Multisystem inflammatory syndrome in children and SARS-CoV-2: A scoping review
Abstract
PURPOSE:
The pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 43 million people resulting in over 1 million deaths. Approximately 2% of cases in the United States are children, and in most cases the child is either asymptomatic or has mild symptoms. However, some pediatric cases can present with Multisystem Inflammatory Syndrome (MIS-C). Understanding the epidemiology, clinical presentation, and management of MIS-C related to SARS-CoV-2 will help to streamline early diagnosis and treatment, particularly in pediatric patients with complex medical conditions.
METHODS:
This scoping review adopted methods from the Joanna Briggs Institute (JBI) manual for evidence synthesis and preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) guidelines. Primary studies of patients meeting the Centers for Disease Control and Prevention (CDC) criteria for MIS-C from December 31
RESULTS:
Of 417 studies identified, 57 met inclusion criteria, accounting for 875 patients from 15 countries. Globally, 57% of children affected with MIS-C were males. The median age was 9 years old, ranging from 6 months to 21 years. Forty-five percent of the patients had underlying comorbidities including obesity and lung disease. Fever, conjunctivitis and GI symptoms were common. Most MIS-C patients had high biomarkers including troponin I, N-terminal prohormone of B-type natriuretic peptide (NT-proBNP), D-dimer, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), white blood cells (WBCs), interleukin 6 (IL-6), procalcitonin, and ferritin. The treatment for most patients included IVIG and inotropic support.
CONCLUSION:
MIS-C can be a unique and potentially life-threatening manifestation of SARS-CoV-2 in children and often requires medical intervention.
1.Introduction
Since the first reported case in Wuhan, China, as of October 5, 2020 there have been over 42 million confirmed cases and 1 million deaths from COVID-19 worldwide. Most patients develop mild respiratory distress and recover without intervention. Older adults and those with underlying diseases are more likely to develop serious illness that involves other systems [1].
Globally, children are far less likely to be affected by and develop severe illness from SARS-CoV-2, with patients less than 18 years old comprising only 2% of confirmed cases in the US, 2.2% in China, 1.2% in Italy and 0.8% in Spain [2, 3, 4]. For the majority of pediatric patients, COVID-19 is asymptomatic or mild. In these cases, the common clinical presentation includes mild fever and cough. Unlike adults, children may experience gastrointestinal (GI) symptoms more often. Studies show that abdominal pain, nausea, and diarrhea are the classic GI presentations in children [5]. The recommended treatment is supportive and oral hydration therapy [6].
However, SARS-CoV-2 can rarely progress to Multisystem Inflammatory Syndrome in Children (MIS-C). The first cases, which consisted of several critically ill children, were brought to attention in April 2020 by National Health Service in United Kingdom. These children presented with overlapping features of Kawasaki Disease, a rare childhood vasculitis that leads to similar mucocutaneous manifestations. Similar to Kawasaki Disease, several organ systems can become inflamed in MIS-C, including the heart, brain, skin, eyes and GI organs. Due to the similarities between MIS-C and autoimmune diseases including Kawasaki Disease, diagnosis may be delayed [4, 5]. In severe cases, the suggested treatment includes supplemental oxygen, mechanical ventilation, antibiotics, or antivirals [7]. Although rare in children, critical cases present with sepsis and multiorgan failure [8]. Case studies worldwide report the severe form of the disease in children, referred to as MIS-C, which presents similarly to Kawasaki Disease and toxic shock syndrome.
According to the CDC, MIS-C is defined as patients under the age of 21 with fever for greater than 24 hours, laboratory evidence of inflammation, severe illness that requires hospitalization, more than two organ systems involved, and positive SARS-CoV-2 infection (RT-PCR, serology, or antigen test) or exposure to a COVID-19 case within 4 weeks prior to symptom onset [9]. In cases of MIS-C, common clinical manifestations include rash, conjunctivitis, GI symptoms, shock, and myocardial dysfunction [7].
Data on symptomatic management of MIS-C patients is rapidly emerging. There exists at least one systematic review on MIS-C which included data through June 2020 from 35 articles relating to 783 patients [10]. Roughly half of the primary studies included in the systematic review by Radia et al. are reported in further detail here, as many did not meet eligibility criteria of this present study or did not report quantitative laboratory data. While the review includes superficial qualitative data on laboratory and radiological investigations, a quantitative synthesis of laboratory findings was not included and several laboratory tests were not reported. Additionally, the review appears to have implemented the World Health Organization (WHO) case definition for MIS-C [11], which differs from the CDC case definition for MIS-C [9] applied in the present study. Another systematic review by Yasuhara et al. on COVID-19 in children was published in July 2020 [12], but MIS-C was not the primary focus as only 17 of the 114 included patients satisfied the CDC definition for MIS-C. All of the studies in that review pertaining to MIS-C are included here in further detail. The shortcomings of existing literature are addressed in this present review by including the following: patient data as recent as October 5, 2020 (which meets the CDC case definition for MIS-C); a rigorous quantitative data synthesis of laboratory investigations; and a discussion of recent literature describing the potential urgency of this condition.
This present study aims to clarify knowledge gaps in the definition, clinical presentation, quantitative laboratory findings, and treatment regimens of MIS-C by providing a broad purview of available primary evidence describing various characteristics of MIS-C. Furthermore, as this review foregoes a quantitative statistical analysis, a critical appraisal of individual sources of evidence, and an assessment of publication bias, a scoping review format was adopted for this study.
2.Methods
The Joanna Briggs Institute (JBI) manual for evidence synthesis was consulted in conducting this scoping review, which was completed using the preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews checklist (PRISMA-ScR) [13, 14]. An a priori protocol was not used in this study. However, study selection criteria were established during the initial literature review process.
2.1Eligibility criteria
All primary studies, including case reports, that positively identified children defined as age less than 21 years with a potential diagnosis of MIS-C in addition to a positive SARS-CoV-2 PCR test result, SARS-CoV-2 serum antibody assay, or epidemiologic link to person who tested positive were included. Only reports published or accepted after December 2019 were eligible, as MIS-C prior to this date is unrelated to SARS-CoV-2. Studies that reported patients with negative SARS-CoV-2 PCR or negative SARS-CoV-2 serum antibody were excluded.
2.2Information sources and search strategy
Sources and search strategies were developed after an initial review of the available literature. Online literature searches were conducted in PubMed and Scopus databases. The latest search was conducted on October 5, 2020. The search terms included were “Multisystem Inflammatory Syndrome in Children.” After removing duplicates, articles were screened for eligibility.
2.3Study selection
Studies were identified and screened for inclusion criteria. They were excluded if they were published before December 31, 2019 (the date the WHO received its first report of the novel coronavirus), had a study population of age greater than 21 years, were unrelated to SARS-CoV-2, were in-vitro/lab studies, or were editorials or commentaries. Remaining articles were assessed for eligibility by analysis of title, abstract, and full-text when necessary. Articles were excluded if they solely discussed management or pathophysiology of MIS-C, contained no lab values, included cases with a diagnosis other than MIS-C, or were time-course studies. Included articles were primary studies, such as case reports, case series, and cohort studies that contained demographical data, symptoms, laboratory values, and management. All articles synthesized in this review contained MIS-C cases that met inclusion criteria.
2.4Data collection process and data items
Demographics including median age, location of study, gender, comorbidities, symptoms, laboratory investigation values, and treatments provided were recorded for each study. The symptoms that were recorded are: fatigue, fever, rash, abdominal pain, nausea, vomiting, nervous system involvement, conjunctivitis, tachycardia, chest pain, ventricular dysfunction, cardiogenic shock, ventricular arrhythmia, coronary artery dilation or ectasia, and upper or lower extremity edema. Recorded lab values included troponin I, NT-proBNP, CRP, ESR, procalcitonin, D-Dimer, WBC, IL-6, and ferritin. The lab values were recorded by median and the first (Q
Figure 1.
PRISMA flow chart for study selection.
