- Department of Computing Technologies, School of Computing, SRM Institute of Science and Technology, Chennai, India
With the SARS-CoV-2's exponential growth, intelligent and constructive practice is required to diagnose the COVID-19. The rapid spread of the virus and the shortage of reliable testing models are considered major issues in detecting COVID-19. This problem remains the peak burden for clinicians. With the advent of artificial intelligence (AI) in image processing, the burden of diagnosing the COVID-19 cases has been reduced to acceptable thresholds. But traditional AI techniques often require centralized data storage and training for the predictive model development which increases the computational complexity. The real-world challenge is to exchange data globally across hospitals while also taking into account of the organizations' privacy concerns. Collaborative model development and privacy protection are critical considerations while training a global deep learning model. To address these challenges, this paper proposes a novel framework based on blockchain and the federated learning model. The federated learning model takes care of reduced complexity, and blockchain helps in distributed data with privacy maintained. More precisely, the proposed federated learning ensembled deep five learning blockchain model (FLED-Block) framework collects the data from the different medical healthcare centers, develops the model with the hybrid capsule learning network, and performs the prediction accurately, while preserving the privacy and shares among authorized persons. Extensive experimentation has been carried out using the lung CT images and compared the performance of the proposed model with the existing VGG-16 and 19, Alexnets, Resnets-50 and 100, Inception V3, Densenets-121, 119, and 150, Mobilenets, SegCaps in terms of accuracy (98.2%), precision (97.3%), recall (96.5%), specificity (33.5%), and F1-score (97%) in predicting the COVID-19 with effectively preserving the privacy of the data among the heterogeneous users.
Introduction
The epidemic of COVID-19 is believed to be one of the most hazardous illnesses that have a devastating effect on the lives of many individuals. Serious acute respiratory syndrome coronavirus (SARS-CoV) affects a huge population (1–3). India has the 2nd highest number of confirmed victims worldwide with 33,678,785 recorded positive cases and the third-highest number of COVID-19 fatalities (4, 5). As soon as, the count of COVID-19 infections rose to unimaginable heights, leaving the government and doctors ill-equipped to deal with them (6, 7). As a result, clinicians were having difficulty in identifying individuals who are COVID-19-positive due to a lack of testing models (8). The diagnosis of COVID-19 disorders relies heavily on clinical symptoms, epidemiological history, computed tomography (CT), and pathogenic testing. Most of the patients with COVID-19 show the same visual symptoms on CT scans as the other lung disorders (9–13) despite the use of a variety of radiological modalities. Symptoms of COVID-19 vary widely across individuals, and the disease is growing at an alarming rate (14).
As a result, hospitals may communicate information on patients with COVID-19 to get an appropriate diagnosis. It is a difficult challenge to securely share data (without exposing the privacy of users) and train a global model to recognize positive cases. As a result, current research is unable to exchange data with each other and train the model correctly. AI-based solutions are hindered by the difficulty of obtaining data from a variety of sources. Because healthcare facilities lack a privacy-preserving methodology, such sensitive information cannot be made available (15).
Currently, various artificial intelligence (AI) techniques are under exploration to solve the ambiguity in diagnosis (16–23). The existing AI techniques often require large data from a single source to train the deep learning model for a more accurate prediction. In contrast, data from a single source lack the feature distribution variance problem (24) that leads to the high misclassification rate affecting the diagnosis outcomes in terms of accuracy. These data unavailability problems can be reduced if many hospitals can share the data. But security and privacy concerns restrict the hospitals to share the data for research even. Hence, the existing AI methods need to be improved (25, 26), so that they can focus on collaborative learning while maintaining privacy.
Motivation
According to the most recent WHO study, COVID-19 is an infectious illness that mostly affects the lungs, giving them a honeycomb-like appearance. Some people who have recovered from COVID-19 are left with long-term lung impairment. The primary goal of our study was to classify the patterns in the lungs caused by COVID-19, so that experienced radiologists do not overlook infection. Second, sharing the information to train a stronger deep learning model while keeping the privacy concerns of data providers (27). A deep learning-based model for automated identification of COVID-19 may be developed for sharing the data.
The first challenge is that owing to a lack of privacy, personal information cannot be made available. The second challenge is to use a blockchain network to train the global model (the Federated model). The third issue is difficult to obtain enough training data and improve the prediction model, which has an impact on the diagnosis ratio. Finally, recognizing the patterns of COVID-19 lung screening is a difficult process.
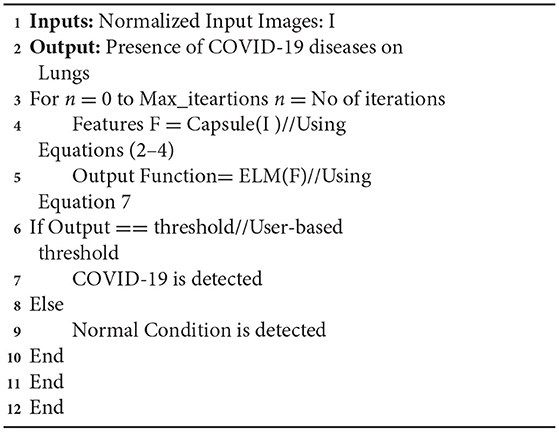
This problem motivated the invention of a collaborative deep learning model that can diagnose COVID-19 instances (28, 29) and share the results while ensuring the privacy and security of the hospitals (30). This paper proposes the FLED-Block framework, which learns jointly from various CT images acquired from various sources. The new capsule-deep extreme learning network is introduced in the suggested framework for enhanced segmentation and classification. A capsule network is developed for its specialization in recognizing abnormalities in medical images. To train a more accurate model using the previous approach, a large amount of data is required. The capsule network enhances the deep learning models' performance at the level of the models' internal layers. To improve classification accuracy and diagnosis, the suggested system combines the strong properties of capsule Networks and extreme learning machines (ELMs) to solve the issues. To better forecast COVID-19, ELM substitutes dense classification layers with capsule networks, which obtain strong feature maps. Privacy concerns are addressed using federated learning methods to distribute the learned model across any node in the network (31).
The main contribution of the paper
• The proposed algorithm detects and classifies the COVID-19 patterns from the CT images from multiple sources using an ensemble of capsule networks. The extreme learning machine in the capsule is used for better feature extraction to achieve better classification.
• This paper introduces the blockchain powered data collection and sharing unit, which collects the data from different heterogeneous sources and employs federated learning to maintain data privacy between the organizations with high-accuracy global model training.
• Finally, the excellence of the proposed algorithm is proved by experimenting with the different sources of datasets in which the performance metrics are evaluated and compared with other existing deep learning algorithms.
This research article proposed a blockchain empowered federated framework to improve the recognition of multiple-source heterogenous CT images and share the data among the hospitals while maintaining privacy and security. Also, ensembling of capsule networks and ELMs are used for effective feature extraction and classification to detect the COVID-19 among the different sources of publicly available heterogenous CT image datasets.
The paper is structured into four sections. The introduction is dealt in the first section and the second section presents the related works on the decentralized network. The data normalization, ensembled learning model, and blockchain-based federated data sharing mechanism are presented in Section FLED-Block Model. The experimentations, results, findings, and comparative analysis are discussed in Section Experimental Results. Finally, the paper is concluded in Section Results and Findings with future enhancement.
Related Works
To detect the diseases as early as possible, Supriya et al. studied the trending medical imaging analysis techniques in terms of prediction, e-treatment, stage classifications, virtual monitoring, and data transmission (24). Various supervised learning classifiers such as SVM, DT, KNN, and ANN are utilized in medical imaging. Similarly, the blockchain is important for public access to medical data and data transfer across worldwide using numerous blocks in a distributed approach. The author concluded that current medical image transmission is ruled by these innovations in the recent days for serving the healthcare industry.
People who are tested for the COVID-19 virus may not get enough instructions on how to manage and decrease both the risk of infection and the spread of the virus. COVID-19 data cannot be widely disseminated because of concerns about patient's privacy. To address the restrictions outlined above, this article offers a privacy architecture based on federated learning and blockchain technology. The proposed infrastructure can improve public communication and provide alternate means of disseminating COVID-19 information. Furthermore, the suggested architecture may effectively address the problem of huge data silos and offer a shared model while respecting the privacy of data owners. It has also been determined that the planned infrastructure can withstand information security and privacy breaches (32).
An open-source software framework based on federated learning for medical imaging is developed by Georgios et al. as PriMIA which deals with multiple sources of pediatric radiology for classification purposes (33). The proposed PriMIA includes the deep convolutional neural network (DCNN) designed to categorize the various stages of cardiac disease using the trained pediatric chest X-ray image database. DCNN is trained with gradient-based model for inversion attacks that detect chest disease at the earliest. Shichang et al. developed the blockchain-based security model for detecting malicious nodes, by merging competing vote authentication techniques and aggregating strategies to a federated model. The key factor of this propounded model is to optimize the communication cost in transferring the big data during the federating learning process and avoided the two standard attacks called “free-riding attacks” and “model poisoning attacks” (34).
Kim et al. (35) suggested a “blockchain federated learning” (BlockFL) architecture for decentralized federated learning. The architecture named “BlockFL” was able to overcome the single point of failure by expanding its federated scope. To entrust the devices in public networks, the local training outcomes are included in the verification procedure. In terms of trust and motivation, Bao et al. (36) presented FLchain, a centralized, publicly audited, and healthy federated learning ecosystem. The traditional federated learning central coordinator is replaced by blockchain in FLchain.
