Abstract
The coronavirus 2019 disease (COVID-19) is characterised by a heterogeneous clinical presentation, a complex pathophysiology and a wide range of imaging findings, depending on disease severity and time course. We conducted a retrospective evaluation of hospitalized patients with proven SARS-CoV-2 infection, clinical signs of COVID-19 and computed tomography (CT) scan-proven pulmonary involvement, in order to identify relationships between clinical, serological, imaging data and disease outcomes in patients with COVID-19. Clinical and serological records of patients admitted to two COVID-19 Units of the Abruzzo region in Italy with proven SARS-CoV-2 pulmonary involvement investigated with CT scan, assessed at the time of admission to the hospital, were retrospectively evaluated. Sixty-one patients (22 females and 39 males) of median age 65 years were enrolled. Fifty-six patients were discharged while death occurred in 5 patients. None of the lung abnormalities detected by CT was different between discharged and deceased patients. No differences were observed in the features and extent of pulmonary involvement according to age and gender. Logistic regression analysis with age and gender as covariates demonstrated that ferritin levels over the 25th percentile were associated with the involvement of all 5 pulmonary lobes (OR = 14.5, 95% CI 2.3–90.9, p = 0.004), the presence of septal thickening (OR = 8.2, 95% CI 1.6–40.9, p = 0.011) and the presence of mediastinal lymph node enlargement (OR = 12.0, 95% CI 1.1–127.5, p = 0.039) independently of age and gender. We demonstrated that ferritin levels over the 25th percentile are associated with a more severe pulmonary involvement, independently of age and gender and not associated with disease outcomes. The identification of reliable biomarkers in patients with COVID-19 may help guiding clinical decision, tailoring therapeutic approaches and ultimately improving the care and prognosis of patients with this disease.
Similar content being viewed by others
Introduction
In late 2019, an outbreak of viral pneumonia caused by a novel coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified in Wuhan, China1; subsequently, the coronavirus 2019 disease (COVID-19) spread worldwide, and on March 11, 2020, the WHO declared COVID-19 a global pandemic2.
COVID-19 clinical presentation ranges from asymptomatic infection to interstitial pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS) and sepsis3. The pathophysiology is complex, involving immune and hematologic systems, epithelial cells and vascular system4. Several pro-inflammatory cytokines and chemokines, interleukin (IL)-1β, IL-2, IL-6, IL-7, IL-10, tumour necrosis factor-α (TNFα), granulocyte colony-stimulating factor (G-CSF), interferon (IFN) γ-induced protein 10 (IP-10/CXCL10), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α/CCL3), have been detected in the bloodstream and target tissue of COVID-19 patients1,3,5. Severe lymphopenia is a peculiar feature of patients with severe disease since the majority of patients with mild disease present without this manifestation6,7,8. The inflammatory cytokine storm is likely one of the main factors behind the observed lymphopenia. The serum level of pro-inflammatory cytokines, such as TNF-α and IL-6, have been closely correlated with lymphopenia, while recovered patients show close to normal levels of such cytokines. Importantly, severe lymphopenia predisposes to ICU stay6 and mortality, where during hospitalization, non‐survivors demonstrated a more significant deterioration in lymphopenia compared with those who survived7,8.
In more detail, T lymphocytes including CD4+ and CD8+ subtypes and NK cells are reduced in patients with severe disease9. Conversely, monocytes and macrophages are increased, explaining elevated levels of some pro-inflammatory cytokines. All this fits with the evidence that the predominant pattern of lung lesions in patients with COVID-19 patients is a diffuse alveolar damage, with the presence of platelet–fibrin thrombi in small arterial vessels, and the great majority of the inflammatory cells infiltrating the lungs are monocytes, macrophages and CD4+ lymphocytes10,11.
Further events lead to activation of the coagulation cascade through endothelial and tissue factor pathways, paralleling the systemic inflammation12, 13. Moreover, some Authors suggested that COVID-19 might be considered as a peculiar cardiovascular (CV) disease based on predisposing patients to arterial and venous thrombosis and particularly as consequence of hypercoagulability14,15 and endothelial dysfunction16,17. Indeed, it has been reported that CV complications are quickly emerging as a key threat in COVID-19 beyond respiratory involvement. The mechanisms underlying the disproportionate effect of SARS-CoV-2 infection on patients with CV comorbidities are not yet fully elucidated. Nevertheless, Iaccarino et al. recently showed that in patients with COVID-19 mortality is predicted by age and comorbidities, particularly diabetes mellitus, chronic obstructive pulmonary disease, and chronic kidney disease but not hypertension18.
