Abstract
Purpose of Review
The COVID-19 pandemic has had a profound impact on athletics, and the question of safely resuming competitive sports at all levels has been a source of significant debate. Concerns regarding myocarditis and the risk of arrhythmias and sudden death in athletes have prompted heightened attention to the role of cardiovascular screening. In this review, we aim to comprehensively outline the cardiovascular manifestations associated with COVID-19 infection, to discuss screening, diagnosis, and treatment strategies, and to evaluate the current literature on the risk to athletes and recommendations regarding return-to-play.
Recent Findings
COVID-19 is known to cause myocarditis, with presentations ranging from subclinical current or prior infection detected on cardiac MRI imaging, to fulminant heart failure and shock. While initial data early in the pandemic suggested that the risk of myocarditis could be significant even in patients with nonsevere COVID-19 infection, recent studies suggest a very low prevalence of clinically significant disease in young athletes.
Summary
While COVID-19 can have significant cardiovascular manifestations, recent data demonstrate that a screening approach guided by severity of COVID-19 infection and cardiovascular symptoms allows the majority of athletes to safely return to play in a timely manner. We must continue to tailor our approach to screening athletes as knowledge grows, and further research on the longitudinal cardiovascular effects of COVID-19 is needed.
Similar content being viewed by others
Introduction
Beginning in early 2020, a pandemic of the highly contagious respiratory virus SARS-CoV-2 (COVID-19) swept through the USA and the world, triggering widespread stay-at-home orders to limit the spread of disease, including the cancellation of team sports seasons from amateur to professional levels. Given that COVID-19 has known potential cardiovascular manifestations, physicians caring for athletes have had a heightened attention to the risks that viral myocarditis poses to athletes and have incorporated additional protocols into the preparticipation evaluation. At the onset of the pandemic, data from hospitalized patients suggested an alarming incidence of cardiac involvement. Although that incidence was felt to be lower in a young, healthy population with mild disease, a paucity of data existed. As we accumulate more data on the athletic population that confirms a low number of cases, we continue to refine how to best minimize risk among exposed populations of athletes.
Cardiovascular manifestations of COVID-19
While the clinical course of COVID-19 infection is dominated by respiratory tract symptoms, its potential effects on the heart and other organ systems have been well documented [1]. Cardiac manifestations can include elevations of cardiac biomarkers, myocarditis, pericarditis, myocardial infarction (type I and type II), left and right ventricular dysfunction, and arrhythmias [2]. Depending on how cardiac involvement is defined, initial studies found that 20–30% of hospitalized patients had some form of cardiac manifestation [3, 4]. The mechanisms of myocardial injury in COVID-19 have multiple potential etiologies, including direct viral infection via binding of the ACE2 receptors on myocardial and endothelial surfaces, and ischemia and necrosis due to the viral induced “cytokine storm.” Diffuse small vessel inflammation and a hypercoagulable state can also contribute to ischemia and thrombotic complications [5, 6]. Elevation of cardiac biomarkers, including troponin-I and B-type natriuretic peptide (BNP), are common in severe illness, as are markers of systemic inflammation, including C-reactive protein (CRP) [1]. Among hospitalized patients, troponin levels positively correlated in a linear fashion with high-sensitivity CRP and NT-proBNP levels and were associated with more frequent ventricular arrhythmias and increased mortality [3, 4].
Since the majority of testing has been performed on hospitalized patients with more severe disease, the true prevalence of myocardial involvement in mild or asymptomatic outpatients is not known. While older age and comorbidities are risk factors for severe disease, young people are not immune from complications. In a study of 3222 young adults age 18–34 who required hospitalization for COVID-19, 21% required intensive care and 2.7% died [7]. Risk factors were similar to the general population in that obesity, hypertension, and diabetes were associated with increased mortality [8]. African Americans have also had disproportionately high rates of hospitalization and mortality [9]. Severity of disease does seem to correlate with increased risk of myocardial involvement; however, large-scale data in the nonhospitalized population is lacking.
Myocarditis and the relevance to athletes
Myocarditis ranks as the third most common etiology of sudden cardiac death in young athletes with an estimated prevalence between 4 and 7.5% and is most commonly caused by antecedent viral infection [10,11,12].