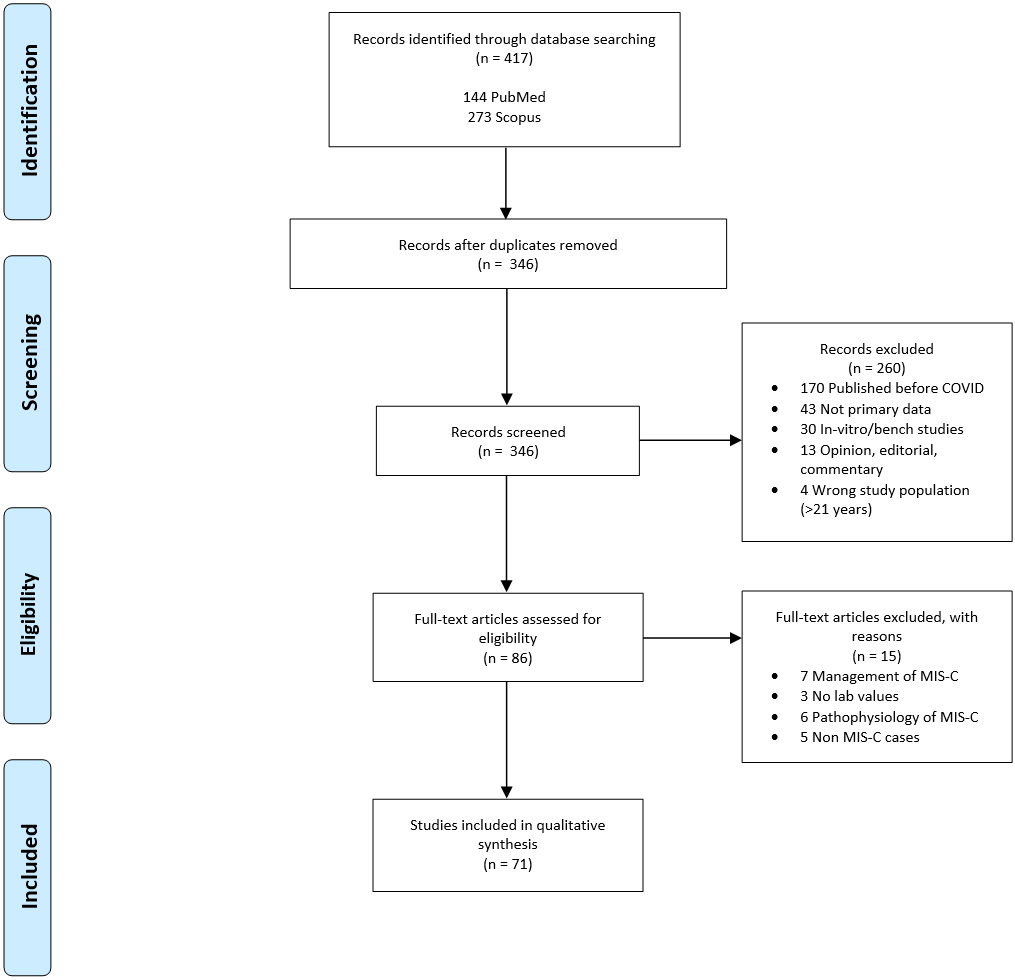
3.Results
3.1Study selection
The PRISMA study selection flow diagram is shown in Fig. 1. Four hundred seventeen articles were identified through PubMed and Scopus. After duplicates were removed, 345 records were screened by title and abstract. Two hundred sixty-one records unrelated to MIS-C due to SARS-CoV-2 were omitted. Eighty-four full-text articles were assessed for eligibility, of which 27 were excluded. Excluded articles comprised of seven that solely discussed clinical management of MIS-C, six that did not report lab values or treatment, six that solely discussed the pathophysiology of the disease, five that did not satisfy criteria for MIS-C, two articles that did not provide evidence of SARS-CoV-2 infection or exposure, and one for which the English version was not available [4, 7, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69].
Of the 57 articles, 28 were case reports, 29 were larger observational studies or case series, and 16 reported whether patients had a comorbidity. Three studies did not specify gender and three did not specify age. One study stratified the data based on MIS-C positive versus SARS-CoV-2 positive patients. The 27 studies that were published in the United States comprised of patients that satisfied the CDC’s criteria for MIS-C. In the 30 studies that were published outside the United States, MIS-C was defined as SARS-CoV-2 positive patients less than 21 years of age, with more than two organs involved and presence of fever.
3.2General characteristics
The general patient and study characteristics including patient number, gender, age, positive testing or epidemiologic link to SARS-CoV-2 as defined by the CDC, and comorbidities are shown in Table 1.
Table 1
Summary of study and patient characteristics
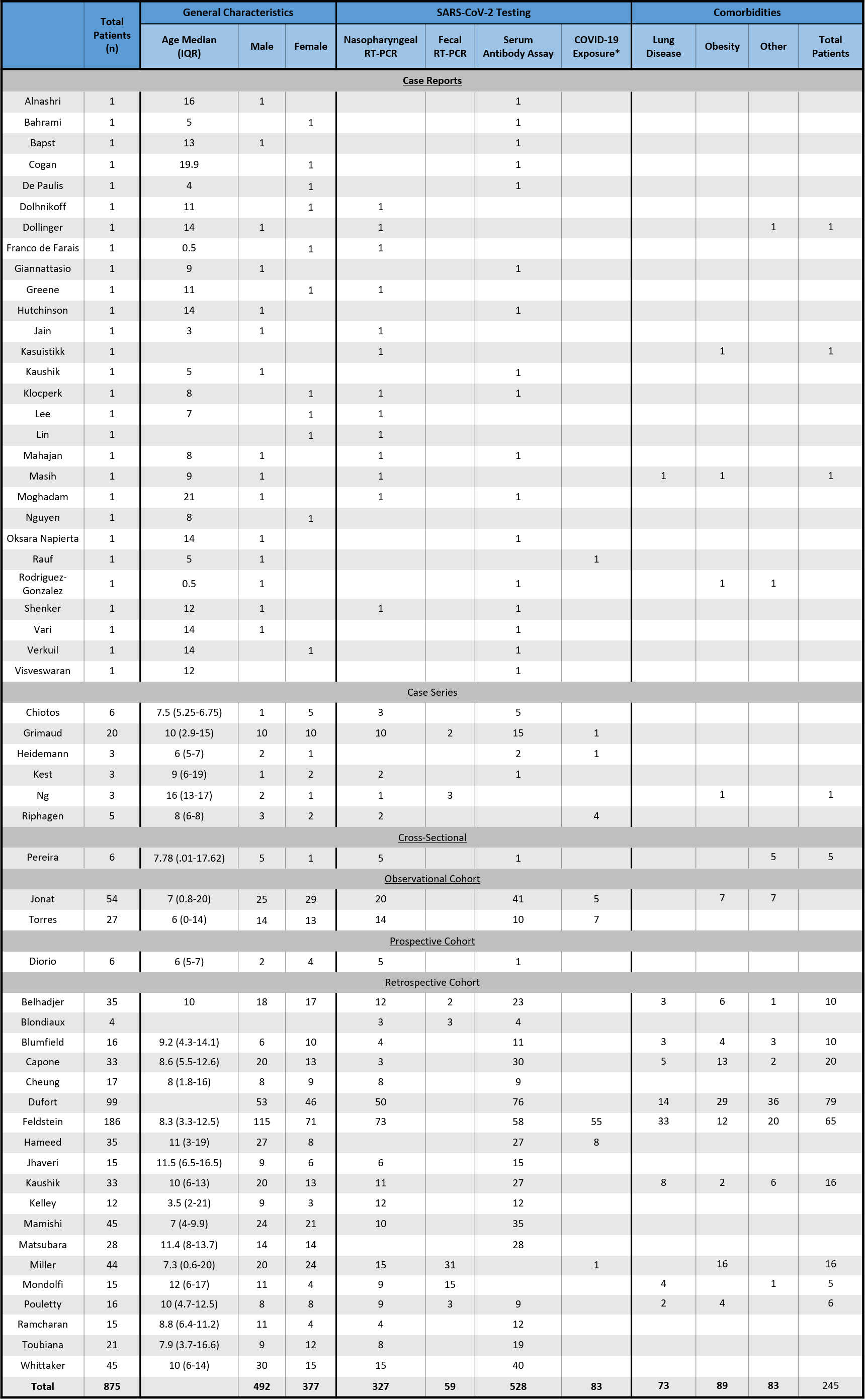
|
3.3Age, gender and comorbidity of MIS-C patients
The patient median age and IQRs are shown in Table 1. The median patient age of the observational studies ranged from 3.5 to 16 years, with Ng et al. [41] reporting the highest median age of 16 years (IQR 13–17). The largest age IQR was reported by Miller et al. [22], with a median age of 7.3 years (IQR 0.6–20), although this was not the largest study (Miller et al.