The blockchain architecture with global models is learned using the concept of channels. The channel-specific ledger-based blockchain architecture was presented by Majeed and Seon (37). Each local parameter of the model is kept as blocks in a channel-specific ledger and followed the decentralized data maintaining procedure. Martinez et al. (38) use a crypto currency and federated learning to solve the issues of data privacy and safety. The authors suggest a detailed methodology, an off-chain record database for scalable gradient recording and reward. Abdul Salam et al. proposed a new federated learning algorithm especially for patients with COVID-19. The proposed neural network is pre-trained with chest X-ray (CXR) images of abnormal patients to predict the death severity and to provide the e-treatment. The limitation of the developed predictor is less sufficient for big data (39).
A model based on capsule networks (COVID-CAPS) was presented to address the limitations of CNN-based models while dealing with short datasets by Parnian et al. (40). This model may be used to detect COVID-19-positive cases from X-ray images. It was observed that the COVID-CAPS model performed better than that of the conventional network when the model parameters were adjusted.
Using the neural architecture search (NAS) algorithm and a federated NAS (FedNAS) algorithm, He et al. (41) proposed an experimental study on automated federated learning (AutoFL), which aims to improve the quality and productivity of local machine learning models that connect their model updates. For non-IID (i.e., not unique ID) clients, they found that the default parameters of local machine learning models did not suit the federated context.
FLED-Block Model
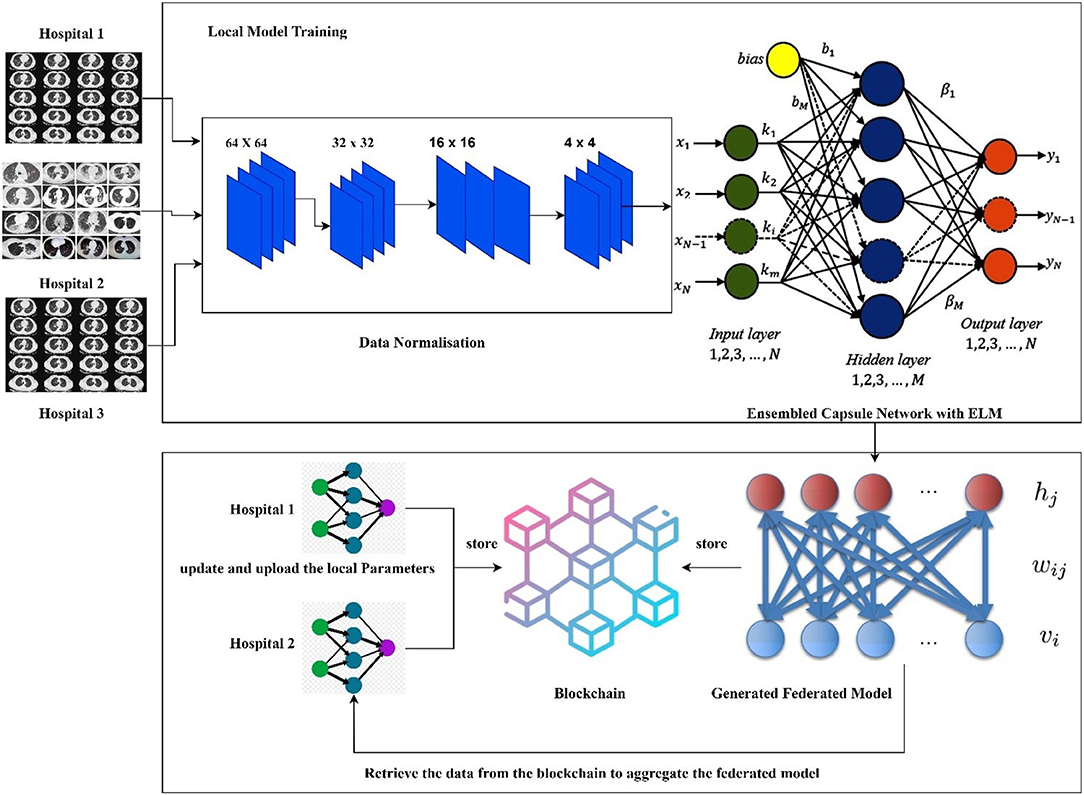
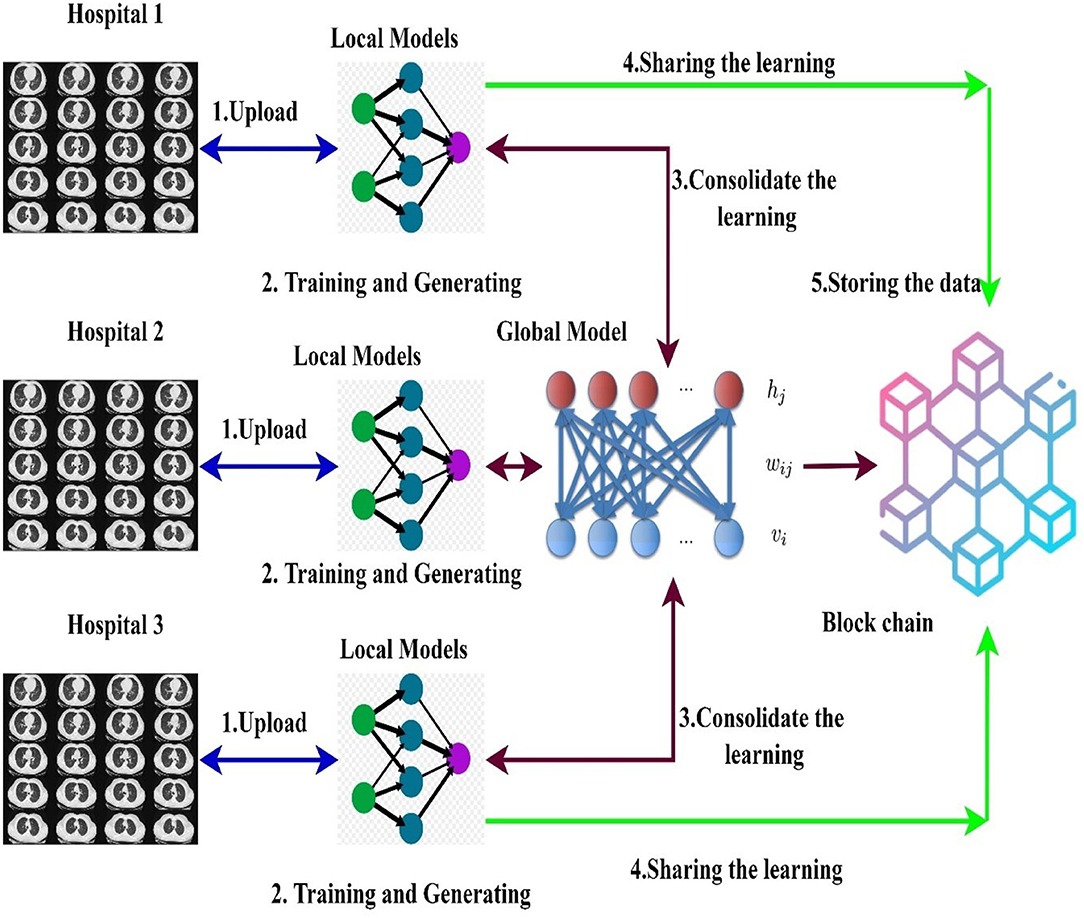
The hospitals and other healthcare organizations are showing a susceptible response to sharing the patients' data with other centers due to the breach of privacy of the patients. On implementing the deep learning models for an effective diagnosis of diseases, a large amount of data is required. The high amount of data processing increases the time complexity and leads to performance degradation most of the times. To handle real-time problems, this research proposes the new model FLED-Block, which can train and share the global models. Figure 1 presents the proposed framework.
The proposed FLED-Block architecture collects data cooperatively from various hospitals with a variety of different CT scanner types. As a first step in the process of data normalization, spatial normalization and signal normalization are being used. Then, deep learning algorithms are utilized to identify COVID-19 patterns in lung CT images. Image segmentation and training are done using the ensembled capsule network for greater generalization. As compared to other learning models, ensembled capsule network performed better.
The COVID-19 images are then classified using the extreme learning machines (ELMs). ELM is a neural network with one hidden layer which can self-tune. Finally, we employ the federated learning technique to build the global model and tackle the privacy issue. It gathers data, trains an adaptive model together, and then distributes this model throughout the public network. However, federated learning enables hospitals to keep their patient's information private while exchanging just weights and gradients via blockchain technology. Data from different hospitals may be communicated safely and securely using a decentralized architecture without compromising the security of patient's information.
More precisely, the proposed framework (1) collects the data from the different sources (medical health care centers), (2) trains the model by the hybrid capsule learning network for segmentation and classification of COVID-19 images, and (3) collaboratively shares the hybrid model using blockchain with federated learning while preserving the privacy of the organization.
Materials and Methodologies
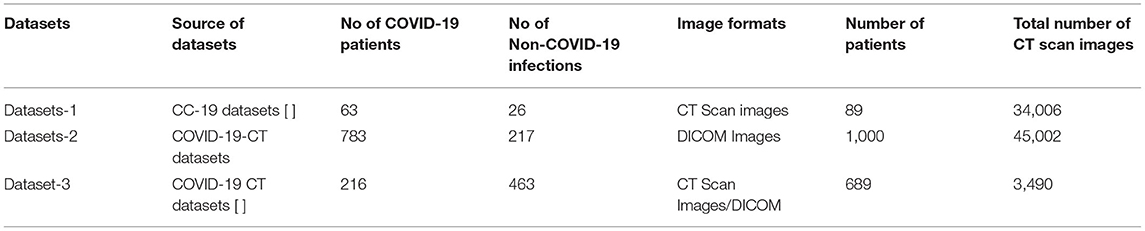
The role of artificial intelligence has occupied an unavoidable position in clinical diagnosis (42). Additionally, the deep learning algorithms need to be trained with the huge amount of data, and data collection is considered to be more important for validating the proposed model (43). The different heterogeneous CT image data sources were used for training the proposed model. The description of the datasets used for evaluating the model is presented as datasets 1, 2, and 3.