There are 3 phases of myocarditis: acute viral phase with direct injury to myocytes; acute immune phase with activation of the immune response, resulting in myocardial inflammation/edema and fibrosis; and in a smaller number of cases, a chronic phase which is felt to be autoimmune-mediated [13]. Acute and chronic myocardial inflammation carries a risk of ventricular arrhythmias, that may be triggered by extreme exertion, which forms the basis for the recommendation for restriction from competitive athletics and vigorous activity for 3–6 months following diagnosis [13]. Most mild cases are self-limited, and severe myocarditis resulting in heart failure is rare in athletes. However, even mild or asymptomatic cases with resultant scar found on autopsy or seen as late gadolinium enhancement (LGE) on MRI have been implicated in sudden death [11].
One of the central questions in return-to-sport following the COVID-19 pandemic has been how to screen and accurately detect current or prior myocardial involvement that may put the athlete at increased risk for malignant arrhythmias. Who to screen, how to screen, and how to interpret the screening tests have all been significantly debated. There are a number of clinical challenges: the number of athletes with prior COVID-19 infection is high, the true prevalence of COVID-19 cardiac involvement is unknown but likely low, the disease course of COVID-19 can be unpredictable, and the most inexpensive screening tests have limited sensitivity and specificity. Cardiac MRI (CMR) is both highly sensitive and specific; however, it is not cost-effective as a screening strategy. Interpretation also requires both expertise in normal findings for athletes, and sufficient volumes and protocols for accurate diagnosis of myocardial pathology.
Diagnosis of myocarditis
The diagnosis of acute myocarditis includes a clinical syndrome of “acute heart failure, angina-type chest pain, or known myopericarditis of less than 3-month duration,” and an otherwise unexplained elevation of troponin, electrocardiogram (ECG) change, arrhythmia or high-grade atrioventricular (AV) block, systolic dysfunction or regional wall motion abnormalities, or pericardial effusion [14]. In the right clinical setting, CMR findings of altered tissue signal on T2- or T1-weighted images or presence of LGE are diagnostic [14]. Symptoms can be nonspecific and include chest pain, palpitations, shortness of breath, and decreased exercise tolerance.
The ECG may show abnormalities in the setting of myocarditis, including ST elevation or depression, T wave inversion, AV or bundle branch block, atrial/ventricular arrhythmias, or abnormal Q waves, but it is neither sensitive nor specific, and there are no pathognomonic findings [15]. In a study involving 45 patients with a histologic diagnosis of active myocarditis, the sensitivity of ECG was 47% [16]. In athletes, benign physiologic changes on ECG are common and include sinus bradycardia, first-degree AV block, incomplete right bundle branch block, early repolarization, and isolated QRS voltage criteria for left ventricular hypertrophy [17]. Early repolarization (a common finding in well-trained athletes) must be distinguished from the diffuse ST-elevation and PR depression of pericarditis. Whenever possible, comparison to prior ECG tracings is helpful.
Troponin-I is specific but not sensitive in the diagnosis of myocarditis. In a study including 53 patients with biopsy-proven myocarditis, an elevated troponin-I level was 89% specific but only 34% sensitive [18]. In athletes, an elevated troponin-I level after intense exercise is common, and it usually returns to the normal range within 48 h following activity. This has been shown in many different athletic populations using earlier generation troponin assays, and is likely even more common with high-sensitivity troponin assays (hs-troponin) although data are lacking [19]. Clinicians should avoid checking a troponin level in athletes who have exercised in the preceding 48 h, and if elevated, recheck, as a persistent elevation warrants more rigorous evaluation.
Echocardiographic findings in myocarditis may range from no overt abnormalities to severe systolic dysfunction, and it is paramount to be able to distinguish physiologic remodeling from pathologic findings. Cardiac remodeling in athletes frequently leads to mild symmetric LV hypertrophy and balanced chamber dilation, particularly in endurance athletes. Marked dilation of the left ventricle (LV end-diastolic dimension > 70 mm in men or > 60 mm in women) or ejection fraction (EF) < 50% are uncommon for athletic remodeling, and should prompt further evaluation for myocarditis or other causes of dilated cardiomyopathy [20•]. Other findings supporting benign athletic changes are normal diastolic parameters, normal LV global longitudinal strain (GLS) (i.e., more negative than − 16%), and normal augmentation of EF with stress [20•]. Regional wall motion abnormalities or asymmetric hypertrophy always warrant further evaluation for pathology, and more than trivial pericardial effusion may be a marker of pericarditis or myopericarditis. Strain can also have prognostic value—in a study involving 45 patients with suspected myocarditis, decreased longitudinal strain, circumferential strain, and strain rate were each associated with decreased event-free survival [21].