The massive release of pro-inflammatory mediators and the aberrant activation of the immune and coagulation systems, resembles the so-called cytokine release syndrome, a group of conditions sharing the same pathogenic mechanism, although with a different aetiology19. This cytokine storm accounts for the two main causes of mortality in COVID-19, ARDS and secondary haemophagocytic lymphohistiocytosis, the latter occurring in a small subset of patients20. Furthermore, since increased levels of ferritin along with a cytokine storm have been described in patients with severe COVID-1921, it has been speculated that COVID-19 may be included in the spectrum of the hyperferritinemic syndromes20.
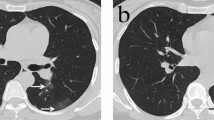
COVID-19 pneumonia displays a wide range of imaging findings, depending on disease severity and time course, and can overlap with a variety of infectious and non-infectious pulmonary diseases. The choice of imaging in COVID-19 is left to the judgement of clinical teams at the point-of-care accounting for the differing attributes of X-ray and computed tomography (CT), local resources, and expertise22. However, CT is more sensitive for early parenchymal lung disease, disease progression, and alternative diagnoses including acute heart failure from COVID-19 myocardial injury and pulmonary thromboembolism23. Typically, COVID-19 pneumonia is characterised by bilateral peripheral patchy ground-glass opacities (GGO) with or without consolidation; superimposed interlobular septal thickening can also be present, resulting in a crazy-paving pattern24,25. Pleural effusion or septal thickening have been rarely described26, while recently an Italian study described lymphadenopathy in up to 58% of cases27. GGO alone, followed by GGO with consolidation, is the most frequent finding in mild cases, while in severe cases with ARDS widespread dense consolidative opacification are present23.
The aim of this study was to describe clinical, serological and CT imaging features of a cohort of patients with COVID-19 pneumonia and identify possible relationships between the variables and disease outcomes (admission to intensive care unit (ICU) and/or death).
Methods
Patient cohort and data collection
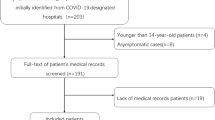
Clinical and serological records of patients admitted to two COVID-19 Units of the Abruzzo region in Italy (S. Salvatore Hospital, L’Aquila and SS Filippo e Nicola Hospital, Avezzano) with proven SARS-CoV-2 pulmonary involvement investigated with CT scan, assessed at the time of admission to the hospital, were retrospectively evaluated. All patients presented the clinical and laboratory signs of COVID-19 infection, as assessed by positive SARS-CoV-2 RT-PCR testing.
Collected data included: age, gender, days of symptoms before the CT scan, red blood cell count, hemoglobin (Hb), platelet (PLT) count, white blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), absolute monocyte count (AMC), lymphocyte subpopulation (CD3+, CD4+, CD8+, CD16+, CD19+), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), ferritin, D-dimer, fibrinogen, lactate dehydrogenase (LDH), procalcitonin, arterial oxygen partial pressure:fractional inspired oxygen ratio (PaO2/FiO2). Neutrophils:lymphocytes (N:L) ratio and ferritin:ESR ratio were calculated based on the available data. Anemia was defined according to the WHO criteria as Hb values below 12 g/dl in females and 13 g/dl in males28. All the other cytopenias were defined according to the reference levels of the local laboratory (leukopenia: WBC < 4.00 × 103/μL; neutropenia: ANC < 2.00 × 103/μL; lymphocytopenia: ALC < 0.80 × 103/μL).
All patients underwent chest CT and the images were examined by an independent radiologist (FB) with more than 10-year experience on chest CT imaging. The information collected were: lobes involved and presence/absence of: GGOs, parenchymal consolidation, septal thickening, pleural effusion, mediastinal lymph node enlargement29. Two semi-quantitative scoring systems were used to quantify the extension of the disease: i. each of the 5 lobes (right upper lobe, right middle lobe, right lower lobe, left upper lobe and left lower lobe) was scored on a scale of 0–3 (0: no lesion, 1: < 1/3 of the lobe volume involved, 2: > 1/3 and < 2/3 of the lobe volume involved, 3: > 2/3 of the lobe volume involved) (score A)30; ii. each of the five lung lobes was assessed for degree of involvement and classified as none (0%), minimal (1–25%), mild (26–50%), moderate (51–75%), or severe (76–100%). No involvement corresponded to a lobe score of 0, minimal involvement to a lobe score of 1, mild involvement to a lobe score of 2, moderate involvement to a lobe score of 3, and severe involvement to a lobe score of 4 (Score B)31. An overall lung “total severity score” was reached by summing the five lobe scores (Score A, range of possible scores, 0–15; Score B range of possible scores, 0–20).