Dataset-1
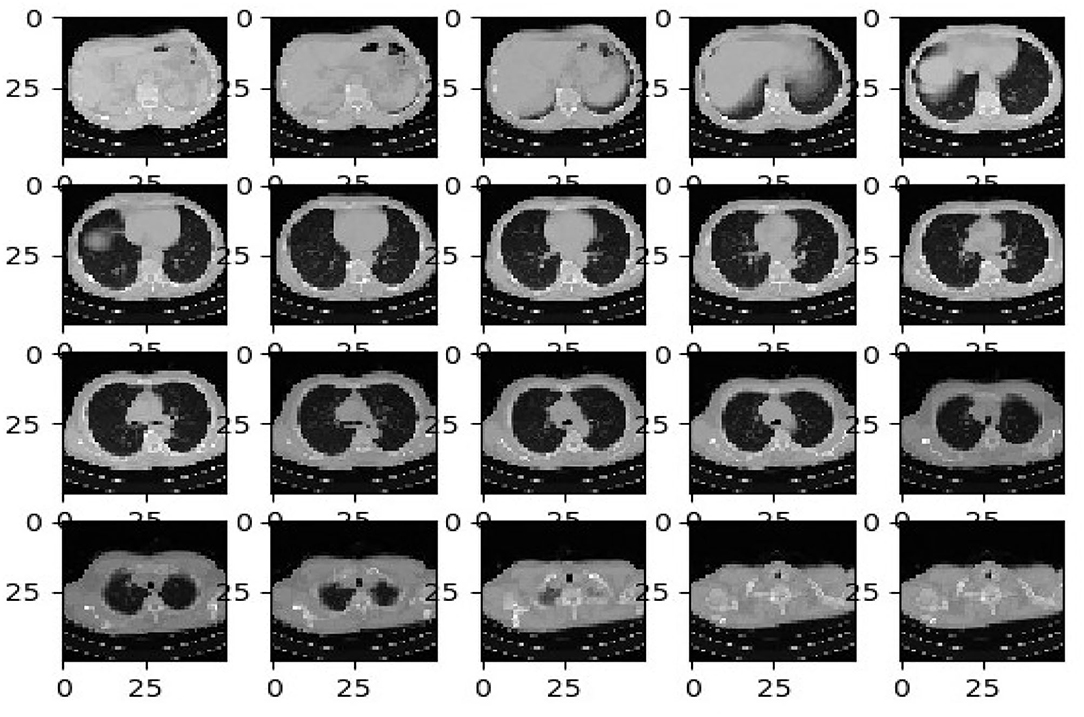
The first dataset holds 34,006 CT scan slices from three hospitals by 89 individuals, with 28,395 of the CT scan slices belonging to patients with COVID-19-positive (44). A total of six separate scanners have scanned the data, which includes the CT scan slices for 89 distinct people. In all, 68 of the 89 participants tested positive for the COVID-19 virus, whereas the other 21 tested negative. The gathered CT image datasets are shown graphically in the below table. Experimentation utilizing image datasets is shown in Figure 2.
Dataset-2
The second dataset is made up of patients with confirmed COVID-19 infections who have unenhanced chest CTs (45). “Hypertension or coronary heart disease, diabetes, and interstitial pneumonia or emphysema” were the most common concomitant disorders mentioned by patients. An in-patient setting was used to collect these images from patients who had positive “Reverse Transcription Polymerase Chain Reaction” (RT-PCR) tests for COVID-19 and accompanying clinical symptoms between March 2020 and January 2021. The images were taken during this period. No intravenous contrast was used during the CT examinations, which were performed in “Helical” mode on a NeuViz 16-slice CT scanner (Neu soft medical systems). All images are in DICOM format and are composed of 512 X 512 pixel 16-bit grayscale images.
Dataset-3
In this dataset 3, there are 349 COVID-19 CT scans from 216 patients and 463 non-COVID-19 CT images. These datasets comprised of initial images and both datasets were taken at the point of care in an epidemic situation from patients having RT-PCR confirmation for the presence of SARS-CoV-2. In (46, 47), the datasets are described in more detail.
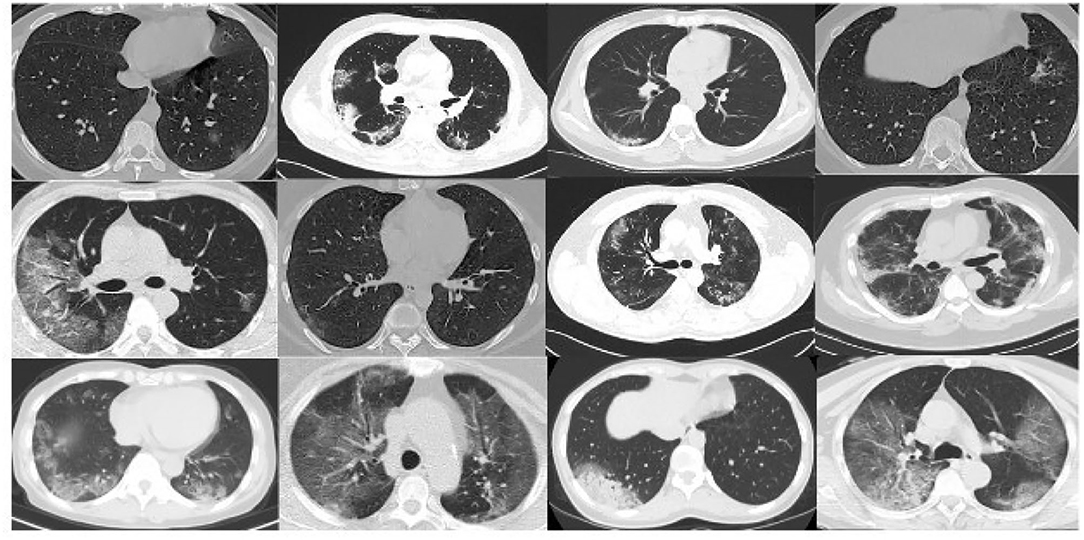
As the data are acquired from heterogeneous data sources, we develop the normalizing approach to adopt the CT scan images for a federated learning mechanism. Table 1 gives an overview of the datasets obtained for assessing the proposed federated learning model. Figure 3 illustrates the sample datasets utilized for experimentation.
Data Normalization
The data normalization technique mentioned in Krizhevsky et al. (48) is adopted by the proposed research. Since the heterogeneous data were used, a strong normalization technique is required to enhance the performance of the proposed federated learning models. As mentioned in Krizhevsky et al. (48), two types of normalization such as signal normalization and spatial normalization techniques are used in handling the CT scan images. The functionalities of these normalization techniques are discussed as the signal normalization technique and spatial normalization technique.
Signal Normalization Technique
Signal normalization calculates with the voxel's intensity based on the lung window. As every CT scan has Hounsfield units (HU), two types of windows such as window level (WL) and window width (WW) are mostly used in medical practices. Based on this window size, the normalized value is calculated using equation 1.
Where Onormalized is the image to be normalized in terms of intensity and O is the input original image. For this experimentation, lower bound window size has been chosen in the range of [−0.05, 0.5].
Spatial Normalization Technique
The spatial normalization is adopted based on the dimension and resolution of the CT scan images. All the CT scan images are converted to a standard resolution of 332 × 332 × 512 mm3 as mentioned in Krizhevsky et al. (48). This normalization technique is applied to all the collected datasets and all formats of images are normalized to standard format (49) which can be applied to federated learning. It achieves better learning and performance.
Ensembled Capsule-Based Model Training
Recently, usage of the deep learning framework has gained its own popularity with the strong feature extraction layers and its classification mechanism. Over the past few years, convolutional neural network (CNN) is predominantly utilized for image classification analysis. Since the pooling layers of CNN do not consider the spatial relationship between the features in an image, this leads to high computational complexity and even affects the classifier's performance. To achieve better classification accuracy and diagnosis, the proposed system ensembles the powerful features of capsule networks and extreme learning machines (ELM) to overcome these above-mentioned problems. In this approach, capsule networks are used for extracting the strong feature maps whereas ELM replaces the traditional dense classification layers for better prediction of COVID-19.
Capsule Networks
Capsule network (50) had “(1) convolutional layer, (2) hidden layer, (3) Primary Caps layer, and (4) DigitCaps layer” which had addressed the limitation. Figure 4 shows the complete architecture for the proposed training model. The input normalized image is given as the input to the proposed capsule networks. It is divided into two phases.
• Probability of existence in entities.
• Entities' instantiation parameters.