Endurance athletes also frequently have right ventricular dimensions above the American Society of Echocardiography cutoffs for normal; however, dilation should be proportionate to the LV. Marked or isolated RV dilation (RV end-diastolic area > 15 cm2/m2, RV end-diastolic volume > 260 ml, or basal RV/LV end-diastolic ratio > 1.0), low RV function (fractional area change < 35%), or segmental wall motion abnormalities are all abnormal and require further workup, which should include cardiac MRI due to its superior imaging of the RV [20•].
Cardiac MRI is an essential tool—in both diagnosis and prognosis of viral myocarditis—as it evaluates active inflammation as well as sequelae of prior infection (LGE). The Lake Louise criteria propose using two main criteria to diagnose suspected myocarditis: myocardial edema, demonstrated by increased T2 values, and nonischemic myocardial injury, demonstrated by increased T1 values, increased extracellular volume (ECV), or LGE [22]. These criteria have shown high diagnostic accuracy in large cohorts, especially with the addition of parametric mapping (native T1, T2, and ECV) [20•, 23]. However, in athletes, cardiac remodeling can frequently confound CMR interpretation. In healthy athlete populations, multiple CMR “abnormalities” have been demonstrated, that are felt to be largely benign. These include chamber dilation and low normal LV/RV function in endurance athletes and focal LGE in the interventricular septum (RV insertion point) [24, 25]. Myocarditis typically leads to a patchy, mid-wall, or subepicardial pattern of LGE that is not usually restricted to the RV insertion sites.
Moulson and Petek et al. classified definite myocardial involvement as either (1) CMR T1 abnormality or LGE + T2 abnormality or (2) CMR T2 abnormality + at least one supportive finding (EF < 45%, pericardial effusion, pericardial enhancement, or troponin > 99% upper limit of normal) [26•]. Probable myocardial involvement was defined as CMR T1 abnormality or LGE + at least one supportive finding. The significance of an isolated CMR T1 abnormality or presence of LGE is less clear and may be possible evidence of myocardial involvement. Clinicians should correlate CMR results with the clinical evaluation. Endomyocardial biopsy, while historically indicated in some cases of fulminant myocarditis of undetermined etiology, has not had a role in COVID-19 myocarditis.
Identifying those at risk of poor outcomes is a topic of continued study. Extent of LGE on CMR has been shown to correlate with prognosis, and among those with no residual LGE, can identify a low risk group with an annual major adverse event rate less than 1% [27]. In a study involving 73 athletes who had previously experienced ventricular arrhythmias, LGE on CMR was shown to predict future adverse events. Thirty-five of the athletes with a prior ventricular arrhythmia had subepicardial/midmyocardial LGE on CMR—of these 35 athletes, six experienced an ICD shock, sustained ventricular tachycardia, or sudden death over three years of follow up [27].
Treatment of myocarditis
Treatment of viral myocarditis is generally supportive, and the treatment of more severe disease in COVID-19 associated with heart failure or arrhythmias does not differ from treatment of other viral myocardial processes. For those with mild symptoms, which make up most of the athlete population, this typically consists of isolation and restriction from activity. In patients with more significant disease, treatment is targeted at specific complications. For acute or chronic heart failure, standard guideline-directed medical therapy is recommended (i.e., beta blockers, ACE inhibitors, etc. for patients with reduced ejection fraction) [28]. Arrhythmias are a common complication of myocarditis, and treatment varies based on the type of arrhythmia. Treatments for severe COVID-19 in hospitalized patients, including drugs like Remdesivir and dexamethasone, as well as convalescent plasma or monoclonal antibodies, may have some benefit in modulating disease severity and/or mortality, but it is unknown whether they affect the risk of myocardial involvement. Historically, antiviral therapies, corticosteroids, and intravenous immune globulin have not consistently shown benefit when used to treat viral myocarditis [15, 29, 30].