All methods were carried out in accordance with Good Clinical Practice guidelines and all patients provided written informed consent in accordance with the declaration of Helsinki. Ethical review and approval (ASL1 Avezzano-Sulmona-L’Aquila) was obtained in accordance with local legislation and institutional requirements.
Statistical analysis
Data were analyzed with STATA/SE 16.1. The Mann Whitney U test was used to compare continuous variables while the χ2 and Fisher’s exact tests were used as needed for categorical variables. Bivariate correlation (Spearman’s ρ) and binary logistic regression analysis were also performed. All tests were two tailed and values of p < 0.05 were considered statistically significant.
Results
Characteristics of the study cohort
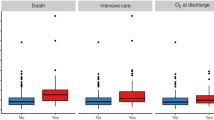
Sixty-one patients (22 females and 39 males) with a median age of 65 years (range 32–93 years) were enrolled (Table 1). Sixty percent of patients displayed at least 2 comorbidities, and one quarter of the overall cohort had four or more comorbidities. Upon admission to the COVID-19 ward patients received supportive therapy, including supplemental oxygen. None of the patients received immunomodulatory treatment (including but not limited to corticosteroids prior the CT scan). Acute phase reactants were abnormal in the majority of patients while with regard to blood cell counts, 26/60 patients (43%) did not display any cytopenia. Anemia was detectable in 22/60 patients (37%), leukopenia in 9 patients (15%) and thrombocytopenia in 11/60 patients (18%). In 27/60 patients (45%) only one cytopenia was present, in 6/60 patients (10%) cytopenia involved 2 cell lines, in only 1 patient involved all the 3 cell lines. A reduction of ANC was observed in 6/60 patients (10%) while ALC was below the lower limit in 15/60 patients (25%). Of interest, 7/60 patients (12%) displayed lymphocytosis. The mean N:L ratio was 5.07. The ferritin value was available in 51 out of 61 patients and increased in 38 of them (74%). The average time between the onset of respiratory symptoms and the chest CT was 5.69 days. Overall, pulmonary involvement was moderate to severe, with 59% of subjects having 5 lobes involved and 58 patients (95%) displaying bilateral disease. About half of the patients displayed only GGO and the other half both GGO and consolidation. Pleural effusion was observed only in a small number of patients (N = 11; 18%) while 46 patients (75%) displayed septal thickening. Mediastinal lymph node enlargement was detected in 20 (33%) of patients. Mean PaO2/FiO2 value was 300.1, with only 5/58 (9%) patients displaying normal values (> 400), 23/58 (40%) patients displaying slight impairment (300–400); 24/58 (41%) patients displaying moderate impairment (200–300) and 6/58 (10%) patients displaying severe impairment. The average values of the two CT severity scores were 5.85 and 6.69 respectively. Table 2 shows the correlations between pulmonary involvement and different serological variables. As far as the disease outcomes are concerned, 56 patients (92%) were discharged while 5 patients (8%) deceased. Of the 56 discharged patients, five (9%) required admission to ICU before discharge.
Subgroup analysis by age and gender
When patients were divided according to the age (below or over 65 years, Table 3) a few variables resulted significantly different. In particular, lower CD8 lymphocyte counts and lower Hb levels along with higher CRP and D-dimer levels were observed in patients over 65 years (all p values < 0.05). As expected by lower Hb values, anemia was more prevalent in patients over the age of 65 (p = 0.032). Of interest, while the prevalence of thrombocytopenia, leukopenia and lymphopenia was similar in the 2 groups, all patients with neutropenia were below the age of 65 (p = 0.009) and all but one patient with lymphocytosis were over the age of 65 (p = 0.05). In addition, the N:L ratio was significantly higher in patients over 65 years of age (p = 0.03). To note, neither the features and extent of pulmonary involvement, nor the functional impairment seemed to be influenced by age of onset. Likewise, no difference in the features and extent of pulmonary involvement, nor in the functional impairment emerged comparing males to females. However, males displayed significantly higher levels of ferritin and, as a consequence, of the ferritin:ESR ratio.