There were a total of 875 patients included in this review comprised of 492 males (56.6%) and 377 females (43.4%). Three studies did not report gender, accounting for six patients (Table 1).
Four case reports, one case series, one observational cohort, and 10 retrospective cohort studies reported comorbidities. Of the 544 patients assessed for comorbidities, 245 (45.0%) had an underlying condition. Furthermore, the most common comorbidities were obesity (89 patients or 16.4%) and lung diseases consisting of asthma and reactive airway disease (73 patients or 13.4%). Eighty-three patients (15.2%) had a different underlying disease. Examples of other comorbidities include diabetes mellitus and autoimmune conditions such as systemic lupus erythematosus and Crohn’s disease (Table 1).
3.4Clinical presentation
The clinical presentations of MIS-C patients are shown in Fig. 2. Of the 875 patients, 803 (91.8%) presented with fever. GI symptoms were common and included abdominal pain (462 patients or 52.8%), vomiting (392 patients or 44.8%), and diarrhea (346 patients or 39.5%). Mucocutaneous symptoms were common in MIS-C patients with 385 (44.0%) presenting with conjunctivitis and 334 (38.2%) with skin rash [70]. Less common in children were respiratory distress (183 patients or 20.9%) and neurological involvement (153 patients or 17.5%).
Figure 2.
Clinical presentation of patients reported.
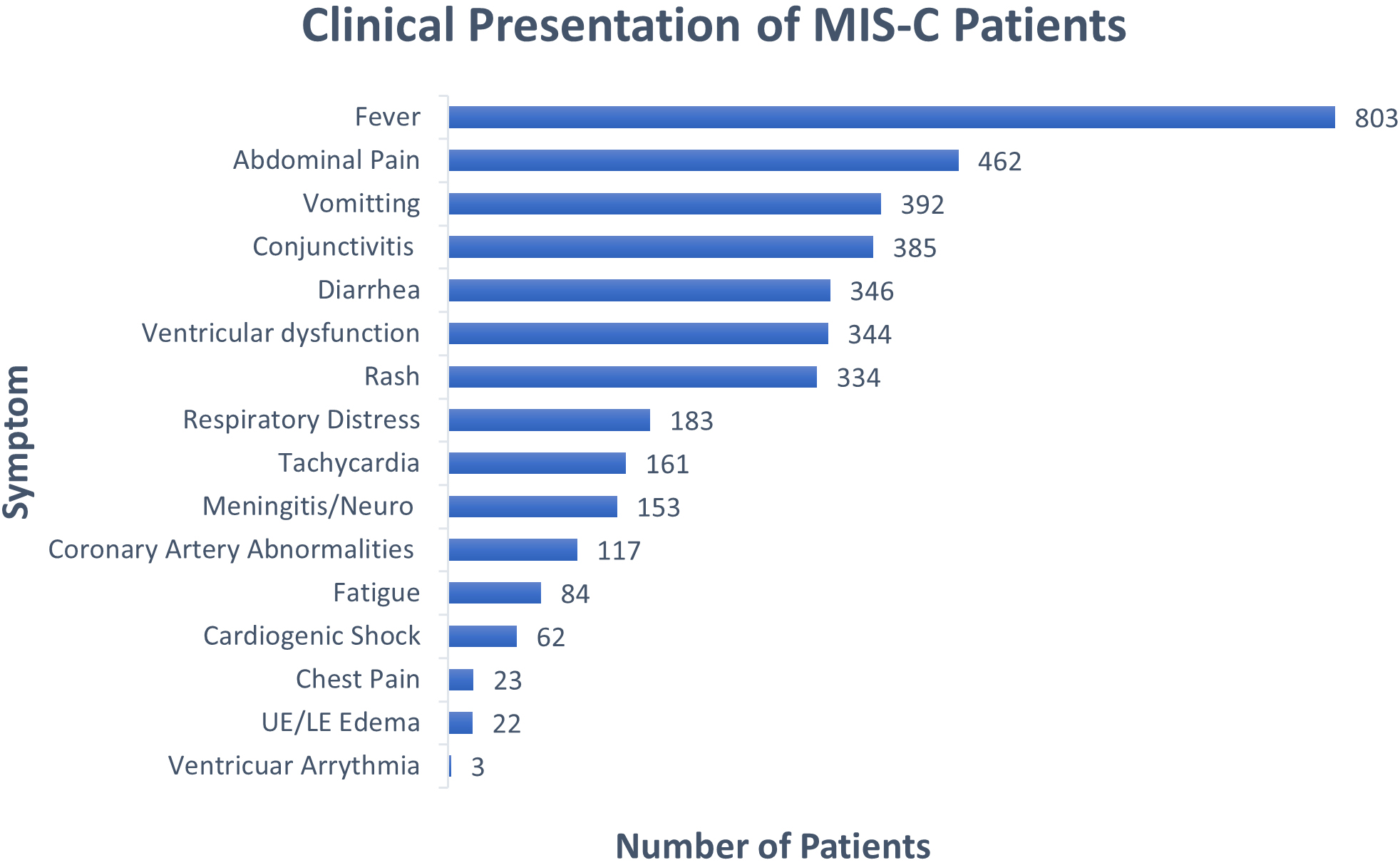
Cardiovascular symptoms included ventricular dysfunction (344 patients or 39.3%), tachycardia (161 patients or 18.4%), coronary artery dilation or ectasia (117 patients or 13.4%), cardiogenic shock (62 patients or 7.1%), chest pain (23 patients or 2.6%), and ventricular arrhythmia (3 patients or 0.3%). Additionally, upper or lower extremity edema was reported in 22 patients (2.5%). The implications of these cardiovascular symptoms associated with MIS-C are addressed in the discussion section, including suggestions from recent literature on this topic.
3.5Laboratory values
The reported laboratory values of all patients included in this review are illustrated in Fig. 3 through 5. Figure 3 displays cardiac biomarkers by study, Fig. 4 displays inflammatory biomarkers by study, and Fig. 5 displays infectious biomarkers by study.
Figure 3.
Median and IQR of cardiac biomarkers of patients by study.
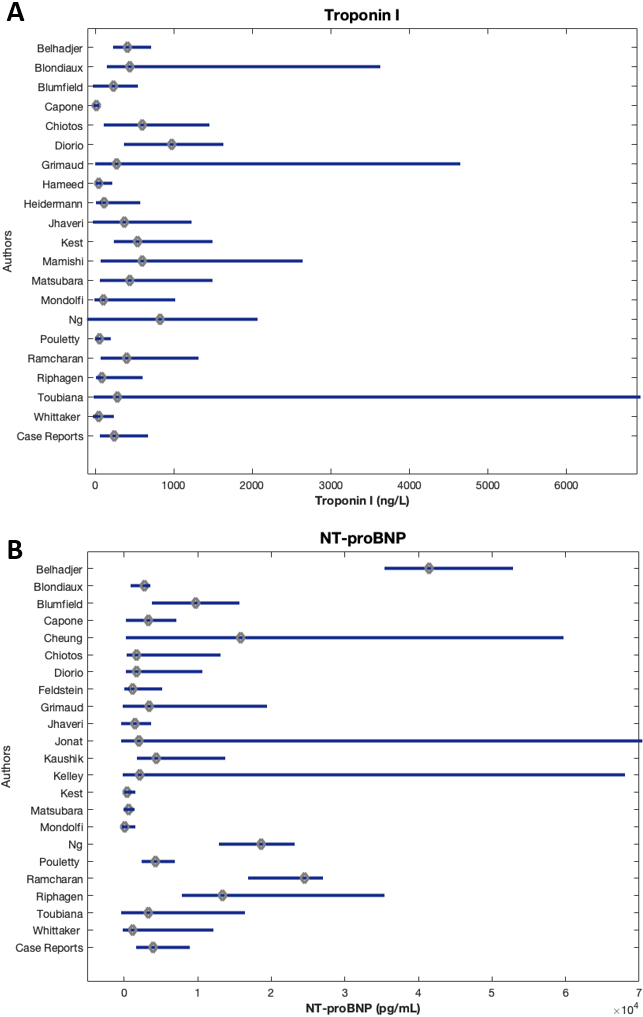
Figure 4.