Athletes with Diagnosed COVID-19 Myocardial Involvement
Per the American College of Cardiology guidelines, athletes diagnosed with myocarditis should be restricted from vigorous exercise and competition for a minimum of 3–6 months after resolution of clinical symptoms [14]. Prior to return, they should undergo an echocardiogram, 24-h Holter monitoring, and an exercise ECG. If LV function has normalized, cardiac biomarkers are negative, and there are no frequent or complex arrhythmias on Holter or exercise ECG, then it is reasonable to gradually return to play. While LGE on CMR may convey an increased risk of arrhythmia, it is unresolved whether athletes should be restricted from play until LGE has fully resolved. Isolated pericarditis warrants restriction from competition during the acute illness, i.e., until asymptomatic, pericardial effusion has resolved, and inflammatory markers have normalized [14].
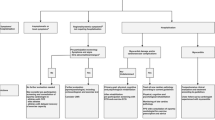
Return to play for athletes diagnosed with COVID-19
Guidance on screening athletes for evidence of COVID-19 cardiac involvement comes primarily from expert recommendations. The most recent expert consensus report from October 2020 proposes an algorithm based on severity of initial COVID-19 infection, and presence of symptoms concerning for cardiac involvement (Fig. 1) [31•]. Athletes with asymptomatic or mild COVID-19 infections without concerning cardiovascular symptoms may gradually return to play after appropriate quarantine. Nonhospitalized athletes with moderate COVID-19 symptoms should undergo ECG, hs-troponin, and echocardiogram. Athletes with severe COVID-19 infection requiring hospitalization should undergo hs-troponin and echocardiogram, ideally during hospitalization, with consideration of CMR. If an athlete has an abnormal test result or if new cardiovascular symptoms develop, then further investigation should be done, including CMR when possible [31•]. As per a previously published recommendation on athletes with myocarditis, if significant clinical suspicion remains but CMR is normal, serial testing may be considered [13].
COVID-19 return-to-play algorithm for adult athletes in competitive sports. Originally published in JAMA Cardiology by Kim et al. [31•]. CV = cardiovascular, hs-cTn = high-sensitivity troponin-I, RTP = return to play. Mild symptoms = anosmia, ageusia, headache, mild fatigue, mild upper respiratory tract illness, and mild gastrointestinal illness. Moderate symptoms = persistent fever, chills, myalgias, lethargy, dyspnea, and chest tightness. CV symptoms = dyspnea, exercise intolerance, chest tightness, dizziness, syncope, and palpitations.
Review of current data on COVID-19 cardiovascular involvement in athletes
One of the first studies to include non-hospitalized patients with mild-to-moderate disease published by Puntmann et al. demonstrated cardiac MRI abnormalities in an alarming number of patients, with 78 of 100 (78%) showing one abnormal finding and 60 with evidence of ongoing myocardial inflammation. This study has very little applicability to the screening of the athletic population as the mean age was 49 years, there was a high prevalence of comorbid conditions, and many patients had new or ongoing symptoms [32]. Since then, several observational studies in athletes with minimal-to-moderate disease severity have been published, with variable protocols and prevalence of myocardial involvement.
Three single-center studies utilizing screening CMR in addition to triad testing (ECG, troponin, and echocardiogram) [26•] on a total of 230 collegiate athletes with prior mild-moderate COVID-19 infection demonstrated a variable prevalence of myocarditis, ranging from 1.4 to 15% (Rajpal, Clark, Starekova) [33,34,35]. Five of the eight individuals diagnosed with CMR criteria for myocarditis had no cardiovascular symptoms and normal initial triad testing. In the largest study, Starekova et al. compared 145 athletes with prior COVID-19 with both athletic and nonathletic healthy controls, finding only finding two athletes (1.4%) meeting criteria for myocardial involvement (1 myopericarditis, 1 myocarditis) [35]. Smaller studies have also been published on elite athletes in Poland and Hungary and found no cases of myocarditis [36, 37]. Brito et al. screened 54 college athletes with mild-to-moderate COVID-19 with ECG, troponin-I, and echocardiography followed by CMR if symptomatic or for an abnormal screening test, 48 of whom underwent CMR. None met criteria for myocarditis; however, 40% had pericardial enhancement with pericardial effusion [38]. This high rate of pericardial involvement has not been replicated in other studies.
