mechanism can also affect the neurons as bystanders. However, the neuroinvasiveness of some coronaviruses, including SARS-CoV, has demonstrated in both mice and humans [36]. Accordingly, there are imaging studies of the brain by MRI, showing a transient increase in volume or hyperintensity of the olfactory bulb and the brain regions that are associated with olfaction [37, 38]. It seems that more pathologic investigations on the involved nasal mucosa or cadaveric brain specimens are needed to find out the exact responsible mechanisms.
A non-enhanced coronal paranasal CT scan of 36-year-old lady presented only with sudden anosmia and headache showing obstruction of olfactory cleft by kissing mucosal swelling B. Chest CT scan of the same patient, after showing COVID-19 symptoms. The sense of smell was recovered after 8 days of onset
Fever has announced as a main symptom of COVID 19, with a prevalence ranging from 38 to 95.8% that may be due to the various factors including severity of the disease [22, 39, 40]. The prevalence of fever in Mao et al. [22] study was 61% while Lechien et al. [23] reported in less than 50% of their patients. In our patients, fever as a presenting symptom reported by 31.5% of the patients, while it was detected in 54.3% of the patients during the hospitalization period.
SARS-CoV-2 showed great potential for dissemination became a pandemic during 2.5 months [20]. Although it may be related to the structure of the virus per se, one explanation could be the shedding and spreading of the virus by the infected people with very mild symptoms, similar to those of an URTI, which could be quite misleading. These patients with subtle symptoms may unintentionally breach the quarantine rules. Hence, those who have olfactory loss without any sinonasal symptoms need to be considered as probable cases of COVID-19 during the pandemic [21, 23]. This may be of great importance in controlling the spreading of the COVID-19. Although we designed a prospective study, data about the presenting symptoms was collected retrospectively. In this study, the olfaction and sinonasal symptoms were evaluated only in patients with COVID-19 that were able to answer the questionnaire and were hospitalized. Therefore, the reported frequency may not exactly be the same in milder or very severe forms of COVID-19. The other limitation is that the patients were not evaluated with a standard olfactory test; this was due to the condition of patients with dyspnea and the preventive protocols that limited unnecessary contacts of persons and instruments during the pandemic. The correlation between olfactory loss and sinonasal symptoms may need to be evaluated in larger studies.
Conclusion
Sudden temporary olfactory loss and URTI symptoms have a considerable prevalence in patients with COVID-19 and need to be considered as the symptoms of this infection. There was, however, no association between URTI symptoms and the prevalence of olfactory loss in these patients, emphasizing that the pathology may not be the generalized mucosal swelling that happens during an URTI with common coronaviruses. The pathophysiology, natural course, prognosis, and therapeutic strategies of olfactory loss caused by SARS-CoV-2 deserve further investigation.
References
Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998
Hamre D, Procknow JJ (1966) A new virus isolated from the human respiratory tract. Proc Soc Exp Biol Med 121:190–193
Fehr AR, Perlman S (2015) Coronaviruses: an overview of their replication and pathogenesis. In Coronaviruses:1-23: Springer. Number of pp 1-23
Consortium CSME (2004) Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science 303:1666–1669
Mackay IM, Arden KE (2015) MERS coronavirus: diagnostics, epidemiology and transmission. Virol J 12:1–21
Al-Tawfiq JA, Zumla A, Memish ZA (2014) Coronaviruses: severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus in travelers. Curr Opin Infect Dis 27:411–417
Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, Ahuja A, Yung MY, Leung CB, To KF, Lui SF, Szeto CC, Chung S, Sung JJY (2003) A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med 348:1986–1994
Zumla A, Hui DS, Perlman S (2015) Middle East respiratory syndrome. Lancet 386:995–1007
Flanagan CE, Wise SK, DelGaudio JM, Patel ZM (2015) Association of decreased rate of influenza vaccination with increased subjective olfactory dysfunction. JAMA Otolaryngol Head Neck Surg 141:225–228
Dai Q, Pang Z, Yu H (2016) Recovery of olfactory function in postviral olfactory dysfunction patients after acupuncture treatment. Evid Based Complement Alternat Med 2016:1–6. https://doi.org/10.1155/2016/4986034
Boesveldt S, Postma EM, Boak D, Welge-Luessen A, Schöpf V, Mainland JD, Martens J, Ngai J, Duffy VB (2017) Anosmia—a clinical review. Chem Senses 42:513–523
Fark T, Hummel T (2013) Olfactory disorders: distribution according to age and gender in 3,400 patients. Eur Arch Otorhinolaryngol 270:777–779