Median and IQR of inflammatory biomarkers of patients by study.
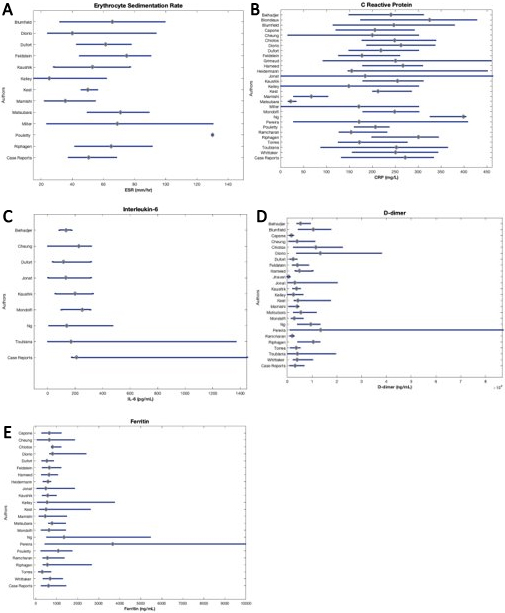
Figure 5.
Median and IQR of infectious biomarkers of patients by study.
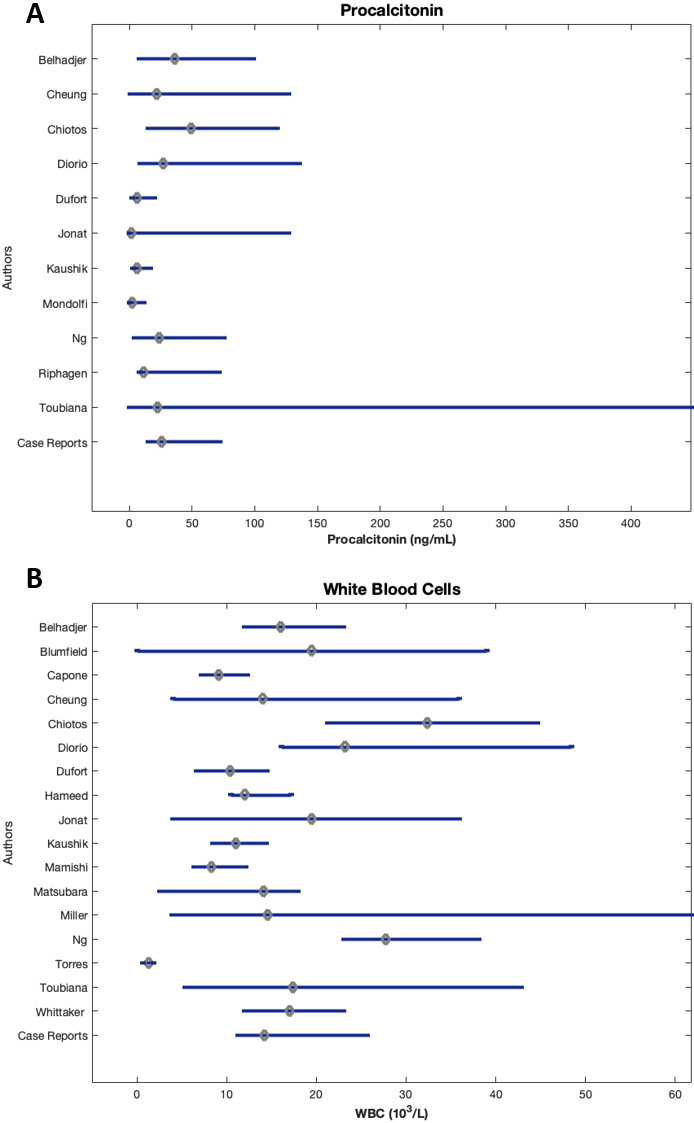
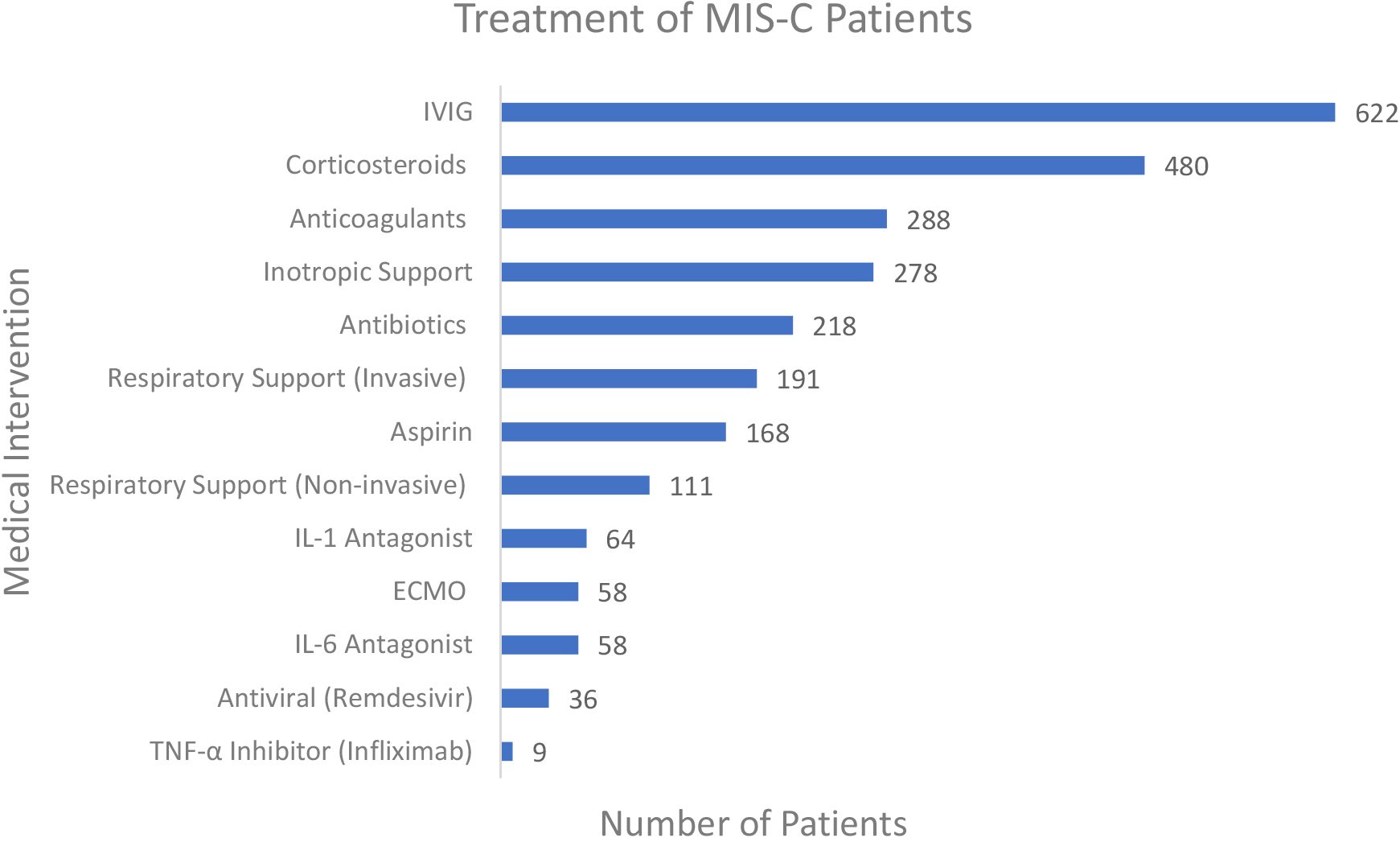
Figure 6.