In March 2021, Martinez et al. published the results of screening 789 North American professional athletes (5 different sports, mean age 25 years, 98.5% men) prior to return to play based on a risk stratification approach and found an exceedingly low number of athletes with myocardial or pericardial inflammation (0.6%) [39•]. Initial testing was performed on all COVID-positive athletes which included clinical evaluation, troponin, ECG, and echocardiogram (median 19 days following test result, range 3–156 days), with additional testing (CMR) if abnormal. Fifty-eight percent were symptomatic during acute COVID-19 infection; none had severe disease but one was hospitalized for observation overnight. Six athletes (0.8%) had elevated troponin levels, 10 (1.3%) had ECG abnormalities, and 20 (2.5%) had abnormal echocardiogram findings, which were largely mild reductions in EF (which normalized on subsequent stress testing or had a normal CMR). Three patients (0.4%) were diagnosed with myocarditis, and two (0.3%) with pericarditis that resulted in restriction from play according to guidelines. Follow-up was relatively short, but at the time of publication, there were no adverse events in those that resumed professional sport.
Finally, in the largest study of college athletes, published in April 2021, Moulson and Petek et al. reported the results of screening 3018 COVID-19-positive collegiate athletes from 42 universities for cardiac involvement (26 sports, mean age 20 years, 68% men) [26•]. The majority of the COVID-positive athletes (2820) underwent at least one triad test, with CMR if clinically indicated, and 198 underwent primary screening CMR. They found definite, probable, or possible COVID myocardial involvement in 0.7% (21/3018) and 0.5% (15/2820) in the clinically driven CMR cohort versus 3.0% (6/198) in the primary screening CMR cohort. Abnormal ECG was found in 0.7% (21/2999), troponin in 0.9% (24/2719), and TTE in 0.9% (24/2556). The majority of the athletes (62%) were asymptomatic or had mild COVID, 13% reported cardiopulmonary symptoms. There were no cardiac events or hospitalizations attributed to COVID myocardial involvement. Ten athletes were hospitalized or visited the ER for noncardiac COVID symptoms, and one athlete had a resuscitated cardiac arrest with no findings of myocarditis on CMR. Predictors of myocardial involvement included having at least one abnormal triad test (OR 37.4, 95% CI 13.3,105.3) or cardiopulmonary symptoms (OR 3.1, 95% CI 1.2,7.7). No athletes were hospitalized or had a cardiac event related to myocardial complications of COVID in the median follow-up time of 113 days. This prospective study was part of the ORCCA registry (Outcomes Registry for Cardiac Conditions in Athletes). The study authors specified definitions for definite, probable, or possible myocardial involvement, however acknowledge that there is not a consensus on these definitions, which is likely one of the reasons for the wide range of myocardial involvement found in previous studies. There was also some heterogeneity in screening protocols, and abnormal study findings were not adjudicated by a central core facility, so were potentially subject to some variation in interpretation. The diagnostic yield of CMR was fourfold higher with primary screening CMR vs. clinically indicated CMR; however, the significance of CMR findings in the absence of symptoms or other abnormal testing is less clear. This study supports the current recommendations for clinically-driven CMR screening of athletes.
Interpretation of these results
These data suggest that there is a very low risk of clinically significant cardiac involvement in young, healthy athletes with no cardiovascular symptoms following nonsevere COVID-19 infection.
There are some considerations to the applicability of these findings. The study populations were comprised of all college or young professional athletes, who were generally healthy. Therefore, caution should be used in extrapolating these findings to other populations, particularly older athletes or those with significant comorbidities. Masters athletes (age ≥ 35 years), and those with cardiovascular risk factors, are likely at higher risk of cardiovascular manifestations based on the results from trials involving the general population. The guidelines on return to play include a separate algorithm for masters athletes—the considerations and process for testing are much the same as college/professional athletes, but clinicians should bear in mind that the pretest probability for COVID-19 cardiac involvement is likely higher in this population, especially if cardiovascular risk factors are present [31•]. Cardiovascular symptoms should still be evaluated for other etiologies, namely, coronary artery disease. Children and high school athletes with nonsevere COVID-19 infection are felt to have an even lower risk of myocardial involvement, and return to play evaluation should follow the proposed algorithm, with athletes ≥ age 15 undergoing symptom-driven screening similar to the adult population [31•].