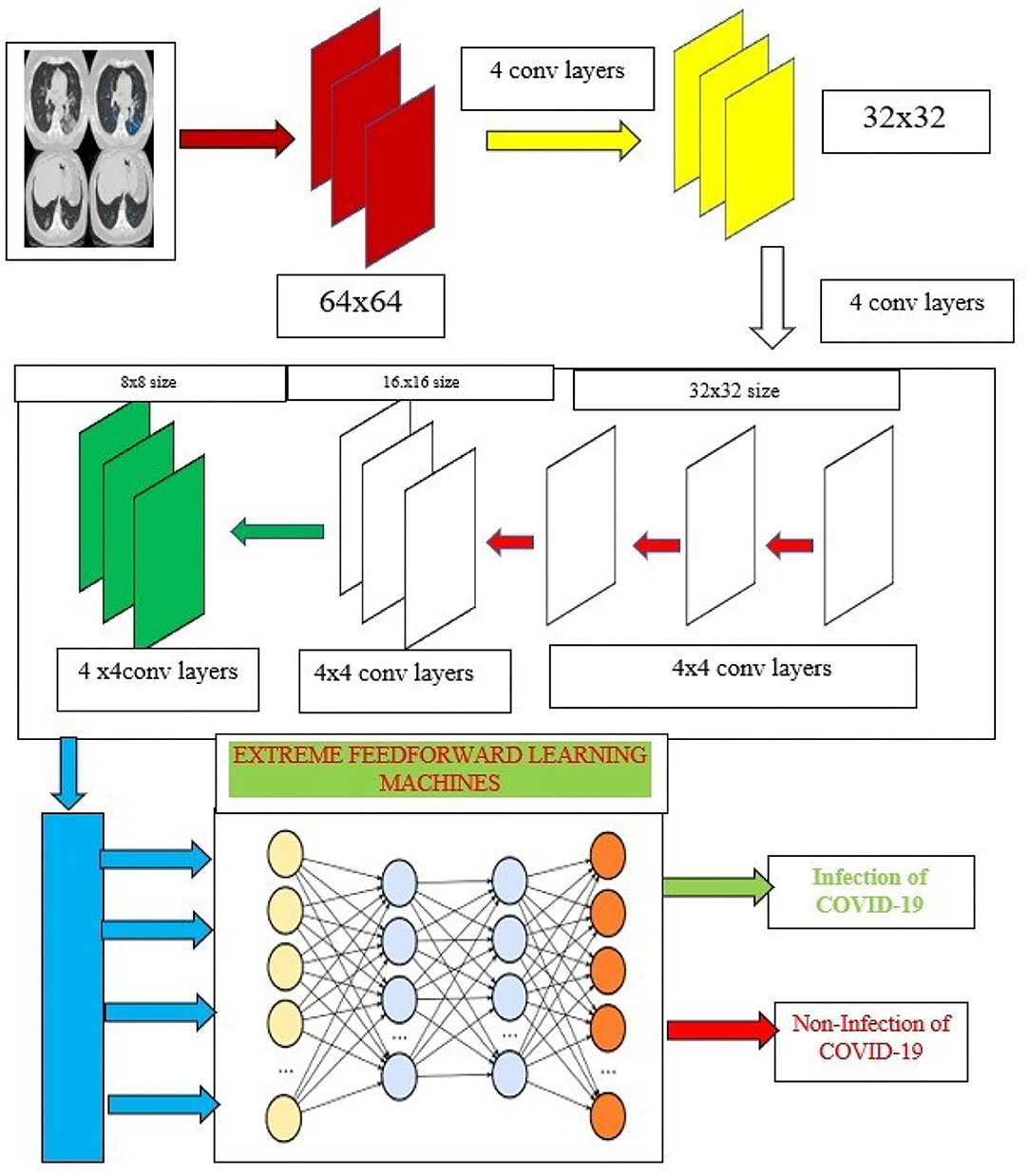
Figure 4. Capsule ensembled ELM layers for achieving the feature extraction and classification accuracy.
To encode the imperative spatial association between low- and high-level features within the image, equation 2 is calculated, whereas the input vectors “s” and the weight matrix “W” and component vector “U.”
The sum of the weighted input vectors is calculated to determine the current capsule “D” using the equation 3
Finally, non-linearity is applied using the squash function using equation 4.
The distribution of the “low level capsule to the high-level capsule” is progressively adjusted according to the outcome until an optimal distribution will be attained.
Extreme Learning Machines
The proposed research incorporates the capsule networks for better feature extraction which can help to achieve the highest accuracy of classification. Additionally, these features are then fed to the extreme learning machines (ELMs) which are used to classify the images. ELM is a kind of neural network that utilizes the single hidden layers and works on the principle of autotuning property which is depicted in Figure 4.
Extreme learning machine exhibits better performance when compared to the other learning models. Performance metrics considered are high speed and less computational overhead. The learning models compared are support vector machines (SVMs), Bayesian classifier (BC), K-nearest neighborhood (KNN), and even random forest (RF) (51).
The neural network utilizes a single hidden layer, that does not require the tuning mandatorily. ELM uses the kernel function to yield good accuracy for better performance. The major advantages of the ELM are minimal training error and better approximation, since ELM uses the autotuning of the weight biases and non-zero activation functions. The detailed working mechanism of the ELM is discussed in Huang et al. (52) and Wang et al. (53).
Mathematically, the characteristic of ELM is represented by
where x → input
β → output weight vector and it is given as follows as:
h(x) output hidden layer which is given by the equation 7
- Hence, the outcome can be found by the equation 8.
where O is output target vectors which are solved by bias weights of hidden layers which are solved by the Moore's pseudo-concepts (52). Based on equation (7), the infectious impact of COVID-19 on lungs is classified effectively. The working mechanism of the propounded network is presented in Algorithm 1.
Federated Learning for Global Training
In this section, decentralized data sharing mechanism with multiple hospitals has been considered. The proposed model adopts federated learning for sharing the hospital's models without sacrificing privacy and also aggregates the different models shared by the different hospitals. For this scenario, we consider the number of hospitals as H and d as the overall datasets. In the proposed federated model, the ensembled learning model is considered as the global model M in which the weights W of the ELM are distributed randomly to the other hospitals. Figure 5 shows the federated learning model adopted in the proposed research.
A blockchain-based federated learning framework that can be used to train and share in a collaborative way (50). The hospital uses a global or collaborative model, which is referred to as “federated learning,” to integrate the weights of the locally trained model (54). Initially, the data are gathered from many sources into the local model devised with a normalization approach to cope with various types of CT scan data. After normalizing the data, the second step is to segment the images using the ensembled capsule network to train the model to recognize COVID-19 suspects. Finally, we distribute the weights of the local model across the blockchain network to train a global model.
Mathematically, let H be the number of hospitals, d be the total datasets which consists of training and testing datasets as in equation 8
Also testing data are represented as in equation 9
Hence, the total dataset used for training the global model is in equation 10.
Since the data D(i) are collected from the heterogeneous source of hospitals, the distribution of data remains unequal. In every round of the communication, weights W of ELM are distributed among the hospitals. The hospitals create the local model with weights obtained and stored in the blockchain network. Then, weights are updated and loaded into the blockchain network for each round. The updated weights are mathematically updated like in equation 11
where Wi and Wl are considered as distributed weights of the global model and weights of the local model, respectively. Finally, the all-local models in the blockchain are aggregated to form the new learning model which works on the principle of ELM which is implemented in the proposed deep learning algorithm.
Blockchain Framework for Federated Learning
The proposed framework incorporates the blockchain architecture for an effective and secured data retrieval and sharing process of federated learning. Multiple hospitals can collaboratively learn and train the models for the better detection of diseases. Based on the multiple-organization blockchain architecture designed by (55, 56), the proposed work discusses about the data retrieval and sharing process adopted in the proposed approach.
Blockchain-Based Data Retrieval Process
Every hospital provides the data (local model) and stores it as a transaction in the block chain network (57). Retrieving the data from the nodes depends on the two parameters such as distance between the nodes(d) and ID of the hospital (ID). Based on the distance between the hospitals, a unique ID is created. The blockchain maintains the log tables to store the unique ID of the user hospital. The data are retrieved from the neighborhood hospitals identified by their unique IDs.
Mathematically, hospitals are denoted by X partitioned as the different communities in which the hospitals are considered as nodes. The expression used to measure the neighborhood distance between the nodes is given by equation 12
where X(i) and X(j) are the neighborhood hospitals located at ith and jth position which are differentiated by their unique ID.
In the next stage, the consensus process is used to train the FLED-Block model using the stored local models. In this way, all of the nodes work together to train the FLED-Block. It gives proof of work, which allows the data to be shared among the various nodes. The consensus approach verifies the quality of the local models during collaborative training by calculating the mean prediction accuracy error (MPAE). The MPAE is a result of a better ability to forecast the future. All data on the node are encrypted using high random Chaotic public (58) and private keys (50, 52, 53) to keep the data confidential. All transactions are subjected to MPAE, which is then recorded in the distributed ledger of the blockchain.
Blockchain-Based Data Sharing Process
Security is a vital role in sharing the data between requester and source hospitals (59). Instead of sharing the complete data, hospitals can provide only learned models with the requesters. The hospitals can communicate with each other, and consensus algorithm is used to learn from federated data. The providers' and requesters' data stored in the blockchain nodes (60). To maintain data privacy, only learning models are shared instead of original data information (61). In the first phase, each hospital uploads the image datasets for collaboratively learning. In the second phase, hospitals share the locally trained model weights with the blockchain and use the federated learning to aggregate all the local models into global models.