Treatment and management of patients reported.
3.6Troponin I
Median troponin I values ranged from 9.14 to 970 ng/L (Fig. 3A). Of the 38 studies that reported Troponin I values, 14 reported median values above the reference range (0–150 ng/L), up to over six times the upper limit of normal (ULN) [71]. Dioro et al. [28] reported the highest median troponin I value at 970 ng/L (IQR 400–1600), and Toubiana et. al. [23] reported the largest IQR of 10–6900 with a median of 282 ng/L.
3.7N-terminal prohormone of B-type natriuretic peptide
Zero studies reported a median NT-proBNP level within the reference range (
3.8Erythrocyte sedimentation rate
The median ESR values for all studies ranged from roughly the ULN to over six times the ULN (Fig. 4A). Furthermore, 11 of 12 observational studies reported the Q
3.9C-reactive protein
All but three studies reported the Q
3.10Interleukin-6
Of the studies that reported median IL-6 values, all were above reference values (0–16.4 pg/mL), ranging from 7 to 15 times the ULN (Fig. 4C). Riollano-Cruz et al. [19] reported the highest median IL-6 value at 253 pg/ml (IQR 99.9–311), and Toubiana et al. [23] reported the largest IQR of 4-1366 pg/mL, with a median of 170 pg/mL. Nine cases studies reported IL-6 values, and all were above the ULN.
3.11D-dimer
All studies that reported D-dimer values had median values substantially greater than reference range (
3.12Ferritin
Most studies reporting serum ferritin had elevated median values compared to the reference range (36–311 ng/ml for males
3.13Procalcitonin
All studies reported a median procalcitonin value substantially greater than reference range (0.5– 1.99 ng/mL), from 3 to nearly 25 times the ULN (Fig. 5A). Chiotos et al. [33] reported the highest median procalcitonin value at 49.19 ng/mL (IQR 15.16–117.64). Toubiana et al. [23] noted the largest IQR of 0.1–448 ng/mL with a median of 22.5 ng/mL.
3.14White blood cell count
Only three studies, Dufort et al. [40], Capone et al. [39], and Mamishi et al. [57] reported WBC count within reference range (4500–11,000
3.15Management
The reported interventions are illustrated in Fig. 6. Of the 875 patients diagnosed with MIS-C, 622 (71.1%) received intravenous immunoglobulin (IVIG), which was the most common treatment. Other anti-inflammatory therapies included corticosteroids in 480 patients (54.9%), aspirin in 168 (19.2%), TNF-alpha inhibitor (infliximab) in 9 (1.0%), Interleukin-1 antagonist in 64 (7.3%) and Interleukin-6 antagonist in 58 (6.6%). Cardiovascular inotropic support was also reported in 287 patients (31.8%). Invasive respiratory support was provided in 191 patients (21.8%) and non-invasive respiratory support was provided in 111 (12.7%). Additionally, Extracorporeal Membrane Oxygenation (ECMO) was reported in 58 patients (6.6%). Anticoagulation therapy was reported in 288 patients (32.9%). The antiviral therapy remdesivir was reported in 36 patients (4.1%) and antibiotics were reported in 218 (24.9%).
4.Discussion
In most cases, SARS-CoV-2 is asymptomatic in children and requires hospitalization in 2% [2, 3, 4]. Severe cases of MIS-C report overlapping features with toxic shock syndrome and Kawasaki vasculitis, which may be considered as differential diagnoses for this condition [73].
In this review, 57 studies of MIS-C patients confirmed to have positive testing or an epidemiologic link to SARS-CoV-2 were compiled. These studies identified a total of 875 patients from 15 countries including the United States, United Kingdom, France, Italy, Switzerland, Brazil, Chile, Saudi Arabia, Iran, Belgium, Norway, Czech Republic, Poland, Spain and India. While this study did not investigate discrepancies based on geographical location, this may be an opportunity for future research.
Of the included studies, more male MIS-C cases were reported than female. Although any age can be affected, it appears that the most commonly affected age is 9 years old. The median ages from the reported studies are between 3.5–16 years old. Of the studies that reported comorbidities in patients, 45% had an underlying medical condition. Although SARS-CoV-2 is known to cause respiratory disease, the most common comorbidity was obesity. Because MIS-C presents similarly to autoimmune diseases, accurate diagnosis may be delayed. While currently lacking evidence, it can be speculated that MIS-C is more likely to affect immunocompromised children. The fatality rate from MIS-C is 2.5%, indicating that with early and proper intervention, recovery is expected.
Spiking fever for several days is a common initial presentation of MIS-C. Interestingly, MIS-C patients commonly presented with GI and mucocutaneous symptoms. This presentation is unique to children, as adults exposed SARS-CoV-2 typically present with respiratory distress. As the common manifestations of mucocutaneous involvement of MIS-C include conjunctivitis and rash, the clinical presentation is sometimes comparable to Kawasaki vasculitis.
The biomarkers of inflammation and infection included in this review (CRP, ESR, WBC, Procalcitonin, Interleukin-6, D-dimer and Ferritin) were elevated in nearly all patients who fit diagnostic criteria for MIS-C. It therefore stands that inflammation of organs is a hallmark of MIS-C, which is a shared feature with both toxic shock syndrome and Kawasaki Disease. Elevated cardiac biomarkers were also reported in several studies. Median troponin I and NT-proBNP values were above the reference ranges in many of the included studies. Acute myocardial injury has been associated SARS-CoV-2 infection and one study reported that 10–20% of adult SARS-CoV-2 patients presented with myocarditis or ejection fraction decline [74]. Additionally, several studies mention elevated cardiac biomarkers as one of the hallmarks of MIS-C [4, 75].
At least three recent studies have specifically addressed the cardiac manifestations of MIS-C, including the potential progression to cardiogenic shock and need for intensive care [72, 76, 77]. In addition to acute myocardial injury and myocarditis as identified in this review, these studies also suggest that other cardiac complications may include pericarditis, coronary artery aneurysm, valve dysfunction, pericardial effusion, arrhythmia, ventricular dilation, decreased ejection fraction, and tachycardia. It has been suggested that the myocardial dysfunction mechanism of heart failure in MIS-C due to SARS-CoV-2 may be closely associated with the inflammatory process resulting in myocardial fiber distention and subsequent activation of BNP, rather than a sequela of direct damage caused by the virus. In either case, it is imperative that relevant diagnostic studies be obtained so that cardiovascular decompensation can be identified before evolving to cardiogenic shock. These may include plasma troponin or NT-proBNP, echocardiography, or electrocardiogram. It is important to note that severe cardiovascular abnormalities may necessitate cardiopulmonary support, immunomodulatory and vasoactive agents, and anticoagulation which can reduce the inflammatory response and mortality. A recent study from a pediatric emergency physician stresses the importance of involving a multidisciplinary team including the emergency department, pediatricians and pediatric specialists, and intensivists where clinical instability is observed [76].
The most common treatment for MIS-C, however, is IVIG. In many patients, IVIG was administered concomitantly with other anti-inflammatory agents. Additional treatment of MIS-C patients may also include inotropic support. In this review, 31% of patients received intravenous inotropes, and management sometimes progressed to ECMO (7.7%).
Antibiotic and anti-viral treatments were also reported for the treatment of MIS-C. Broad spectrum antibiotics were initially administered in cases where SARS-CoV-2 was not suspected to be the offending agent. In other cases, antibiotics were administered to treat a co-infection after SARS-CoV-2.
As mentioned previously, two systematic reviews have been recently published that include patients with MIS-C [10, 12]. However, this scoping review provides additional insight into this rapidly evolving condition as it includes several studies not included in either previous review, with MIS-C data through October 5, 2020 (compared to May 31, 2020 and June 20, 2020 in previous reviews). Furthermore, a thorough, quantitative synthesis of laboratory investigations has not been previously reported and is performed here, including nine serum biomarkers. In addition, recent literature describing cardiac manifestations of MIS-C and the potential urgency of associated cardiovascular complications is presented. This information may provide distinct value to the field of pediatric physical medicine and rehabilitation. For example, in patients who are unable to communicate or where COVID-19 precautions may be compromised due to physical limitations as a result of an underlying condition, a prompt and accurate diagnosis of MIS-C when appropriate may allow physiatrists to intervene before complications such as cardiovascular decompensation ensue.
