Defining the CD39/CD73 Axis in SARS-CoV-2 Infection: The CD73- Phenotype Identifies Polyfunctional Cytotoxic Lymphocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Intracellular Cytokine Staining
2.3. Data Evaluation and Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
3.2. Increased Expression of Granzyme B and Perforin by Cytotoxic Lymphocytes from COVID-19 Patients
3.3. Expression of CD39 and CD73 by Lymphocyte Subsets from COVID-19 Patients and Healthy Controls
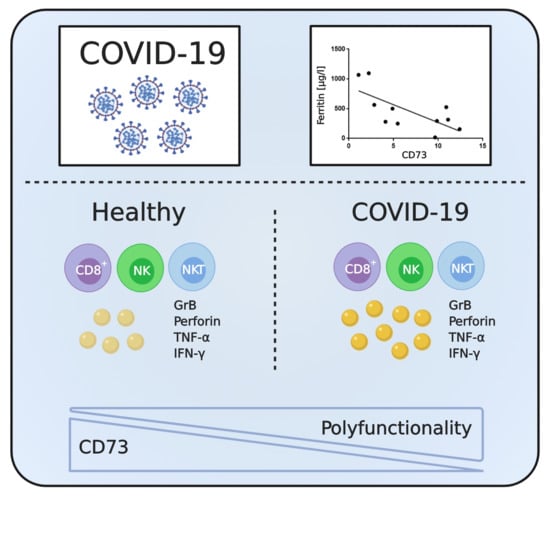
3.4. Lack of CD73 Expression on CD8+ T Cells and NKT Cells in COVID-19 Patients Correlates with Clinically-Manifested Systemic Inflammation
3.5. Absence of CD73 Identifies Highly-Functional Cytotoxic Lymphocytes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. New Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019-Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China: a descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- WHO. Report of the Who-China Joint Mission on Coronavirus Disease 2019 (covid-19). Available online: https://www.Who.Int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (accessed on 27 May 2020).
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [Green Version]
- Cao, X. COVID-19: Immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020, 20, 269–270. [Google Scholar] [CrossRef] [Green Version]
- Rouse, B.T.; Sehrawat, S. Immunity and immunopathology to viruses: What decides the outcome? Nat. Rev. Immunol. 2010, 10, 514–526. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.-S.; Ackermann, C.; Tóth, I.; Dierks, P.; Eberhard, J.M.; Wroblewski, R.; Scherg, F.; Geyer, M.; E Schmidt, R.; Beisel, C.; et al. Down-regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J. Leukoc. Boil. 2017, 101, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Wiesch, J.S.Z.; Thomssen, A.; Hartjen, P.; Tóth, I.; Lehmann, C.; Meyer-Olson, D.; Colberg, K.; Frerk, S.; Babikir, D.; Schmiedel, S.; et al. Comprehensive Analysis of Frequency and Phenotype of T Regulatory Cells in HIV Infection: CD39 Expression of FoxP3+ T Regulatory Cells Correlates with Progressive Disease. J. Virol. 2010, 85, 1287–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tóth, I.; Le, A.Q.; Hartjen, P.; Thomssen, A.; Matzat, V.; Lehmann, C.; Scheurich, C.; Beisel, C.; Busch, P.; Degen, O.; et al. Decreased frequency of CD73+ CD8+ T cells of HIV-infected patients correlates with immune activation and T cell exhaustion. J. Leukoc. Boil. 2013, 94, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Abouelkhair, M.A. Targeting Adenosinergic Pathway and Adenosine A2A receptor Signaling for the Treatment of COVID-19: A hypothesis. Med. Hypotheses 2020, 144, 110012. [Google Scholar] [CrossRef] [PubMed]
- Lauer, G.M.; Ouchi, K.; Chung, R.T.; Nguyen, T.N.; Day, C.L.; Purkis, D.R.; Reiser, M.; Kim, A.Y.; Lucas, M.; Klenerman, P.; et al. Comprehensive Analysis of CD8+-T-Cell Responses against Hepatitis C Virus Reveals Multiple Unpredicted Specificities. J. Virol. 2002, 76, 6104–6113. [Google Scholar] [CrossRef] [Green Version]
- Roederer, M.; Nozzi, J.L.; Nason, M.C. SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011, 79, 167–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 827. [Google Scholar] [CrossRef]
- Moncrieffe, H.; Nistala, K.; Kamhieh, Y.; Evans, J.; Eddaoudi, A.; Eaton, S.; Wedderburn, L. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J. Immunol. 2010, 185, 134–143. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [Green Version]
- Schneider, E.; Rissiek, A.; Winzer, R.; Puig, B.; Rissiek, B.; Haag, F.; Mittrücker, H.-W.; Magnus, T.; Tolosa, E. Generation and Function of Non-cell-bound CD73 in Inflammation. Front. Immunol. 2019, 10, 1729. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhou, S.; Liu, G.; Kong, F.; Chen, S.; Yan, H. Multiple steps determine CD73 shedding from RPE: Lipid raft localization, ARA1 interaction, and MMP-9 up-regulation. Purinergic Signal. 2018, 14, 443–457. [Google Scholar] [CrossRef]