Experimental Results
The proposed model was developed using opensource TensorFlow federated version 2.1.0, whereas the classical models are developed using TensorFlow version 1.8. with both the models using Keras as backend. The complete experimentation is carried out on a PC workstation with Intel Xeon CPU, NVIDIA Titan GPU, 16GB RAM, and 3.5 GHZ operating frequency. Each dataset is splitted into 70% for training, 20% for testing, and 10% for validation, respectively. The 70% of all the three datasets (23,804, 31,501, and 2,443 images) are used for training the proposed model using TensorFlow federated version 2.1.0, and it is also used to train the other classical model using TensorFlow version 1.8. These models are implemented on the federated blockchain using python 3.9.1. The user interface for the blockchain runs on CSS script. This setup is utilized for validating and testing the algorithm. The proposed architecture is evaluated by the various performance metrics such as accuracy, precision, recall, specificity, and F1-score. These metrics are the representation of the model's ability that how correctly the model differentiates between the COVID-19 and non-COVID infections among the subjects using the proposed federated model and other classical models (62). Table 2 presents the mathematical expressions used for calculating the performance metrics.
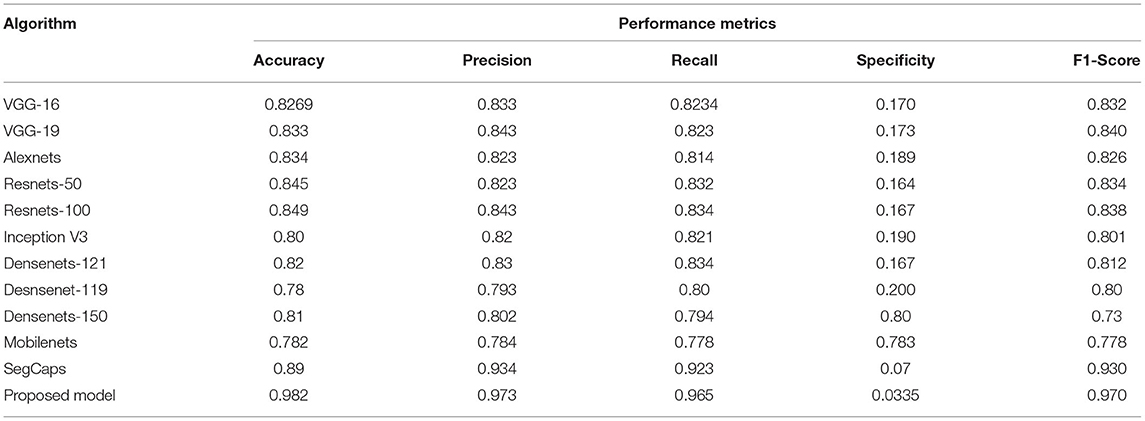
Table 2. Comparative analysis of the different algorithms in detecting the COVID-19 using dataset 1.
A medical diagnosis-based system needs to have “high accuracy, high precision and recall.” To solve the model's overfitting problem and improve the generalization problem, the early stopping method (63) is used. This method can be used to end the proposed network training. when the validation performance shows no improvement for N consecutive times.
Results And Findings
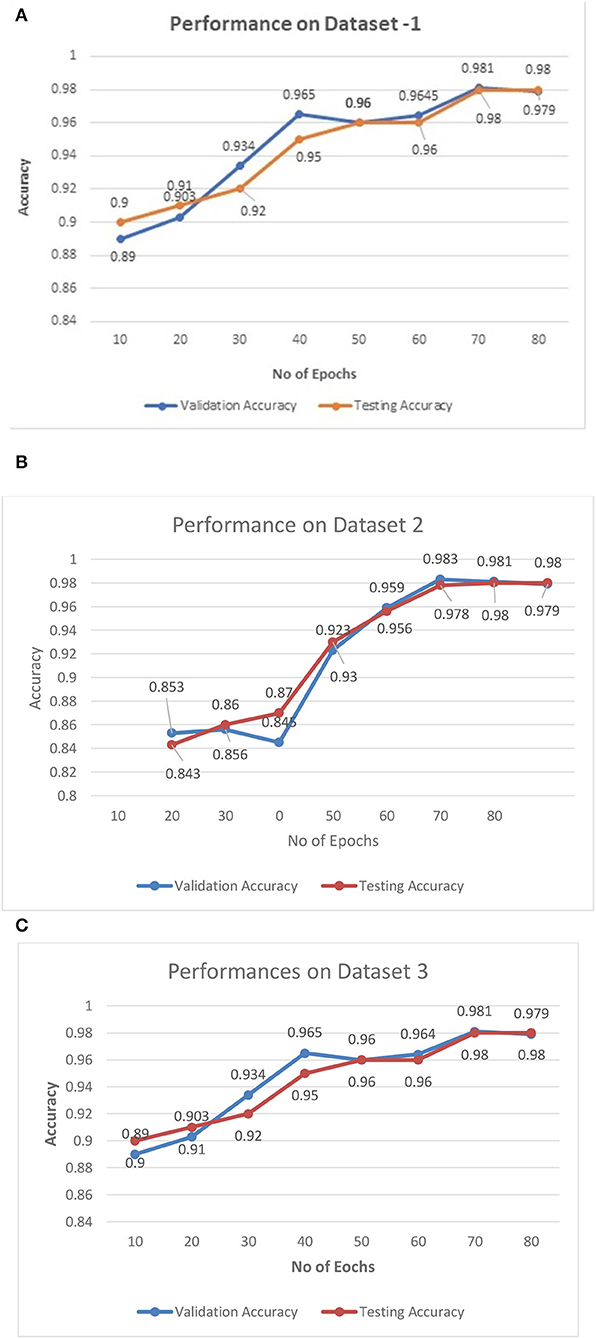
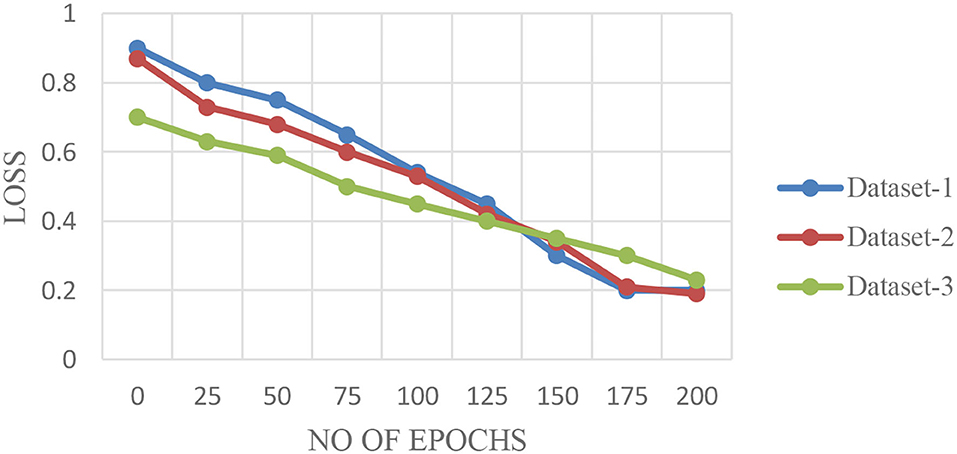
The performance of the proposed architecture is validated in three folds. In the first fold, the performance metrics of the proposed algorithm are calculated for the different CT datasets. Additionally, loss validation curves (LVC) are calculated for validating the performance of the proposed architecture. Finally, the excellence of the proposed algorithm has been proved by comparing it with the other existing deep learning algorithms. This section presents the performances of the proposed model using the different datasets as depicted in Figures 6A–C.
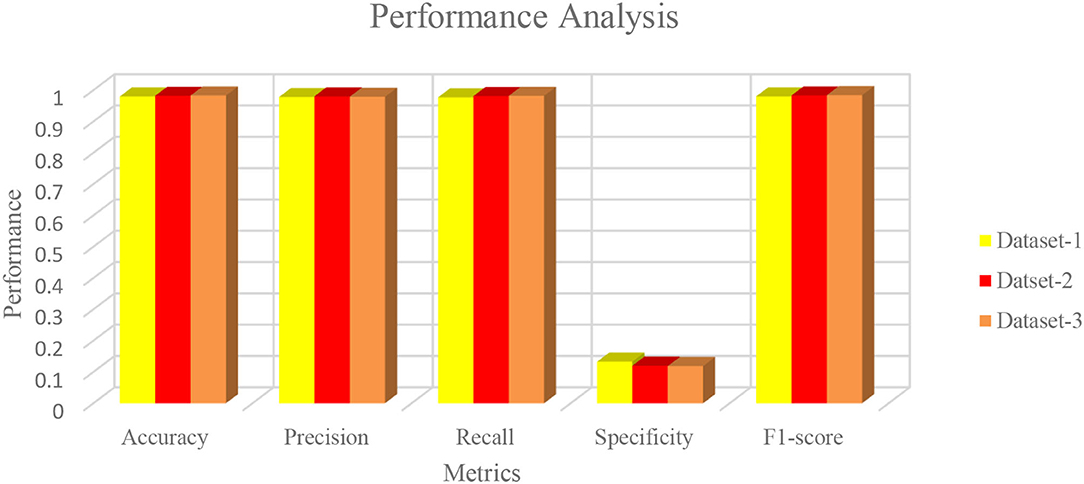
Figures 6A–C illustrate the validation curves for training the proposed model with the different datasets whereas Figure 7 shows the loss validation curves of the model. From Figure 6A, it is found that the root mean square error (RMSE) between the training and validation model is found to be 0.001. A similar fashion of characteristics is found in the Figure 6C. As more datasets are involved in dataset 2, RMSE is a little higher in Figure 6B, i.e., RMSE is 0.0014. Hence, the average performance of the proposed model in detecting the COVID-19 is found to have 98.5 (dataset 1), 98.3 (dataset 2), and 98.5% (dataset 3), respectively.