Subgroup analysis by ferritin levels
No difference in the extent or severity of pulmonary manifestations was observed. We divided the 51 patients with available ferritin values according to percentiles (Table 4) rather than using the reference level of the local laboratory to ensure generalizability of the results. Patients with ferritin values over the 25th percentile were more frequently males and also displayed higher levels of fibrinogen, LDH and procalcitonin compared to those with ferritin levels below the 25th percentile. To note, the extent of pulmonary involvement (number of lobes affected, bilateral involvement, and severity scores) as well as the prevalence of septal thickening and mediastinal lymph node enlargement were significantly higher in patients with higher levels of ferritin while the functional impairment (PAO2/FiO2) was more pronounced in this group.
We subsequently performed logistic regression analysis and since older age and male gender have been identified as predictors of more severe disease in previous studies and ferritin was significantly higher in males in our cohort, we included both age and gender as covariates. Ferritin levels over the 25th percentile were associated with the involvement of all 5 pulmonary lobes (Odds ratio (OR) = 14.5, 95% CI 2.3–90.9, p = 0.004), the presence of septal thickening (OR = 8.2, 95% CI 1.6–40.9, p = 0.011) and the presence of mediastinal lymph node enlargement (OR = 12.0, 95% CI 1.1–127.5, p = 0.039) independently of age and gender. The N:L ratio was also associated with the presence of septal thickening (OR = 2.0, 95% CI 1.2–3.5, p = 0.008) but at multivariate analysis only ferritin remained significantly associated with this pulmonary manifestation (OR = 5.1, 95% CI 1.1–24.3, p = 0.043).
Subgroup analysis by disease outcomes
When comparing discharged patients according to their need to be admitted to ICU before discharge, we observed significantly higher severity scores and significantly lower PAO2/FiO2 values in patients needing ICU, as well as higher D-dimer and LDH values (Table 5). However, none of the other variables was significantly different between the groups. When comparing discharged and deceased patients it emerged that deceased patients were older, with higher WBC and ANC counts and higher N:L ratios along with higher LDH and procalcitonin levels and lower PaO2/FiO2 values. None of the lung abnormalities detected by CT was different between discharged and deceased patients.
Discussion
Although in the majority of patients COVID-19 is characterized by mild to moderate symptoms, it is burdened by consistent morbidity and mortality due to respiratory failure, ARDS and sepsis3.
In our cohort the prognosis was rather good despite the severity of pulmonary involvement, with only a small number of subjects needing to be transferred to ICU and/or eventually deceased. In addition, we demonstrated and quantified for the first time a strong association between ferritin levels and the severity of pulmonary involvement independently of age and gender.
Since December 2019 an increasing number of articles describing the clinical, serological, radiological and histological features of patients with COVID-19 across the world have been published32. Of interest, in addition to chest X-ray and CT scan, lung ultrasonography has been pointed as a cost-effective non-invasive approach to assess pulmonary involvement in the scenario of a pandemic33,34.
Italy has been early and dramatically affected by COVID-19 although with a large diversity across the Country35. The Abruzzo region is within the 10 less affected regions per number of cases but with a relatively high case fatality ratio of 13%. However, despite the severity of the pulmonary involvement, the overall prognosis in our cohort was good with most of the patients requiring ICU being subsequently discharged and only 8% patients deceased. Furthermore, our data regarding the prevalence of different pulmonary lesions in COVID-19 partially fit with the previous literature36,37.
It has been speculated that pulmonary oedema and hyaline membrane formation may be the underlying pathological driver of GGO sign38,39. GGO may develop into reticular interlobular septa thickening and crazy paving pattern, indicating that the infection leads to diffuse alveolar oedema and interstitial inflammation40. Of interest, septal thickening was the most frequent lesion in our cohort observed in 75% of patient. Multifocal, patchy, or segmental consolidation, distributed in subpleural areas or along bronchovascular bundles, is usually detected in the 2–64% of COVID-19 pneumonia26,37 and may relate to cellular fibromyxoid exudates in alveoli38, 39. Several data suggest that the presence of consolidation and its increase overtime could be an indicator of disease progression40,41,42, however this was not the case in our cohort. We confirmed that pleural effusion is a rare imaging finding and none of our patients displayed coexisting lymphadenopathy. Based on the experience of other viral pneumonia such as influenza and other coronavirus diseases, the presence of pleural effusion may suggest a poor prognosis in COVID-19 patients37,38,42,43,44,45,46,47 and if coexisting with lymphadenopathy bacterial superinfection or another diagnosis should be ruled out36,38. Among demographic and serological features, several parameters, such as older age and male gender, have been associated with more severe COVID-19 prognosis18. Furthermore, higher ferritin levels have been described in patients with more severe disease and deceased21,48,49,50.