- Boivin, W.A.; Cooper, D.M.; Hiebert, P.R.; Granville, D.J. Intracellular versus extracellular granzyme B in immunity and disease: Challenging the dogma. Lab. Investig. 2009, 89, 1195–1220. [Google Scholar] [CrossRef] [Green Version]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, P.J.; Macatangay, B.; Saze, Z.; Jackson, E.K.; Riddler, S.A.; Buchanan, W.G.; Hilldorfer, B.B.; Mellors, J.W.; Whiteside, T.L.; Rinaldo, C.R. CD4⁺CD73⁺ T cells are associated with lower T-cell activation and C reactive protein levels and are depleted in HIV-1 infection regardless of viral suppression. AIDS 2013, 27, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, M.; Dong, Y.; Zhou, H.; Zhang, Z.; Tian, C.; Qin, R.; Wang, H.; Shen, Y.; Du, K.; et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab. Res. Rev. 2020, e3319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peric, S.; Stulnig, T.M. Diabetes and COVID-19. Wien. Klin. Wochenschr. 2020, 1–6. [Google Scholar] [CrossRef]
- Tak, E.; Ridyard, D.; Kim, J.-H.; Zimmerman, M.; Werner, T.; Wang, X.X.; Shabeka, U.; Seo, S.-W.; Christians, U.; Klawitter, J.; et al. CD73-Dependent Generation of Adenosine and Endothelial Adora2b Signaling Attenuate Diabetic Nephropathy. J. Am. Soc. Nephrol. 2013, 25, 547–563. [Google Scholar] [CrossRef] [Green Version]
- Tai, N.; Wong, F.S.; Wen, L. TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice. J. Immunol. 2013, 191, 2926–2937. [Google Scholar] [CrossRef] [Green Version]
- Perrot, I.; Michaud, H.A.; Giraudon-Paoli, M.; Augier, S.; Docquier, A.; Gros, L.; Courtois, R.; Dejou, C.; Jecko, D.; Becquart, O.; et al. Blocking antibodies targeting the cd39/cd73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep. 2019, 27, 2411–2425. [Google Scholar] [CrossRef] [Green Version]
- Slaats, J.; Ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. Il-1beta/il-6/crp and il-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathogens 2016, 12, e1005973. [Google Scholar] [CrossRef]







| Patient | Days Since Symptom Onset | Age | Sex | Relevant Pre-Existing Medical Conditions | Relevant Medication | CRP [mg/L] | IL-6 [ng/L] | Serum Ferritin [µg/L] | Oxygen Demand * | Lowest SpO2 [%] |
|---|---|---|---|---|---|---|---|---|---|---|
| C19-01 | 9 | 61 | m | None | Ampicillin/sulbactam, Piperacillin/tazobactam, Levofloxacin | 58 | n/a | n/a | – | 89 |
| C19-02 | 8 | 51 | m | None | None | 53 | 40.2 | 501 | – | 95 |
| C19-03 | 6 | 78 | m | COPD GOLD 2B, Coronary heart disease, hypertension | Ampicillin/sulbactam | 97 | 63.3 | 1096 | 2 L/min | 82 |
| C19-04 | 13 | 58 | m | None | Ampicillin/sulbactam, Piperacillin/tazobactam, meropenem | 21 | 21 | 1140 | 6 L/min | 75 |
| C19-05 | 5 | 58 | m | None | None | 108 | 38.3 | 316 | – | 95 |
| C19-06 | 12 | 49 | m | NSCLC, Hodgkin’s disease 1991 (CR) | Hydroxychloroquine, azithromycin, cisplatin, paclitaxel, pembrolizumab (03/2020) | 33 | 9.1 | 155 | 3 L/min | 93 |
| C19-07 | 10 | 75 | m | Myocardial infarction (2004), urothelial carcinoma | None | 56 | 335 | 564 | – | 95 |
| C19-08 | 12 | 50 | m | Asthma | None | 12 | 1.9 | 280 | 2 L/min | 95 |
| C19-09 | 11 | 63 | m | Asthma, type 2 diabetes | None | 133 | 125 | 1068 | 4 L/min | 84 |
| C19-10 | 9 | 46 | m | Hypertension | None | 8 | 5.4 | n/a | – | 94 |
| C19-11 | 20 | 39 | m | None | Ampicillin/sulbactam | 46 | 4.7 | 249 | – | 94 |
| C19-12 | 20 | 53 | m | Type 2 diabetes | None | 7 | 1.5 | 526 | – | – |
| C19-13 | 14 | 44 | w | Multiple sclerosis, Breast cancer (2010) | Rituximab (last dose: 01/2020) | 33 | 371.9 | 292,7 | – | 94 |
| C19-14 | 10 | 56 | w | None | Hydroxychloroquine | 38 | 19.9 | 16,7 | – | 93 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi, P.; Hartjen, P.; Kohsar, M.; Kummer, S.; Schmiedel, S.; Bockmann, J.-H.; Fathi, A.; Huber, S.; Haag, F.; Schulze zur Wiesch, J. Defining the CD39/CD73 Axis in SARS-CoV-2 Infection: The CD73- Phenotype Identifies Polyfunctional Cytotoxic Lymphocytes. Cells 2020, 9, 1750. https://doi.org/10.3390/cells9081750
Ahmadi P, Hartjen P, Kohsar M, Kummer S, Schmiedel S, Bockmann J-H, Fathi A, Huber S, Haag F, Schulze zur Wiesch J. Defining the CD39/CD73 Axis in SARS-CoV-2 Infection: The CD73- Phenotype Identifies Polyfunctional Cytotoxic Lymphocytes. Cells. 2020; 9(8):1750. https://doi.org/10.3390/cells9081750
Chicago/Turabian StyleAhmadi, Parimah, Philip Hartjen, Matin Kohsar, Silke Kummer, Stefan Schmiedel, Jan-Hendrik Bockmann, Anahita Fathi, Samuel Huber, Friedrich Haag, and Julian Schulze zur Wiesch. 2020. "Defining the CD39/CD73 Axis in SARS-CoV-2 Infection: The CD73- Phenotype Identifies Polyfunctional Cytotoxic Lymphocytes" Cells 9, no. 8: 1750. https://doi.org/10.3390/cells9081750