Pawełczyk M, Kowalski ML (2017) The role of human parainfluenza virus infections in the immunopathology of the respiratory tract. Curr Allergy Asthma Rep 17:16
Hwang C (2006) Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica 15:26
Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB Jr (2016) Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 213:712–722
Organization WHO (2020) Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020, World Health Organization
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395:497–506
Lim W, Van der Eerden M, Laing R, Boersma W, Karalus N et al (2003) Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax 58:377–382
Krajewska J, Krajewski W, Zub K, Zatoński T (2020) COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol:1–13
Organization WHO (2020) Coronavirus disease 2019 (COVID-19): situation report, 72
Moein S, Hashemian S, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RSmell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. https://doi.org/10.1002/alr.22587
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol:1–11
Bagheri SH, Asghari A, Farhadi M, Shamshiri AR, Kabir A et al (2020) Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Med J Islam Republic Iran (MJIRI) 34:446–452
Parma V, Ohla K, Veldhuizen MG, Nim MY, Kelly CE, et al (2020) More than just smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. COVID-19 Res https://doi.org/10.1093/chemse/bjaa041
Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, Antinori S, Galli M (2020) Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa330
Menni C, Valdes A, Freydin MB, Ganesh S, Moustafa JE-S et al (2020) Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. MedRxiv. https://doi.org/10.1038/s41591-020-0916-2
Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, de Vito A, Babudieri S, Petrocelli M, Serra A, Bussu F, Ligas E, Salzano G, de Riu G (2020) Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 42:1252–1258
Trotier D, Bensimon JL, Herman P, Tran Ba Huy P, Døving KB, Eloit C (2007) Inflammatory obstruction of the olfactory clefts and olfactory loss in humans: a new syndrome? Chem Senses 32:285–292
Nordin S, Brämerson A (2008) Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol 8:10–15
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, Murakami S (2007) Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 117:272–277
Fujikura D, Miyazaki T (2018) Programmed cell death in the pathogenesis of influenza. Int J Mol Sci 19:2065
Brann D, Tsukahara T, Weinreb C, Logan DW, Datta SR (2020) Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. BioRxiv. https://doi.org/10.1101/2020.03.25.009084
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8
Eliezer M, Hautefort C, Hamel A-L, Verillaud B, Herman P, Houdart E, Eloit C (2020) Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg. https://doi.org/10.1001/jamaoto.2020.0832
Calcagno N, Colombo E, Maranzano A, Pasquini J, Keller Sarmiento IJ, et al (2020) Rising evidence for neurological involvement in COVID-19 pandemic. Neurol Sci:1–3
Laurendon T, Radulesco T, Mugnier J, Gérault M, Chagnaud C, el Ahmadi AA, Varoquaux A (2020) Bilateral transient olfactory bulbs edema during COVID-19-related anosmia. Neurology. https://doi.org/10.1212/WNL.0000000000009850
Politi LS, Salsano E, Grimaldi M (2020) Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (covid-19) and anosmia. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.2125
Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T et al. ( 2020) Smell and taste alterations in Covid-19: a cross-sectional analysis of different cohorts. In Int Forum Allergy Rhinol. https://doi.org/10.1002/alr.22610
Kaye R, Chang CD, Kazahaya K, Brereton J, Denneny III JC (2020) COVID-19 anosmia reporting tool: initial findings. Otolaryngol Head Neck Surg. https://doi.org/10.1177/0194599820922992
Acknowledgments
We acknowledge all medical staff involved in the diagnosis and treatment of patients with COVID-19 in Hazrat Rasool Akram Hospital. We thank Miss Mahsa Jalesi and Farzaneh Ezati for helping in data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval (include appropriate approvals or waivers)
The protocol of the study was approved by the Institutional Review Board and ethics committee of Iran University of Medical Sciences (code number 1399.052). The study protocol was in accordance with the 1964 Helsinki declaration and its later amendments comparable ethical standards.
Consent to participate
Written informed consent was obtained from all participants.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jalessi, M., Barati, M., Rohani, M. et al. Frequency and outcome of olfactory impairment and sinonasal involvement in hospitalized patients with COVID-19. Neurol Sci 41, 2331–2338 (2020). https://doi.org/10.1007/s10072-020-04590-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-04590-4