These findings fit with the concept of hyperinflammation in COVID-19, and since hyperferritinemia has been associated with inflammatory states in SARS-CoV-2 infection, it is plausible that ferritin may be a useful parameter to predict disease severity and the extent of the cytokine storm.
Ferritin has been well characterised as an acute phase reactant, as well as a mediator of immune dysregulation in severe COVID-1951. Therefore, ferritin may be not only a marker of the inflammatory milieu but also an active player in the "cytokine storm" scenario that characterizes severe COVID-19. Complex feedback mechanisms between ferritin and cytokines in the control of pro-inflammatory and anti-inflammatory mediators might exist as cytokines can induce ferritin expression, but ferritin can induce the expression of pro- and anti-inflammatory cytokines as well. In this regard, it has been speculated that COVID-19 with pulmonary involvement may be included within the spectrum of hyperferritinemic syndrome20,52.
In our cohort, age was not related to ferritin levels but male patients displayed significantly higher levels. Of interest, however, when stratifying patients according to the levels of ferritin, the association between higher ferritin levels and a more sever clinical picture independently of age and gender became evident. Hyperferritinemic syndromes share clinical and serological features such as fever, cytopenias, multiorgan involvement and coagulopathy53, most of which can also be observed in COVID-19. In fact, despite a lung-centric immunopathology, currently available data suggest that the clinical spectrum of COVID-19 is not limited to lung involvement, but rather represents a multisystem illness4. Patients with higher ferritin levels displayed a higher N:L ratio, which has been associated with more severe pulmonary involvement in COVID-1954,55. In addition, higher D-dimer levels, hinting coagulation abnormalities, were also detected in COVID-19 patients with higher ferritin levels. The most intriguing finding is that ferritin was not associated with the disease outcome (discharge, admission to ICU or death) and neither was D-dimer, the latter in contrast with a retrospective Chinese study3. It is therefore tempting to speculate that ferritin may have a pathogenic role in the development of lung damage in COVID-19 but the exact mechanisms need to be fully elucidated. Inflammation is an essential part of an effective immune response and it has also clearly been indicated as a specific promoter of endothelial dysfunction and enhancer of the atherosclerotic process with increasing the CV risk56. Since SARS-CoV-2 can induce excessive and prolonged cytokine/chemokine responses that may eventually lead to death, the identification of reliable biomarkers enabling early stratification of patients developing cytokine storm and at which extent is warranted. This would also allow tailoring of immunomodulatory treatment strategies. This bulk of evidence may also support the “endothelial” hypothesis with the thrombotic or thromboembolic implications COVID-19-dependent associated to the cytokine storm57,58.
We acknowledge that our study displays some limitations, such as its retrospective nature, the small number of patients and the lack of pro-inflammatory cytokine levels and follow-up data.
Conclusions
We demonstrated for the first time that ferritin levels over the 25th percentile are associated with severe pulmonary involvement as detected by CT scan but not with the disease outcome. We also confirmed that CT findings of GGO with or without consolidation are the earliest and common visible manifestation of COVID-19 pneumonia, highlighting its central role in the diagnosis and severity evaluation of the disease. An accurate assessment of radiologic and pathologic features in COVID-19 patients is warranted to gain further knowledge on disease pathogenesis, clinical course and prognosis. This, along with the implementation of reliable biomarkers of lung disease severity in COVID-19 patients will allow early diagnosis, tailored treatment and ultimately improve patient care.
References
Huang, C. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China. Lancet. 395(10223), 497–506 (2020).
WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19, 11-march-2020.
Zhou, F. et al. Lancet Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229), 1054–1062 (2020).
Robba, C., Battaglini, D., & Pelosi, P., et al. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev. Respir. Med. 14(9), 865–868 (2020).