The overall number of athletes with findings requiring restriction from play were low. The clinical significance of isolated pericardial LGE or transient isolated myocardial edema with no symptoms, negative biomarkers, normal ECG, and a normal echocardiogram is uncertain. In these clinical “gray zones,” an expedited return to play evaluation may be considered, taking into account the unknowns, and utilizing a shared-decision-making approach. There is a small chance that subclinical myocardial inflammation will be missed with the current strategy. The ideal timing for testing has also not been defined—exclusion of myocardial involvement during or directly following illness should provide some reassurance; however, a small number of athletes may develop delayed manifestations. The studies differ in protocols (timing, controls, mandatory CMR vs. only if otherwise indicated), and although 2 studies reported significantly higher percentages of athletes with myocardial and pericardial disease, these findings have not been demonstrated in other studies. Lastly, interpretation of CMR findings in athletes does require expertise and is best performed at higher-volume tertiary care centers; however, it is acknowledged that this is not always feasible.
Other Post-COVID-19 sequalae
For physicians that care for athletes, evaluation and management of lingering COVID-19 symptoms that affect exercise performance and overall well-being are also commonly encountered in clinical practice. Dyspnea on exertion, resting tachycardia and/or exaggerated heart rate response to exercise, lightheadedness, nonspecific chest tightness, headaches, sleep disturbances, and difficulty concentrating may be common, even in the absence of overt pathologic cause. Even mild-moderate cases have had prolonged “long-hauler” symptoms including profound fatigue and exercise intolerance that can be multifactorial. A full medical evaluation including investigations for myocarditis/pericarditis (as above), pulmonary disease, pulmonary embolism, and myocardial ischemia (when appropriate) should be conducted, and after exclusion, cardiopulmonary exercise testing is a useful tool to assess the etiology of the limitation. A multidisciplinary approach should be employed, and while there are no blanket or COVID-19-specific treatments, therapies are targeted toward individual symptoms or diagnoses. Postviral sequelae may include asthma/bronchospasm, POTS/dysautonomia, or chronic fatigue syndrome [40,41,42]. The pandemic has also taken a huge toll on the mental health of athletes; from a physical and emotional perspective, the loss of a season, time away from training and competition, isolation from the team, and missed opportunities for scouting or professional play cannot be underestimated.
Gaps in knowledge, future directions
There are still several unanswered questions regarding the cardiovascular effects of COVID-19 in athletes that warrant further investigation. Primary CMR screening of all young, healthy athletes with prior COVID infection does not appear to be warranted, although would likely pick up a small number of additional cases. Why some young adults—athletes or not—develop severe cardiovascular illness, while the majority recover with minor or no cardiovascular injury is not well understood. There needs to be a better understanding of both risk factors—preexisting comorbidities, abnormal cardiac biomarkers, ECG and echocardiogram abnormalities—and potentially protective factors, with respect to the development of major adverse cardiovascular events following COVID-19. Analysis of large, ongoing registries of COVID-19 patients may better help to identify specific risk factors.
While COVID-19 has been at the forefront of the minds of practitioners in sports medicine and cardiology, it is important that the fundamental principles in the screening, treatment, and prevention of cardiovascular disease in athletes remain intact. Even when looking for signs of COVID-19 cardiovascular involvement, physicians should be careful not to miss other potentially dangerous conditions such as hypertrophic cardiomyopathy, channelopathies (Brugada, long QT), and anomalous coronary arteries when screening young athletes. Finally, the importance of sound emergency action planning with training of coaches, trainers, and teachers in how to handle the collapsed athlete and easy accessibility of external defibrillator therapy cannot be overstated.
Conclusion
Since the publication of the proposed return to play guidelines, there have been multiple observational studies evaluating the efficacy of screening athletes who had COVID-19 for cardiovascular complications. In aggregate, these studies have shown a very low prevalence of clinically relevant cardiovascular involvement. Research is needed to further tailor screening strategies, to assess the cardiovascular risk of COVID-19 to masters-level athletes, and to assess the longitudinal effects of COVID-19 on cardiovascular health.
This review covers available data and observations from the first year of the pandemic with a focus on adult athletes. With widespread vaccination underway and new COVID-19 variants emerging, it is unknown how these factors will impact the approach to screening.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus Disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–93. https://doi.org/10.1001/jama.2020.12839.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395(10223):497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, et al. Cardiovascular implications of fatal outcomes of patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):811–8. https://doi.org/10.1001/jamacardio.2020.1017.
Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol. 2020;5(7):802–10. https://doi.org/10.1001/jamacardio.2020.0950.
Fox SE, Lameira FS, Rinker EB, Vander Heide RS. Cardiac endotheliitis and multisystem inflammatory syndrome after COVID-19. Ann Intern Med. 2020;173(12):1025–7. https://doi.org/10.7326/L20-0882.
Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5(7):831–40. https://doi.org/10.1001/jamacardio.2020.1286.
Cunningham JW, Vaduganathan M, Claggett BL, Jering KS, Bhatt AS, Rosenthal N, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2020. https://doi.org/10.1001/jamainternmed.2020.5313.
Lighter J, Phillips M, Hochman S, Sterling S, Johnson D, Francois F, et al. Obesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. 2020;71(15):896–7. https://doi.org/10.1093/cid/ciaa415.
Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–2. https://doi.org/10.1001/jama.2020.6548.
Corrado D, Basso C, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden cardiac death? J Cardiovasc Med (Hagerstown). 2006;7(4):228–33. https://doi.org/10.2459/01.JCM.0000219313.89633.45.
Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo-Rojas LM, et al. Sudden death in young adults: an autopsy-based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58(12):1254–61. https://doi.org/10.1016/j.jacc.2011.01.049.
Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980–2006. Circulation. 2009;119(8):1085–92. https://doi.org/10.1161/CIRCULATIONAHA.108.804617.
Eichhorn C, Biere L, Schnell F, Schmied C, Wilhelm M, Kwong RY, et al. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. J Am Coll Cardiol Img. 2020;13(2 Pt 1):494–507. https://doi.org/10.1016/j.jcmg.2019.01.039.
Maron BJ, Udelson JE, Bonow RO, Nishimura RA, Ackerman MJ, Estes NA 3rd, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132(22):e273–80. https://doi.org/10.1161/CIR.0000000000000239.
Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34(33):2636–48, 48a-48d. https://doi.org/10.1093/eurheartj/eht210.
Morgera T, Di Lenarda A, Dreas L, Pinamonti B, Humar F, Bussani R, et al. Electrocardiography of myocarditis revisited: clinical and prognostic significance of electrocardiographic changes. Am Heart J. 1992;124(2):455–67. https://doi.org/10.1016/0002-8703(92)90613-z.
Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31(2):243–59. https://doi.org/10.1093/eurheartj/ehp473.
Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates Circulation. 1997;95(1):163–8.
Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, et al. Exercise-induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56(3):169–76. https://doi.org/10.1016/j.jacc.2010.03.037.
Phelan D, Kim JH, Elliott MD, Wasfy MM, Cremer P, Johri AM, et al. Screening of potential cardiac involvement in competitive athletes recovering from COVID-19: an expert consensus statement. J Am Coll Cardiol Img. 2020;13(12):2635–52. https://doi.org/10.1016/j.jcmg.2020.10.005. This article was the first expert consensus regarding screening athletes for cardiac involvement post-COVID-19.
Hsiao JF, Koshino Y, Bonnichsen CR, Yu Y, Miller FA Jr, Pellikka PA, et al. Speckle tracking echocardiography in acute myocarditis. Int J Cardiovasc Imaging. 2013;29(2):275–84. https://doi.org/10.1007/s10554-012-0085-6.
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol. 2018;72(24):3158–76. https://doi.org/10.1016/j.jacc.2018.09.072.
Kotanidis CP, Bazmpani MA, Haidich AB, Karvounis C, Antoniades C, Karamitsos TD. Diagnostic accuracy of cardiovascular magnetic resonance in acute myocarditis: a systematic review and meta-analysis. J Am Coll Cardiol Img. 2018;11(11):1583–90. https://doi.org/10.1016/j.jcmg.2017.12.008.
La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, et al. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36(30):1998–2010. https://doi.org/10.1093/eurheartj/ehv202.
Baggish AL, Battle RW, Beaver TA, Border WL, Douglas PS, Kramer CM, et al. Recommendations on the use of multimodality cardiovascular imaging in young adult competitive athletes: a report from the American Society of Echocardiography in collaboration with the Society of Cardiovascular Computed Tomography and the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2020;33(5):523–49. https://doi.org/10.1016/j.echo.2020.02.009.
Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, et al. SARS-CoV-2 Cardiac Involvement in Young Competitive Athletes. Circulation. 2021. https://doi.org/10.1161/CIRCULATIONAHA.121.054824. This study involves the largest cohort of COVID-positive athletes to date (3,018 collegiate athletes from 42 universities) and reports a very low prevalence of myocardial and pericardial involvement. The predominant screening strategy used was a triggered approach with CMR only if clinically indicated, with a small cohort who underwent primary screening CMR.