Moreover, MIS-C has several features, both in clinical presentation and laboratory findings, that may overlap with typical findings in patients with neurological and neuromuscular conditions common to physical medicine and rehabilitation. As many as 90 percent of patients with cerebral palsy, for example, have clinically significant GI symptoms [78], and a gastrostomy tube may complicate the recognition of GI symptom etiology [79]. To further challenge physiatrists, several common medications may also introduce confounding factors that the healthcare team may have to decipher. As just one example, baclofen used for spasticity may adversely produce GI symptoms in over 10% of patients [80]. In addition to potentially masking the clinical symptoms of MIS-C, conditions in patients with severe motor and intellectual disabilities (SMID) may have elevated laboratory findings. Lower extremity deep vein thromboses, for example, have been found to cause asymptomatic elevated D-dimer findings in patients with SMID [81, 82]. In addition to laboratory findings secondary to prolonged rest, decreased exercise as a result of the COVID-19 pandemic may cause worsening musculoskeletal function and subsequent decline in physical ability – presenting yet another long-term challenge to the physiatrist and other members of the rehabilitation care team.
Other constraints imposed as a result of epidemiological factors during the COVID-19 pandemic may also delay the diagnosis of MIS-C in pediatric patients undergoing physical rehabilitation. For example, in response to the COVID-19 pandemic, the emergence of telemedicine has helped physical and occupational therapies remain accessible. However, this has created new barriers, as objective measurements or maneuvers typically performed by a skilled professional may now be vulnerable to the subjectivity of the patient’s caregiver [83]. It may therefore be beneficial for physicians to discuss with their team and their patients’ caregivers the common clinical symptoms of MIS-C outlined in this review so that suspected cases may be swiftly assessed and managed accordingly.
The limitations of this scoping review, due to both the study itself and the available data, are important to consider. One concern might be that the data included in this review did not report the stage of SARS-CoV-2 infection in relation to MIS-C diagnosis. For example, some studies include subjects admitted to the hospital during a “multisystem inflammatory state,” and were tested for SARS-CoV-2 upon arrival. In other studies, the method of SARS-CoV-2 testing was inconsistent or undefined, including RT-PCR, serology, and serum antigen testing. It remains unclear whether the subjects had an active infection upon admission or had previously recovered from SARS-CoV-2 infection. Although patients with negative SARS-CoV-2 PCR or negative SARS-CoV-2 serum antibody were excluded in this review, MIS-C can arise as a manifestation of a current or previous infection of up to 4 weeks prior to the onset of symptoms, as defined by the CDC. Additionally, the immunological statuses of the study subjects were not reported, which may obscure the differences in MIS-C presentation between SARS-CoV-2 infected patients. Homogeneity of SARS-CoV-2 testing could aid in eliminating confounding variables for patients whose multisystem inflammatory states are, or are not, a direct sequela of the virus.
Another potential limitation may include studies that do not demarcate whether patients received one or multiple interventions. Some studies include the number of subjects receiving a given intervention, but do not address overlapping management plans that include a combination of one or more interventions. This information, if reported in future studies, could strengthen the external validity of review studies on this topic.
The design and implementation of this current study offers another possible source of uncertainty. While the JBI manual for evidence synthesis was consulted and a PRISMA-ScR checklist was used, an a priori protocol was not established, study selection and data extraction was not run in duplicate, and individual study funding was not reported or investigated. Future studies that address these concerns are warranted and will further benefit the scientific community.
5.Conclusion
In this scoping review, 57 articles including pediatric patients diagnosed with MIS-C were reviewed. Due to diagnostic criteria, nearly all patients will show laboratory evidence of inflammation, and some may also have elevated cardiac enzymes. Differential diagnoses to consider may include toxic shock syndrome and Kawasaki Disease. Most patients are treated with IVIG, and MIS-C rarely progresses to death.
Considering the potential severity of this condition, including fatality, it is our recommendation that children follow CDC guidelines on preventative measures in order to protect themselves and others. As of the date of submission of this manuscript, current CDC guidelines for children 2 years and older include wearing a mask that covers the nose and mouth when in public settings where it is difficult to maintain social distancing guidelines. The CDC also recommends cleaning and disinfecting high touch surfaces daily, which include tables, chairs, doorknobs, light switches, handles, toilets, and sinks. Laundering items including washable plush toys is also recommended. Diligently following CDC guidelines may reduce the risk of MIS-C, and more research is encouraged on this condition.
Conflict of interest
The authors have no conflicts of interest to report.
Ethical considerations
This study, as a literature review, is exempt from Institutional Review Board approval.
References
[1] | Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. (2020) Feb 15; 395: (10223): 470–473. doi: 10.1016/S0140-6736(20)30185-9. |
[2] | Wu Q, Xing Y, Shi L, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. (2020) Jul; 146: (1): e20200961. doi: 10.1542/peds.2020-0961. |
[3] | Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: an overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. (2020) ; 39: : 355–368. |
[4] | Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. (2020) ; 383: : 334–346. |
[5] | Loke Y-HY-H, Berul CICI, Harahsheh ASAS. Multisystem inflammatory syndrome in children: is there a linkage to Kawasaki disease? Trends Cardiovasc Med. (2020) ; 30: : 389–396. |
[6] | Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) Feb 15; 395: (10223): 497–506. doi: 10.1016/S0140-6736(20)30183-5. |
[7] | Riphagen S, Gomez X, Gonzalez-Martinez C, et al. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. (2020) May 23; 395: (10237): 1607–1608. doi: 10.1016/S0140-6736(20)31094-1. |
[8] | Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2. J Pediatric Infect Dis Soc. (2020) Apr 22; piaa045. doi: 10.1093/jpids/piaa045. |
[9] | Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C) |
[10] | Radia T, Williams N, Agrawal P, et al. Multi-system inflammatory syndrome in children & adolescents (MIS-C): a systematic review of clinical features and presentation. Paediatr Respir Rev. (2020) Aug 11; S1526-0542(20)30117-2. doi: 10.1016/j.prrv.2020.08.001. |
[11] | Freedman S, Godfred-Cato S, Gorman R, et al. Multisystem inflammatory syndrome in children and adolescents with COVID-19: Scientific Brief. World Heal Organ. |
[12] | Yasuhara J, Kuno T, Takagi H, et al. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. (2020) ; 55: : 2565–2575. |
[13] | Peters M, Godfrey C, McInerney P, et al. Chapter 11: Scoping Reviews. In: JBI Manual for Evidence Synthesis. JBI. Epub ahead of print 2020. doi: 10.46658/JBIMES-20-12. |