Conti, P. et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost Agents. 34(2), 327–331 (2020).
Fan, B. E. et al. Hematologic parameters in patients with COVID-19 infection. Am. J. Hematol. 95(6), E131–E134 (2020).
Wang, D. et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China. JAMA. 323(11), 1061–1069 (2020).
Terpos, E. et al. Hematological findings and complications of COVID-19. Am. J. Hematol. 95, 834 (2020).
Zhang, W. et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID19): The experience of clinical immunologists from China. Clin. Immunol. 214, 108393 (2020).
Carsana, L. et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. S1473–3099(20), 30434–30435 (2020).
Ackermann, M. et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383(2), 120–128 (2020).
Tang, N. et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 18(4), 844–847 (2020).
Levi, M. et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet. Haematol. 7(6), e438–e440 (2020).
Panigada, M. et al. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 18, 1738–1742 (2020).
Fan, B. E. et al. COVID-19 associated coagulopathy in critically ill patients: A hypercoagulable state demonstrated by parameters of haemostasis and clot waveform analysis. J. Thromb. Thrombolysis https://doi.org/10.1007/s11239-020-02318-x (2020).
Bikdeli, B. et al. COVID-19 and thrombotic or thromboembolic disease: Implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J. Am. Coll. Cardiol. 75(23), 2950–3297 (2020).
Sardu, C. et al. Hypertension, thrombosis, kidney failure, and diabetes: is COVID-19 an endothelial disease? A comprehensive evaluation of clinical and basic evidence. J. Clin. Med. 9(5), 1417 (2020).
Iaccarino, G., Grassi, G., & Borghi, C., et al, SARS-RAS Investigators. Age and multimorbidity predict death among COVID-19 patients: Results of the SARS-RAS study of the Italian society of hypertension. Hypertension 76(2), 366–372 (2020).
Mehta, P., McAuley, D. F., & Brown, M., et al. HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression Lancet. 395(10229), 1033–1034 (2020).
Alunno, A., Carubbi, F. & Rodriguez-Carrio, J. Storm, typhoon, cyclone or hurricane in patients with COVID-19? Beware of the same storm that has a different origin. RMD Open. 6(1), e001295 (2020).
Ruan, Q. et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan. China. Intensive Care Med. 46(5), 846–848 (2020).
Rubin, G. D. et al. The role of chest imaging in patient management during the COVID-19 pandemic: A multinational consensus statement from the Fleischner Society. Radiology 296(1), 172–180 (2020).
El Homsi, M. et al. Review of chest CT manifestations of COVID-19 infection. Eur. J. Radiol. Open. 7, 100239 (2020).
Ai, T. et al. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 296(2), E32–E40 (2020).
Hani, C. et al. COVID-19 pneumonia: A review of typical CT findings and differential diagnosis. Diagn. Interv. Imaging. 101(5), 263–268 (2020).
Bernheim, A. et al. Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology 295(3), 200463 (2020).
Caruso, D. et al. Chest CT features of COVID-19 in Rome. Italy. Radiology. 296(2), E79–E85 (2020).
Valent, P. Low blood counts: Immune mediated, idiopathic, or myelodysplasia hematology. Am. Soc. Hematol. Educ. Program. 2012, 485–491 (2012).
Hansell, D. M. et al. Fleischner Society: Glossary of terms for thoracic imaging. Radiology 246, 697–722 (2008).
Shen, C. et al. Quantitative computed tomography analysis for stratifying the severity of Coronavirus Disease 2019. J. Pharm. Anal. 10(2), 123–129 (2020).
Chung, M. et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 295(1), 202–207 (2020).
The Johns Hopkins Coronavirus resource center, mortality analysis. https://coronavirus.jhu.edu/data/mortality
Buonsenso, D., Pata, D. & Chiaretti, A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir. Med. 8(5), e27 (2020).
Pata, D. et al. Chest computed tomography and lung ultrasound findings in COVID-19 pneumonia: A pocket review for non-radiologists. Front Med (Lausanne). 26(7), 375 (2020).
The Italian Ministry of Health. http://www.salute.gov.it/
Bao, C. et al. Coronavirus disease 2019 (COVID-19) CT findings: A systematic review and meta-analysis. J. Am. Coll. Radiol. 17(6), 701–709 (2020).
Li, K. et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 55(6), 327–331 (2020).