The characteristics of capsule feature extraction and ELM as the classification layer in the proposed model help to maintain the uniform performance for the multi-source heterogeneous datasets. Figure 8 depicts the performance metrics of the propounded model in handling the different datasets. It is found that the average performance of the model ranges from 98.4 to 985% of accuracy, 97 to 98% of precision, and 97.5 to 98% of recall. Furthermore, it is found that the proposed model exhibits high false alarm rates and low specificity for all the three datasets as depicted in Figure 8.
To prove the excellence of the proposed model, a comprehensive comparative analysis between the deep learning algorithms such as VGG-16/VGG-19 (64–66), AlexNETS (67), DenseNETS (68–70), ResNETS-50/100 (59, 71), and even SegCAP (72) modules is done. Table 2 presents the performance of the different deep learning models and the proposed model in handling the dataset 1 From Table 2, it is found that the proposed model has shown the highest performance and topped over SegCAP networks and Resnets-100 which has produced the considerable good performances. Table 3 performs the divergent models with the proposed model.
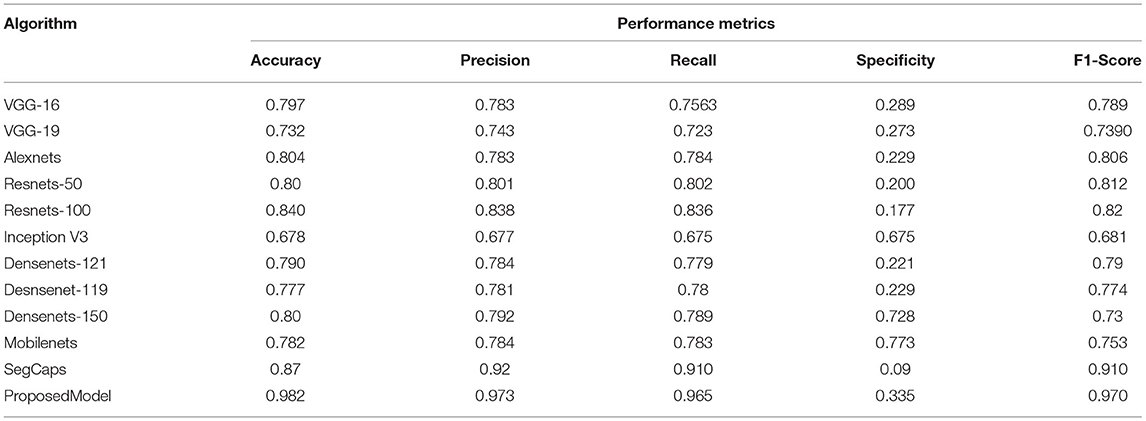
Table 3. Comparative analysis of the different algorithms in detecting the COVID-19 using dataset 2.
As the datasets are high, all deep learning models have shown dip in performance of prediction whereas SegCAP and Resenets-100, the proposed model has shown promising performance for increased datasets in which the proposed model has outperformed the other model in the rocketing style. Also, a similar fashion of performance as in Table 2 is observed in Table 4, which clearly shows that the proposed model has outperformed the other models. The ensemble of capsule networks and extreme learning machines has produced the best accuracy in detecting COVID-19 from the multiple sources of datasets and proves its superiority over the other algorithms.
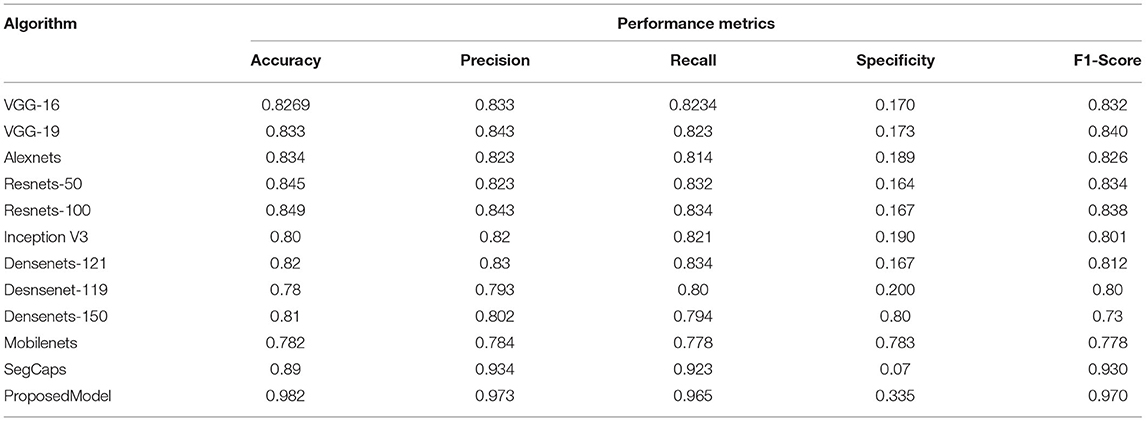
Table 4. Comparative analysis of the different algorithms in detecting the COVID-19 using dataset 3.
Finally, comparison of a proposed model with the other blockchain-based learning models is used in terms of detection accuracy, trust level, and number of datasets. Table 5 presents the comparative analysis of the blockchain-based learning model used for data sharing and data retrieval. Tables 5–7 show that the proposed model has found its good place of suitability in the blockchain for a better data sharing and retrieval process without sacrificing the data's privacy and security.
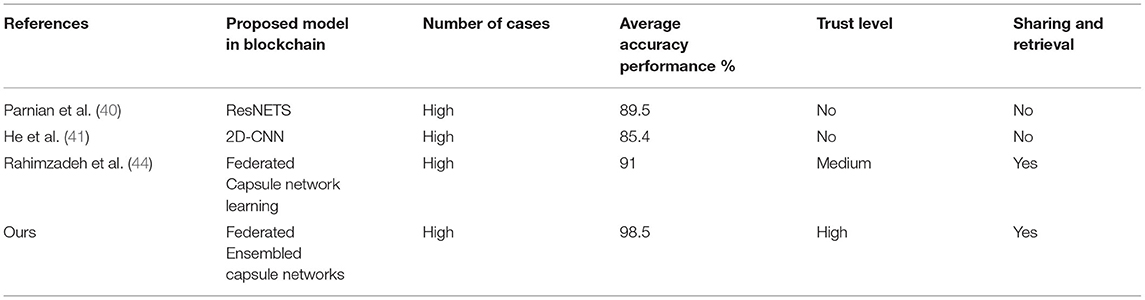
Table 5. Comparative analysis between the blockchain-based learning models for COVID-19 detection of diseases using dataset 1.

Table 6. Comparative analysis between the blockchain-based learning models for COVID-19 detection of diseases using dataset 2.
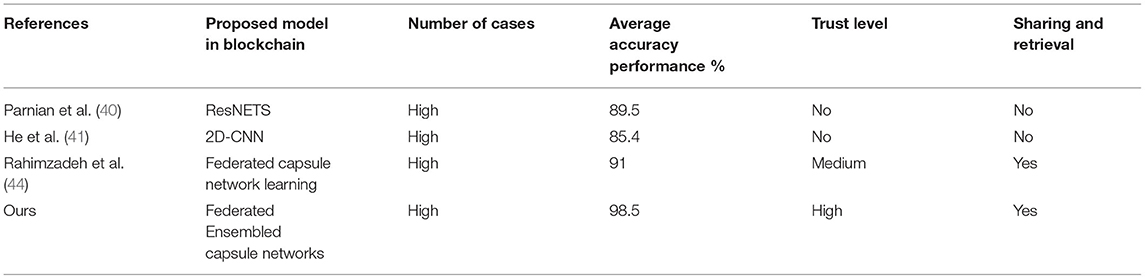
Table 7. Comparative analysis between the blockchain-based learning models for COVID-19 detection of diseases using dataset 3.
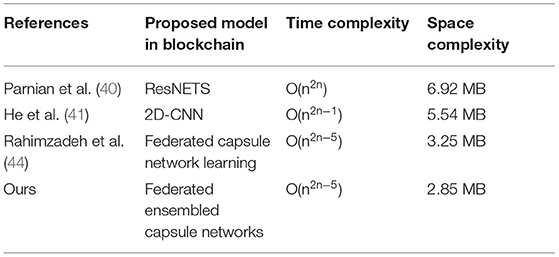
The proposed model and federated model proposed in He et al. (41) have shown the same characteristics, but the proposed method has shown a slight edge with a 7% increment of trust. It is due to the inclusion of chaotic encryptions and detection accuracy in terms of integration of capsule and ELM.
Furthermore, the time complexity and space complexity of the proposed model are compared with the other state-of-the-art classical models. The time complexity is calculated based on big-oh notation, which is generally mentioned as O(n). Since all the classical learning models execute on the centralized system, the number of N computations increases with the power of two. The proposed model uses the distributed system, and the number of execution times decreases based on the number of nodes utilized for the training. In the experimentation, we used 5 nodes which are utilized for training, in which N is reduced to five. Table 8 represents the comparative analysis of different time complexity with the proposed and other existing classical models. Space complexity is measured by memory utilized by the algorithm.
The table represents the time complexity of the different algorithms. From Table 8, it is proved that the federated learning model has achieved less complexity than the other classical models due to a distributed system. The space complexity is the amount of the memory consumed by the model for better execution. The federated model consumes less memory due to its distributive nature whereas the proposed federated model with the usage of an extreme learning machine has reduced the space complexity because of the feed forward nature.