[14] | Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) Jan 1; 4: (1): 1. doi: 10.1186/2046-4053-4-1. |
[15] | Rauf A, Vijayan A, John STST, et al. Multisystem inflammatory syndrome with features of atypical kawasaki disease during COVID-19 pandemic. Indian J Pediatr. (2020) Sep; 87: (9): 745–747. doi: 10.1007/s12098-020-03357-1. |
[16] | Giannattasio A, Maglione M, Zenzeri L, et al. A child with a severe multisystem inflammatory syndrome following an asymptomatic COVID-19 infection: a novel management for a new disease? J Med Virol. (2020) Jun 17; 10.1002/jmv.26189. doi: 10.1002/jmv.26189. |
[17] | Vari D, Miller JMM, Rellosa N, et al. Severe cardiac dysfunction in a patient with multisystem inflammatory syndrome in children associated with COVID-19: retrospective diagnosis of a puzzling presentation. A case report. Prog Pediatr Cardiol. (2020) Sep; 58: : 101270. doi: 10.1016/j.ppedcard.2020.101270. |
[18] | Visveswaran GK, Morparia K, Narang S, et al. SARS-CoV-2 infection and thrombosis: phlegmasia cerulea dolens presenting with venous gangrene in a child. J Pediatr. (2020) Jul 13; 226: : 281–284.e1. doi: 10.1016/j.jpeds.2020.07.032. |
[19] | Riollano-Cruz M, Akkoyun E, Briceno-Brito E, et al. Multisystem Inflammatory Syndrome in Children (MIS-C) Related to COVID-19: A New York City Experience. J Med Virol. (2020) Jun 25; 10.1002/jmv.26224. doi: 10.1002/jmv.26224. |
[20] | Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. (2020) ; 79: : 999–1006. |
[21] | Greene AG, Saleh M, Roseman E, et al. Toxic shock-like syndrome and COVID-19: a case report of multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med. (2020) Jun 6; S0735-6757(20)30492-7. doi: 10.1016/j.ajem.2020.05.117. |
[22] | Miller J, Cantor A, Zachariah P, et al. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children (MIS-C) that is related to COVID-19: a single center experience of 44 cases. Gastroenterology. (2020) Oct; 159: (4): 1571–1574.e2. doi: 10.1053/j.gastro.2020.05.079. |
[23] | Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. (2020) Jun 3; 369: : m2094. doi: 10.1136/bmj.m2094. |
[24] | Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. (2020) Jul 21; 324: (3): 259–269. doi: 10.1001/jama.2020.10369. |
[25] | Moghadam P, Blum L, Ahouach B, et al. Multisystem inflammatory syndrome with particular cutaneous lesions related to COVID 19 in a young adult. Am J Med. (2020) Jul 23; S0002-9343(20)30608-2. doi: 10.1016/j.amjmed.2020.06.025. |
[26] | Cheung EWEW, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. (2020) Jul 21; 324: (3): 294–296. doi: 10.1001/jama.2020.10374. |
[27] | Bapst T, Romano F, Müller M, et al. Special dermatological presentation of paediatric multisystem inflammatory syndrome related to COVID-19: erythema multiforme. BMJ Case Rep. (2020) Jun 29; 13: (6): e236986. doi: 10.1136/bcr-2020-236986. |
[28] | Diorio C, Henrickson SEE, Vella LAA, et al. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. (2020) Oct 5; 140970. doi: 10.1172/JCI140970. |
[29] | Kest H, Kaushik A, DeBruin W, et al. Multisystem inflammatory syndrome in children (MIS-C) associated with 2019 novel coronavirus (SARS-CoV-2) infection. Case Rep Pediatr. (2020) Jul 18; 2020: : 8875987. doi: 10.1155/2020/8875987. |
[30] | Hutchison L, Plichta AMAM, Lerea Y, et al. Neuropsychiatric Symptoms in an Adolescent Boy With Multisystem Inflammatory Syndrome in Children. Psychosomatics. (2020) Jun 30; S0033-3182(20)30201-2. doi: 10.1016/j.psym.2020.06.015. |
[31] | Dolinger MTT, Person H, Smith R, et al. Pediatric crohn’s disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. (2020) Aug; 71: (2): 153–155. doi: 10.1097/MPG.0000000000002809. |
[32] | Hameed S, Elbaaly H, Reid CEL, et al. Spectrum of Imaging Findings on Chest Radiographs, US, CT, and MRI Images in Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with COVID-19. Radiology. (2020) Jun 25; 202543. doi: 10.1148/radiol.2020202543. |
[33] | Chiotos K, Bassiri H, Behrens EMEM, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. (2020) ; 9: : 393–398. |
[34] | Bahrami A, Vafapour M, Moazzami B, et al. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. (2020) Jul 8; 10.1111/jpc.15048. doi: 10.1111/jpc.15048. |
[35] | Shenker J, Trogen B, Schroeder L, et al. Multisystem Inflammatory Syndrome in Children Associated with Status Epilepticus. J Pediatr. (2020) Jul 24; S0022-3476(20)30961-6. doi: 10.1016/j.jpeds.2020.07.062. |
[36] | Kaushik S, Aydin SISI, Derespina KRKR, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2 infection: a multi-institutional study from New York City. J Pediatr. (2020) ; 224: : 24–29. |
[37] | Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: Case Series. Radiology. (2020) Jun 9; 202288. doi: 10.1148/radiol.2020202288. |
[38] | Nguyen DC, Haydar H, Pace ER, et al. Pediatric case of severe COVID-19 with shock and multisystem inflammation. Cureus. (2020) Jun 29; 12: (6): e8915. doi: 10.7759/cureus.8915. |
[39] | Capone CA, Subramony A, Sweberg T, et al. Characteristics, Cardiac involvement, and Outcomes of Multisystem Inflammatory Disease of Childhood (MIS-C) Associated with SARS-CoV-2 Infection. The Journal of pediatrics. Epub ahead of print June 2020. doi: 10.1016/j.jpeds.2020.06.044. |
[40] | Dufort EMEM, Koumans EHEH, Chow EJEJ, et al. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. (2020) ; 383: : 347–358. |
[41] | Ng KFF, Kothari T, Bandi S, et al. COVID-19 multisystem inflammatory syndrome in three teenagers with confirmed SARS-CoV-2 infection. J Med Virol. (2020) ; 92: : 2880–2886. |
[42] | Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. (2020) May 17. doi: 10.1161/CIRCULATIONAHA.120.048360. |
[43] | Ramcharan T, Nolan O, Lai CYCY, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr Cardiol. (2020) Oct; 41: (7): 1391–1401. doi: 10.1007/s00246-020-02391-2. |
[44] | Carter MJ, Fish M, Jennings A, et al. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. (2020) Aug 18. doi: 10.1038/s41591-020-1054-6. |
[45] | Domico M, McCanta AC, Hunt JL, et al. High-grade heart block requiring transvenous pacing associated with multisystem inflammatory syndrome in children during the COVID-19 pandemic. HeartRhythm Case Rep. (2020) Aug 25. doi: 10.1016/j.hrcr.2020.08.015. |
[46] | Kofman AD, Sizemore EK, Detelich JF, et al. A young adult with COVID-19 and multisystem inflammatory syndrome in children (MIS-C)-like illness: a case report. BMC Infect Dis. (2020) Sep 29; 20: (1): 716. doi: 10.1186/s12879-020-05439-z. |
[47] | Minocha PK, Phoon CKL, Verma S, et al. Cardiac Findings in Pediatric Patients With Multisystem Inflammatory Syndrome in Children Associated With COVID-19. Clin Pediatr (Phila). (2020) Sep 25; 9922820961771. doi: 10.1177/0009922820961771. |
[48] | Swann OV, Holden KA, Turtle L, et al. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. (2020) Aug 27; 370: : m3249. doi: 10.1136/bmj.m3249. |
[49] | Biko DM, Ramirez-Suarez KI, Barrera CA, et al. Imaging of children with COVID-19: experience from a tertiary children’s hospital in the United States. Pediatr Radiol. (2020) Sep 18; 1–9. doi: 10.1007/s00247-020-04830-x. |
[50] | Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Heal. (2020) ; 4: : 669–677. |
[51] | Blumfield E, Levin TL. COVID-19 in pediatric patients: a case series from the Bronx, NY. Pediatr Radiol. (2020) Sep; 50: (10): 1369–1374. doi: 10.1007/s00247-020-04782-2. |
[52] | Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Ann Intensive Care. (2020) Jun 1; 10: (1): 69. doi: 10.1186/s13613-020-00690-8. |