Ye, Z. et al. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur. Radiol. 30(8), 4381–4389 (2020).
Xu, Z. et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet. Respir. Med. 8(4), 420–422 (2020).
Song, F. et al. Emerging 2019 novel coronavirus (2019-nCoV) pneumonia. Radiology 295(1), 210–217 (2020).
Pan, F. et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 295(3), 715–721 (2020).
Shi, H. et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 20(4), 425–434 (2020).
Das, K. M. et al. Acute Middle East respiratory syndrome coronavirus: Temporal lung changes observed on the chest radiographs of 55 patients. AJR Am. J. Roentgenol. 205, W267–W274 (2015).
de Wit, E. et al. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 14, 523–534 (2016).
Hui, D. S. C. & Zumla, A. Severe acute respiratory syndrome: Historical, epidemiologic, and clinical features. Infect. Dis. Clin. N. Am. 33, 869–889 (2019).
Zu, Z. Y. et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology 296(2), E15–E25 (2020).
Kooraki, S. et al. Coronavirus (COVID-19) outbreak: What the department of radiology should know. J. Am. Coll. Radiol. 17, 447–451 (2020).
Lin, Z. et al. Serum ferritin as an independent risk factor for severity in COVID-19 patients. J. Infect. S0163–4453(20), 30434–30435 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062 (2020).
Gandini, O. et al. Serum ferritin is an independent risk factor for acute respiratory distress syndrome in COVID-19. J Infect. 81(6), 979–997 (2020).
Kappert, K., Jahić, A. & Tauber, R. Assessment of serum ferritin as a biomarker in COVID-19: bystander or participant? Insights by comparison with other infectious and non-infectious diseases. Biomarkers 25(8), 616–625 (2020).
McGonagle, D. et al. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun. Rev. 19(6), 102537 (2020).
Goldhaber, G., Segal, G. & Dagan, A. Hyperferritinemia in the elderly can differentiate the bad from the worst: A retrospective cohort analysis. Medicine 99(31), e21419 (2020).
Rosário, C. et al. The hyperferritinemic syndrome: Macrophage activation syndrome, Still’s disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med. 11, 185 (2013).
Kong, M. et al. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 148, e139 (2020).
Liu, J. et al. Netrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 18(1), 206 (2020).
Grassi, D., Desideri, G. & Ferri, C. Cardiovascular risk and endothelial dysfunction: The preferential route for atherosclerosis. Curr. Pharm. Biotechnol. 12(9), 1343–1353 (2011).
Jung, F. et al. COVID-19 and the endothelium. Clin. Hemorheol. Microcirc. 75(1), 7–11 (2020).
Funding
This study did not receive specific funding.
Author information
Authors and Affiliations
Contributions
F.C., L.S., A.A., D.G., conceived and developed the study, supervised the acquisition of the data, analyzed and interpreted the data. Fa.M. analyzed CT scan. E.B., R.M., Fr.M., M.P., assisted in collecting clinical information about the patients and helped to interpret the data. C.F., G.D., S.C. critically reviewed the manuscript. All authors edited and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carubbi, F., Salvati, L., Alunno, A. et al. Ferritin is associated with the severity of lung involvement but not with worse prognosis in patients with COVID-19: data from two Italian COVID-19 units. Sci Rep 11, 4863 (2021). https://doi.org/10.1038/s41598-021-83831-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83831-8
This article is cited by
-
Point-of-care lung ultrasound predicts hyperferritinemia and hospitalization, but not elevated troponin in SARS-CoV-2 viral pneumonitis in children
Scientific Reports (2024)
-
Artificial intelligence guided HRCT assessment predicts the severity of COVID-19 pneumonia based on clinical parameters
BMC Infectious Diseases (2023)
-
Immunological and biochemical biomarker alterations among SARS-COV-2 patients with varying disease phenotypes in Uganda
BMC Infectious Diseases (2023)
-
A Pilot Study on COVID-19 Positive Subjects: An Excerpt of Post-Infection-Pro-Diabetic Disposition & Related Consequences in Correlation to Hepato-Pancreatic Bio-Markers, Pro-Inflammatory Cytokines and Other Risk Factors
Indian Journal of Clinical Biochemistry (2023)
-
Reduced circulating FABP2 in patients with moderate to severe COVID-19 may indicate enterocyte functional change rather than cell death
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.