Conclusion
The existing classical AI techniques often require centralized data storage and training for the predictive model development which leads to computational complexity and also affects privacy. To overcome the problem, this paper proposes the blockchain empowered federated framework to enhance the perception of multiple sources of heterogeneous CT images. It shares the data among the hospitals while maintaining privacy and security. Also, an ensemble of capsule networks and extreme learning machines are used for effective feature extraction and classification to detect the COVID-19 among the different sources of publicly available heterogenous CT image datasets. Furthermore, federated learning is adopted for the collaborative training of hospitals backed with blockchain technology. Also, the addition of chaotic encryption keys in the process of data retrieval and sharing process has added more trust in terms of maintaining privacy and security. Comprehensive experimentation has been conducted and compared with the other deep learning algorithms. Additionally, the performance of the proposed algorithm has been compared with the other blockchain powered federated deep learning modules. The results demonstrate that the proposed model has shown the highest performance in terms of accuracy, precision, recall, and high F1-score and proves to be more vital than the other algorithms in terms of trust and datasets. Though the proposed method has shown better performance, it needs improvisation in handling the real-time clinical databases. In the future, an effort will be done to reduce the latency of the blockchain and optimize the cost-effectiveness of the solution.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
RD contributed to the conception and design of the study. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ritchie H, Ortiz-Ospina E, Beltekian D, Mathieu E, Hasell J, Macdonald B. Coronavirus Pandemic (COVID-19). Our World in Data. Statistics and Research. (2021). Available online at: https://ourworldindata.org/coronavirus (accessed September 30, 2021).
2. MOHFW. Home. Ministry of Health Family Welfare. GOI. Available online at: mohfw.gov.in (accessed September 30, 2021).
3. MyGov.in. Govt of India (2021). Retrieved from: https://www.mygov.in/covid-19 (accessed June 12, 2021).
4. Scroll Staff. Coronavirus: India Records 25,166 New Cases in 24 Hours—Lowest in 154 Days. Available online at: Scroll.in (accessed August 17, 2021).
5. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. (2020) 20:533–4. doi: 10.1016/S1473-3099(20)30120-1
6. Podder P, Khamparia A, Mondal MRH, Rahman MA, Bharati S. Forecasting the spread of COVID-19 and ICU requirements. Int J Online Biomed Eng. (2021) 17:81–99. doi: 10.3991/ijoe.v17i05.20009
7. Mahmoudi MR, Heydari MH, Qasem SN, Mosavi A, Band SS. Principal component analysis to study the relations between the spread rates of COVID-19 in high risks countries. Alexandria Eng J. (2021) 60:457–64. doi: 10.1016/j.aej.2020.09.013
8. BBC News. Jump up to:a b “India Coronavirus: New Record Deaths as Virus Engulfs India”. Toronto, ON: BBC News (2021).
9. Das NN, Kumar N, Kaur M, Kumar V, Singh D. Automated deep transfer learning-based approach for detection of covid-19 infection in chest. Ing Rech Biomed. (2020) 43:114–9. doi: 10.1016/j.irbm.2020.07.001
10. Zakria, Deng J, Cai J, Aftab MU, Khokhar MS, Kumar R. Visual features with spatio-temporal-based fusion model for cross-dataset vehicle re-identification. Electronics. (2020) 9:1083. doi: 10.3390/electronics9071083
11. Pathak Y, Shukla PK, Tiwari A, Stalin S, Singh S. Deep transfer learning based classification model for covid-19 disease. IRBM. (2020) 43:87–92. doi: 10.1016/j.irbm.2020.05.003
12. Kumar R, Wang W, Kumar J, Yang T, Khan A, Ali W, et al. An integration of blockchain and ai for secure data sharing and detection of CT images for the hospitals. Comput Med Imaging Graph. (2021) 87:101812. doi: 10.1016/j.compmedimag.2020.101812
13. Khan AA, Shafiq S, Kumar R, Kumar J, Haq AU. H3dnn: 3D deep learning based detection of covid-19 virus using lungs computed tomography. In: 2020 17th International Computer Conference on Wavelet Active Media Technology Information Processing (ICCWAMTIP). (2020). p. 183–6. doi: 10.1109/ICCWAMTIP51612.2020.9317357
14. Podder P, Bharati S, Mondal MRH, Khamparia A. Rethinking the transfer learning architecture for respiratory diseases and COVID-19 diagnosis. In: Khamparia A, Gupta D, Khanna A, Balas VE, editors. Biomedical Data Analysis and Processing Using Explainable (XAI) and Responsive Artificial Intelligence (RAI) Intelligent Systems Reference Library. Singapore: Springer (2022). p. 105–21.
15. Bharati S, Podder P, Mondal M, Prasath VB. CO-ResNet: optimized ResNet model for COVID-19 diagnosis from X-ray images. Int J Hybrid Intell Syst. (2021) 17:71–85. doi: 10.3233/HIS-210008
16. Choe J, Lee SM, Do KH, Lee G, Lee JG, Lee SM, et al. Deep learning–based image conversion of CT reconstruction kernels improves radiomics reproducibility for pulmonary nodules or masses. Radiology. (2019) 292:365–73. doi: 10.1148/radiol.2019181960
17. Kermany DS, Goldbaum M, Cai W, Valentim CC, Liang H, Baxter SL, et al. Identifying medical diagnoses and treatable diseases by image-based deep learning. Cell. (2018) 172:1122–31.e9. doi: 10.1016/j.cell.2018.02.010
18. Negassi M, Suarez-Ibarrola R, Hein S, Miernik A, Reiterer A. Application of artificial neural networks for automated analysis of cystoscopic images: a review of the current status and future prospects. World J. Urol. (2020) 38:2349–58. doi: 10.1007/s00345-019-03059-0
19. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. (2016) 316:2402–10. doi: 10.1001/jama.2016.17216
20. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
21. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
22. Kothai G, Poovammal E, Dhiman G, Ramana K, Sharma A, Alzain MA, et al. A new hybrid deep learning algorithm for prediction of wide traffic congestion in smart cities. Wireless Commun Mob Comput. (2021) 2021:5583874. doi: 10.1155/2021/5583874
23. Wang X, Deng X, Fu Q, Zhou Q, Feng J, Ma H, et al. A weakly-supervised framework for COVID-19 classification and lesion localization from chest CT. In: IEEE Transactions on Medical Imaging, Vol. 39. IEEE (2010). p. 2615–25. doi: 10.1109/TMI.2020.2995965
24. Supriya M, Chattu VK. A review of artificial intelligence, big data, and blockchain technology applications in medicine and global health. Big Data Cogn Comput. (2021) 5:41. doi: 10.3390/bdcc5030041
25. Mondal MRH, Bharati S, Podder P. CO-IRv2: optimized inceptionresnetv2 for COVID-19 detection from chest CT images. PLoS ONE. (2021) 16:e0259179. doi: 10.1371/journal.pone.0259179
26. Bharati S, Subrato Hossain M, Rubaiyat M. 12 Applications challenges of AI-driven IoHT for combating pandemics: a review. In: Khamparia A, Mondal RH, Podder P, Bhushan B, Hugo V, de Albuquerque C, Kumar S, editors. Computational Intelligence for Managing Pandemics. Berlin, Boston, MA: De Gruyter (2021). p. 213–30. doi: 10.1515/9783110712254-012
27. Podder P, Bharati S, Mondal MRH, Utku K. 9 - Application of Machine Learning for the Diagnosis of COVID-19. Academic Press (2021)
28. Mondal MRH, Bharati S, Podder P. Diagnosis of COVID-19 using machine learning and deep learning: a review. Curr Med Imaging. (2021) 17:1403–18. doi: 10.2174/1573405617666210713113439
29. Bharati S, Podder P, Mondal MR, Prasath V. Medical imaging with deep learning for COVID- 19 diagnosis: a comprehensive review. arXiv Preprint. (2021). doi: 10.48550/arXiv.2107.09602
30. Zhang R, Xue R, Liu L. Security and privacy on blockchain. ACM Comput Surv. (2019) 52:34. doi: 10.1145/3316481
31. Shi F, Xia L, Shan F, Wu D, Wei Y, Yuan H, et al. Large-scale screening of covid-19 from community acquired pneumonia using infection size-aware classification. arXiv preprint. (2020) arXiv:2003.09860. doi: 10.1088/1361-6560/abe838
32. Samuel O, Omojo AB, Onuja AM, Sunday Y, Tiwari P, Gupta D, et al. IoMT: A COVID-19 healthcare system driven by federated learning and blockchain. IEEE J Biomed Health Inform. doi: 10.1109/JBHI.2022.3143576. [Epub ahead of print].