[53] | Heidemann SM, Tilford B, Bauerfeld C, et al. Three cases of pediatric multisystem inflammatory syndrome associated with COVID-19 due to SARS-CoV-2. Am J Case Rep. (2020) Aug 13; 21: : e925779. doi: 10.12659/AJCR.925779. |
[54] | Jhaveri S, Ahluwalia N, Kaushik S, et al. Longitudinal Echocardiographic Assessment of Coronary Arteries and Left Ventricular Function following Multisystem Inflammatory Syndrome in Children. J Pediatr. (2020) Aug 5; S0022-3476(20)30984-7. doi: 10.1016/j.jpeds.2020.08.002. |
[55] | Jonat B, Gorelik M, Boneparth A, et al. Multisystem Inflammatory Syndrome in Children Associated With Coronavirus Disease 2019 in a Children’s Hospital in New York City: Patient Characteristics and an Institutional Protocol for Evaluation, Management, and Follow-Up. Pediatr Crit Care Med. (2020) Sep 29. doi: 10.1097/PCC.0000000000002598. |
[56] | Kelly MS, Valle CW, Fernandes ND, et al. Multisystem inflammatory syndrome in children: cardiac biomarker profiles and echocardiographic findings in the acute and recovery phases. J Am Soc Echocardiogr. (2020) ; 33: : 1288–1290. |
[57] | Mamishi S, Movahedi Z, Mohammadi M, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol Infect. (2020) Aug 28; 148: : e196. doi: 10.1017/S095026882000196X. |
[58] | Matsubara D, Kauffman HL, Wang Y, et al. Echocardiographic findings in pediatric multisystem inflammatory syndrome associated with COVID-19 in the United States. J Am Coll Cardiol. (2020) Oct 27; 76: (17): 1947–1961. doi: 10.1016/j.jacc.2020.08.056. |
[59] | Alnashri H, Aljohani N, Tayeb S, et al. A challenging case of multisystem inflammatory syndrome in children related to coronavirus disease-19 hospitalized under adult medical service. IDCases. (2020) ; 22: : e00957. doi: 10.1016/j.idcr.2020.e00957. |
[60] | Cogan E, Foulon P, Cappeliez O, et al. Multisystem inflammatory syndrome with complete kawasaki disease features associated with SARS-CoV-2 infection in a young adult. A case report. Front Med (Lausanne). (2020) Jul 14; 7: : 428. doi: 10.3389/fmed.2020.00428. |
[61] | De Paulis M, Oliveira DBL, Vieira RP, et al. Multisystem inflammatory syndrome associated with covid-19 with neurologic manifestations in a child: a brief report. Pediatr Infect Dis J. (2020) Oct; 39: (10): e321–e324. doi: 10.1097/INF.0000000000002834. |
[62] | Dolhnikoff M, Ferreira Ferranti J, de Almeida Monteiro RA, et al. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc Heal. (2020) ; 4: : 790–794. |
[63] | Farias ECF, Justino MCA, Mello MLFMF. Multisystem inflammatory syndrome in a child associated with coronavirus disease 19 in the brazilian amazon: fatal outcome in an infant. Rev Paul Pediatr. (2020) ; 38: : e2020165. doi: 10.1590/1984-0462/2020/38/2020165. |
[64] | Jain S, Sen S, Lakshmivenkateshiah S, et al. Multisystem Inflammatory Syndrome in Children With COVID-19 in Mumbai, India. Indian Pediatr. (2020) Aug 11; S097475591600230. |
[65] | Rojahn AE, Gammelsrud KW, Brunvand LI, et al. Multiorgan inflammatory syndrome associated with SARS-CoV-2 in a child. Tidsskr Nor Laegeforen. (2020) Jun 25; 140: (11). doi: 10.4045/tidsskr.20.0485. |
[66] | Klocperk A, Parackova Z, Dissou J, et al. Case report: systemic inflammatory response and fast recovery in a pediatric patient with COVID-19. Front Immunol. (2020) Jul 3; 11: : 1665. doi: 10.3389/fimmu.2020.01665. |
[67] | Lee PY, Day-Lewis M, Henderson LA, et al. Distinct clinical and immunological features of SARS-CoV-2-induced multisystem inflammatory syndrome in children. J Clin Invest. (2020) Oct 5; 141113. doi: 10.1172/JCI141113. |
[68] | Lin J, Lawson EC, Verma S, et al. Cytotoxic Lesion of the Corpus Callosum in an Adolescent with Multisystem Inflammatory Syndrome and SARS-CoV-2 Infection. AJNR Am J Neuroradiol. (2020) Aug 20. doi: 10.3174/ajnr.A6755. |
[69] | Mahajan N, Chang HT, Leeman R, et al. Case of multisystem inflammatory syndrome in children presenting as fever and abdominal pain. BMJ Case Rep. (2020) Sep 8; 13: (9): e237306. doi: 10.1136/bcr-2020-237306. |
[70] | Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. (2020) Apr 7; 323: (13): 1239–1242. doi: 10.1001/jama.2020.2648. |
[71] | Andropoulos DB. Appendix B: Pediatric Normal Laboratory Values. In: Gregory’s Pediatric Anesthesia. Oxford, UK: Wiley-Blackwell, (2011) , pp. 1300–1314. |
[72] | Pereira MFB, Litvinov N, Farhat SCL, et al. Severe clinical spectrum with high mortality in pediatric patients with covid-19 and multisystem inflammatory syndrome. Clinics. (2020) ; 75: : 1–7. |
[73] | Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. (2020) May 17. doi: 10.1161/CIRCULATIONAHA.120.048360. |
[74] | Mahajan K, Chand Negi P, Ganju N, et al. Cardiac biomarker-based risk stratification algorithm in patients with severe COVID-19. Diabetes Metab Syndr. (2020) ; 14: : 929–931. |
[75] | Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. (2020) Jul 1; 5: (7): 831–840. doi: 10.1001/jamacardio.2020.1286. |
[76] | Simon Junior H, Sakano TMS, Rodrigues RM, et al. Multisystem inflammatory syndrome associated with COVID-19 from the pediatric emergency physician’s point of view. J Pediatr (Rio J). (2020) Sep 11; S0021-7557(20)30203-5. doi: 10.1016/j.jped.2020.08.004. |
[77] | Sperotto F, Friedman KG, Son MBF, et al. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. (2020) Aug 15; 1–16. doi: 10.1007/s00431-020-03766-6. |
[78] | Cerebral palsy: Clinical features and classification – UpToDate. Availablef rom: https://www.uptodate.com/contents/cerebral-palsy-clinical-features-and-classification?search=cerebralpalsy&source=search_result&selectedTitle=1∼150&usage_type=default&display_rank=1 (accessed 31 October 2020). |
[79] | Eisenberg PG. Causes of diarrhea in tube-fed patients: a comprehensive approach to diagnosis and management. Nutr Clin Pract. (1993) Jun; 8: (3): 119–23. doi: 10.1177/0115426593008003119. |
[80] | Baclofen: Pediatric drug information – UpToDate. Available from: https://www.uptodate.com/contents/baclofen-pediatric-drug-information?search=baclofenpump&source=search_result&selectedTitle=4∼150&usage_type=default&display_rank=4#F138920 (accessed 31 October 2020). |
[81] | Ohmori H, Kanaoka Y, Murata Y, et al. Deep vein thrombosis in patients with severe motor and intellectual disabilities, especially diagnosis and prevention of recurrence for chronic thrombosis-serial changes of sonography and d-dimer. Ann Vasc Dis. (2015) ; 8: : 290–296. |
[82] | Ohmori H, Kanaoka Y, Yamasaki M, et al. Prevalence and characteristic features of deep venous thrombosis in patients with severe motor and intellectual disabilities. Ann Vasc Dis. (2018) ; 11: : 281–285. |
[83] | Nulle J, Nelson VS. Video visits and access to care in pediatric rehabilitation therapies in the time of a pandemic. J Pediatr Rehabil Med. (2020) Oct 13. doi: 10.3233/PRM-200759. |
Appendices
Appendix: Search strategy
Revised Research Question: What are the epidemiology factors, clinical presentation, and laboratory characteristics for multisystem inflammatory syndrome in children (MIS-C) with exposure to coronavirus disease (COVID-19)?
Revised Hypothesis: MIS-C related to SARS-CoV-2 has a distinct presentation detectable through clinical findings and laboratory investigations, and is treated with a regimen of immunosuppressants, IVIG and inotropes.
A document search was conducted on Scopus (www.scopus.com) using the query string: TITLE-ABS-KEY (multisystem AND inflammatory AND syndrome AND in AND children) and limiting results to after 12/31/2019.
Citations were exported as a BibTeX file and imported into Mendeley Reference Management Software. The “Check for Duplicates” function was used after compiling all reference data and subsequently reviewed by title and abstract.