Grani C, Eichhorn C, Biere L, Murthy VL, Agarwal V, Kaneko K, et al. Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. 2017;70(16):1964–76. https://doi.org/10.1016/j.jacc.2017.08.050.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239. https://doi.org/10.1016/j.jacc.2013.05.019.
Chen HS, Wang W, Wu SN, Liu JP. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013(10):CD004471. https://doi.org/10.1002/14651858.CD004471.pub3.
Thakkar S, Arora S, Kumar A, Jaswaney R, Faisaluddin M, Ammad Ud Din M, et al. A systematic review of the cardiovascular manifestations and outcomes in the setting of Coronavirus-19 Disease. Clin Med Insights Cardiol. 2020;14:1179546820977196. https://doi.org/10.1177/1179546820977196.
Kim JH, Levine BD, Phelan D, Emery MS, Martinez MW, Chung EH, et al. Coronavirus Disease 2019 and the athletic heart: emerging perspectives on pathology, risks, and return to play. JAMA Cardiol. 2021;6(2):219–27. https://doi.org/10.1001/jamacardio.2020.5890. This article provides the latest expert recommendations regarding screening athletes for myocardial involvement following COVID-19 infection.
Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–73. https://doi.org/10.1001/jamacardio.2020.3557.
Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, et al. COVID-19 myocardial pathology evaluation in athletes with cardiac magnetic resonance (COMPETE CMR). Circulation. 2021;143(6):609–12. https://doi.org/10.1161/CIRCULATIONAHA.120.052573.
Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, et al. Cardiovascular magnetic resonance findings in competitive athletes recovering from COVID-19 infection. JAMA Cardiol. 2021;6(1):116–8. https://doi.org/10.1001/jamacardio.2020.4916.
Starekova J, Bluemke DA, Bradham WS, Eckhardt LL, Grist TM, Kusmirek JE, et al. Evaluation for myocarditis in competitive student athletes recovering from Coronavirus Disease 2019 with cardiac magnetic resonance imaging JAMA Cardiol. 2021. https://doi.org/10.1001/jamacardio.2020.7444
Malek LA, Marczak M, Milosz-Wieczorek B, Konopka M, Braksator W, Drygas W, et al. Cardiac involvement in consecutive elite athletes recovered from Covid-19: A magnetic resonance study. J Magn Reson Imaging. 2021. https://doi.org/10.1002/jmri.27513.
Vago H, Szabo L, Dohy Z, Merkely B. Cardiac magnetic resonance findings in patients recovered from COVID-19: initial experiences in elite athletes. J Am Coll Cardiol Img. 2020. https://doi.org/10.1016/j.jcmg.2020.11.014.
Brito D, Meester S, Yanamala N, Patel HB, Balcik BJ, Casaclang-Verzosa G, et al. High prevalence of pericardial involvement in college student athletes recovering from COVID-19. J Am Coll Cardiol Img. 2020. https://doi.org/10.1016/j.jcmg.2020.10.023.
Martinez MW, Tucker AM, Bloom OJ, Green G, DiFiori JP, Solomon G, et al. Prevalence of inflammatory heart disease among professional athletes with prior COVID-19 infection who received systematic return-to-play cardiac screening. JAMA Cardiol. 2021. https://doi.org/10.1001/jamacardio.2021.0565. This article involves the second largest cohort of athletes with COVID-19 to date. The study reports the results of screening 789 North American professional athletes for cardiovascular involvement of COVID-19 prior to return to play and found a very low prevalence of myocardial and pericardial disease.
Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2020. https://doi.org/10.1016/j.hrthm.2020.12.007.
Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. 2020;370: m3026. https://doi.org/10.1136/bmj.m3026.
Wilson MG, Hull JH, Rogers J, Pollock N, Dodd M, Haines J, et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br J Sports Med. 2020;54(19):1157–61. https://doi.org/10.1136/bjsports-2020-102710.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Jack Goergen declares that he has no conflicts of interest. Aakash Bavishi declares that he has no conflict of interest. Micah Eimer declares that she has no conflict of interest. Allison Zielinski declares that she has no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Topical Collection on Sports Cardiology
Rights and permissions
About this article
Cite this article
Goergen, J., Bavishi, A., Eimer, M. et al. COVID-19: the Risk to Athletes. Curr Treat Options Cardio Med 23, 68 (2021). https://doi.org/10.1007/s11936-021-00941-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s11936-021-00941-2