33. Georgios K, Alexander Z, Jonathan P. End-to-end privacy preserving deep learning on multi-institutional medical imaging. Nat Mach Intell. (2021) 3:473–84. doi: 10.1038/s42256-021-00337-8
34. Shichang X, Ming J, Xin L, Yao Z, Yang W, Man D. DAM-SE: a blockchain-based optimized solution for the counterattacks in the internet of federated learning systems. Secur Commun Netw. (2021) 2021:14. doi: 10.1155/2021/9965157
35. Kim H, Hong PJ, Mehdi B, Seong-Lyun K. Blockchained on-device federated learning. IEEE Commun Lett. (2020) 24:1279–83. doi: 10.1109/LCOMM.2019.2921755
36. Bao X-L, Su C, Xiong Y, Huang W, Hu Y. FLChain: a blockchain for auditable federated learning with trust and incentive. In: Proceedings of the 5th International Conference on Big Data Computing and Communications (Bigcom). Qing Dao: IEEE (2019). p. 151–9. doi: 10.1109/BIGCOM.2019.00030
37. Majeed U, Seon HC. FLchain: federated learning via MEC-enabled blockchain network. In: Proceedings of the 20th Asia-Pacific Network Operations and Management Symposium (APNOMS). Matsue: IEEE (2019). p. 1–4. doi: 10.23919/APNOMS.2019.8892848
38. Martinez I, Francis S, Hafid AS. Record and reward federated learning contributions with blockchain. In: Proceedings of the 2019 International Conference on Cyber-Enabled Distributed Computing and Knowledge Discovery (CyberC). Guilin: IEEE (2019). p. 50–7. doi: 10.1109/CyberC.2019.00018
39. Abdul Salam M, Taha S, Ramadan M. COVID-19 detection using federated machine learning. PLoS ONE. (2021) 16:e0252573. doi: 10.1371/journal.pone.0252573
40. Parnian A, Shahin H, Farnoosh N, Plataniotis AO, Konstantinos N, Arash M. Covid-caps: a capsule network-based framework for identification of covid-19 cases from x-ray images. Patt Recogn Lett. (2020) 138:638–43. doi: 10.1016/j.patrec.2020.09.010
41. He C, Annavaram M, Avestimehr S. Fednas: federated deep learning via neural architecture search. arXiv preprint. (2020) arXiv:2004.08546.
42. Bharati S, Podder P, Mondal MRH. Hybrid deep learning for detecting lung diseases from X-ray images. Inform Med Unlock. (2020) 20:100391. doi: 10.1016/j.imu.2020.100391
43. Bharati S, Podder P, Mondal MRH, Gandhi N. Optimized NASNet for diagnosis of COVID-19 from lung CT images. In: 20th International Conference on Intelligent Systems Design and Applications. Springer (2020). p. 647–56.
44. Rahimzadeh M, Attar A, Sakhaei SM. A fully automated deep learning-based network for detecting COVID-19 from a new and large lung CT scan dataset. Biomed Signal Process Cont. (2021) 68:102588. doi: 10.1016/j.bspc.2021.102588
45. Zhao J, Zhang Y, He X, Xie P. Covid-ct-dataset: a ct scan dataset about covid-19. arXiv preprint. (2020) arXiv:2003.13865.
46. Bellemare F, Jeanneret A, Couture J. Sex differences in thoracic dimensions and configuration. Am J Respir Crit Care Med. (2003) 168:305–12. doi: 10.1164/rccm.200208-876OC
47. Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. In: Bengio Y, LeCun Y, editors. 3rd International Conference on Learning Representations. San Diego, CA (2015).
48. Krizhevsky A, Sutskever I, Hinton GE. Imagenet classification with deep convolutional neural networks. In: Bartlett PL, editor. Advances in Neural Information Processing Systems 25: 26th Annual Conference on Neural Information Processing Systems 2012. (2012). p. 1106–14.
49. Wang Y, Huang L, Jiang S, Wang Y, Zou J, Fu H, et al. Capsule networks showed excellent performance in the classification of herg blockers/nonblockers. Front Pharmacol. (2020) 10:1631. doi: 10.3389/fphar.2019.01631
50. Yang Q, Liu Y, Chen T, Tong Y. Federated machine learning: concept and applications. ACM Trans Intell Syst Technol. (2019) 10:19. doi: 10.1145/3298981
51. Joloudari JH, Joloudari EH, Saadatfar H, Ghasemigol M, Razavi SM, Mosavi A, et al. Coronary artery disease diagnosis; ranking the significant features using a random trees model. Int J Environ Res Public Health. (2020) 17:731. doi: 10.3390/ijerph17030731
52. Huang G-B, Zhu Q-Y, Siew C-K. Extreme learning machine: theory and applications. Neurocomputing. (2006) 70:489–501. doi: 10.1016/j.neucom.2005.12.126
53. Wang B, Huang S, Qiu J, Liu Y, Wang G. Parallel online sequential extreme learning machine based on MapReduce. Neurocomputing. (2015) 149:224–32. doi: 10.1016/j.neucom.2014.03.076
54. Durga R, Poovammal E. Federated learning model for healthchain system. In: 2021 6th IEEE International Conference on Recent Advances and Innovations in Engineering (ICRAIE). (2021). p. 1–6. doi: 10.1109/ICRAIE52900.2021.9703948
55. Maymounkov P, Mazieres D. Kademlia: A peer-to-peer informa- ‘ tion system based on the XOR metric. In: Computer Science (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). (2002). doi: 10.1007/3-540-45748-8_5
56. Rahman A, Islam MJ, Montieri A, Nasir MK, Reza MM, Band SS, et al. SmartBlock-SDN: an optimized blockchain-SDN framework for resource management in IoT. IEEE Access. (2021) 9:28361–76. doi: 10.1109/ACCESS.2021.3058244
57. Sohaib Latif A, Fang B, Wen X, Iwendi C, Wang LF, Muhammad Mohsin S, et al. AI-empowered, blockchain and SDN integrated security architecture for IoT network of cyber physical systems. Comp Commun. (2022) 181:274–83, doi: 10.1016/j.comcom.2021.09.029
58. Kothai G, Poovammal E. Conventional and hybrid encryption schemes for security against attacks in vehicular adhoc network. In: 2021 4th International Conference on Computing and Communications Technologies (ICCCT). (2021). p. 489–93.
59. Bharat S, Poongodi M, Gunasekaran M, Mounir H. Multi-Tier stack of block chain with proxy re-encryption method scheme on the internet of things platform. ACM Trans Internet Technol. (2021) 22:20. doi: 10.1145/3421508
60. Islam MJ, Rahman A, Kabir S, Karim MR, Acharjee UK, Nasir MK, et al. Blockchain-SDN-based energy-aware distributed secure architecture for IoT in smart cities. IEEE Internet Things J. (2022) 9:3850–64. doi: 10.1109/JIOT.2021.3100797
61. Durga R, Poovammal E, Ramana K, Jhaveri RH, Singh S, Yoon B. CES Blocks—a novel chaotic encryption schemes-based blockchain system for an IoT environment. IEEE Access. (2022) 10:11354–71. doi: 10.1109/ACCESS.2022.3144681
62. Anichur R, Muaz R, Dipanjali K, Karim MR, Shahab Band S, Mehdi S. Study on IoT for SARS-CoV-2 with healthcare: present and future perspective. Math Biosci Eng. (2021) 18:9697–726. doi: 10.3934/mbe.2021475
63. Chollet F. Xception: Deep learning with depthwise separable convolutions. In: 2017 IEEE Conference on Computer Vision and Pattern Recognition. Honolulu, HI: IEEE Computer Society. (2017). p. 1800–7. doi: 10.1109/CVPR.2017.195
64. He K, Zhang X, Ren S, Sun J. Deep residual learning for image recognition. arXiv preprint. (2015) arxiv:1512.03385
65. Szegedy C, Vanhoucke V, Ioffe S, Shlens J, Wojna Z. Rethinking the inception architecture for computer vision. In: 2016 IEEE Conference on Computer Vision and Pattern Recognition. Las Vegas, NV: IEEE (2016). p. 2818–26. doi: 10.1109/CVPR.2016.308
66. Howard G, Zhu M, Chen B, Kalenichenko D, Wang W, Weyand T, et al. Mobilenets: Efficient convolutional neural networks for mobile vision applications. arXiv preprint. (2017) arXiv:1704.04861.
67. Sandler M, Howard AG, Zhu M, Zhmoginov A, Chen L. Mobilenetv2: inverted residuals and linear bottlenecks. In: 2018 IEEE Conference on Computer Vision and Pattern Recognition. Salt Lake City, UT: IEEE (2018). p. 4510–20. doi: 10.1109/CVPR.2018.00474
68. Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In: Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition. (2017). p. 4700–8. doi: 10.1109/CVPR.2017.243
69. Kuzdeuov A, Baimukashev D, Karabay A, Ibragimov B, Mirzakhmetov A, Nurpeiissov M, et al. A network-based stochastic epidemic simulator: controlling COVID-19 with regionspecific policies. IEEE J Biomed Health Inform. (2020) 24:2743–54. doi: 10.1109/JBHI.2020.3005160
70. Chen J, Wu L, Zhang J, Zhang L, Gong D, Zhao Y, et al. Deep learning-based model for detecting 2019 novel coronavirus pneumonia on high-resolution computed tomography. Sci Rep. (2020) 10:19196. doi: 10.1038/s41598-020-76282-0
71. Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, et al. Using artificial intelligence to detect covid-19 and community-acquired pneumonia based on pulmonary CT: evaluation of the diagnostic accuracy. Radiology. (2020) 296:E65–71. doi: 10.1148/radiol.2020200905
Keywords: image processing, artificial intelligence, extreme learning machine, privacy preservation, capsule learning model
Citation: Durga R and Poovammal E (2022) FLED-Block: Federated Learning Ensembled Deep Learning Blockchain Model for COVID-19 Prediction. Front. Public Health 10:892499. doi: 10.3389/fpubh.2022.892499
Received: 10 March 2022; Accepted: 09 May 2022;
Published: 17 June 2022.
Edited by:
Enrico Capobianco, University of Miami, United StatesReviewed by:
Subrato Bharati, Bangladesh University of Engineering and Technology, BangladeshShahab S. Band, National Yunlin University of Science and Technology, Taiwan
Copyright © 2022 Durga and Poovammal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: R. Durga, dr8529@srmist.edu.in
 R. Durga
R. Durga E. Poovammal
E. Poovammal