Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1102
Peer-review started: April 18, 2021
First decision: July 27, 2021
Revised: August 9, 2021
Accepted: February 15, 2022
Article in press: February 15, 2022
Published online: March 21, 2022
Coronavirus disease 2019 (COVID-19) is, at present, one of the most relevant global health problems. In the literature hepatic alterations have been described in COVID-19 patients, and they are mainly represented by worsening of underlying chronic liver disease leading to hepatic decompensation and liver failure with higher mortality. Several potential mechanisms used by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to cause liver damage have been hypothesized. COVID-19 primary liver injury is less common than secondary liver injury. Most of the available data demonstrate how liver damage in SARS-CoV-2 infection is likely due to systemic inflammation, and it is less likely mediated by a cytopathic effect directed on liver cells. Moreover, liver alterations could be caused by hypoxic injury and drugs (antibiotics and non-steroidal anti-inflammatory drugs, remdesivir, tocilizumab, tofacitinib and dexamethasone). SARS-CoV-2 infection can induce multiple vascular district atherothrombosis by affecting simultaneously cerebral, coronary and peripheral vascular beds. Data in the literature highlight how the virus triggers an exaggerated immune response, which added to the cytopathic effect of the virus can induce endothelial damage and a prothrombotic dysregulation of hemostasis. This leads to a higher incidence of symptomatic and confirmed venous thrombosis and of pulmonary embolisms, especially in central, lobar or segmental pulmonary arteries, in COVID-19. There are currently fewer data for arterial thrombosis, while myocardial injury was identified in 7%-17% of patients hospitalized with SARS-CoV-2 infection and 22%-31% in the intensive care unit setting. Available data also revealed a higher occurrence of stroke and more serious forms of peripheral arterial disease in COVID-19 patients. Hemostasis dysregulation is observed during the COVID-19 course. Lower platelet count, mildly increased prothrombin time and increased D-dimer are typical laboratory features of patients with severe SARS-CoV-2 infection, described as “COVID-19 associated coagulopathy.” These alterations are correlated to poor outcomes. Moreover, patients with severe SARS-CoV-2 infection are characterized by high levels of von Willebrand factor with subsequent ADAMTS13 deficiency and impaired fibrinolysis. Platelet hyperreactivity, hypercoagulability and hypofibrinolysis during SARS-CoV-2 infection induce a pathological state named as “immuno-thromboinflammation.” Finally, liver dysfunction and coagulopathy are often observed at the same time in patients with COVID-19. The hypothesis that liver dysfunction could be mediated by microvascular thrombosis has been supported by post-mortem findings and extensive vascular portal and sinusoidal thrombosis observation. Other evidence has shown a correlation between coagulation and liver damage in COVID-19, underlined by the transaminase association with coagulopathy, identified through laboratory markers such as prothrombin time, international normalized ratio, fibrinogen, D-dimer, fibrin/fibrinogen degradation products and platelet count. Other possible mechanisms like immunogenesis of COVID-19 damage or massive pericyte activation with consequent vessel wall fibrosis have been suggested.
Core Tip: Evidence in the literature suggests a contribution of the severe acute respiratory syndrome coronavirus 2 to the pathophysiology of liver injury, atherothrombosis and coagulation disorders. This minireview explores the possible mechanisms by which these alterations are generated during coronavirus disease 2019 according to current knowledge.
- Citation: D’Ardes D, Boccatonda A, Cocco G, Fabiani S, Rossi I, Bucci M, Guagnano MT, Schiavone C, Cipollone F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J Gastroenterol 2022; 28(11): 1102-1112
- URL: https://www.wjgnet.com/1007-9327/full/v28/i11/1102.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i11.1102
Coronavirus disease 2019 (COVID-19) is, at present, one of the most relevant global health problems, declared a pandemic on March 11, 2020 by the World Health Organization[1]. It is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a novel positive-sense single-stranded RNA betacoronavirus[2,3]. The pulmonary manifestation of COVID-19, including pneumonia and acute respiratory distress syndrome, are well known, but it is necessary to emphasize that the coronavirus deleterious effects are also exerted on many other organ systems and are responsible for extrapulmonary manifestations[4].
SARS-CoV-2 can cause both direct and indirect cardiovascular sequelae (myocardial injury, acute coronary syndromes, cardiomyopathy, acute cor pulmonale, arrhythmias and cardiogenic shock), acute kidney injury and gastrointestinal symptoms, such as diarrhea, nausea, vomiting, abdominal pain and anorexia[4]. Neurological complications include headaches, dizziness, ageusia, myalgia, anosmia up to stroke, Guillain-Barré and encephalopathy, and dermatological signs have also been described (petechiae, urticaria, vesicles, erythematous rash, livedo reticularis)[4]. Hepatobiliary manifestations can be observed especially in patients with severe presentations of COVID-19 and occur mainly with increased plasma levels of transaminases and bilirubin[4]. Thromboembolic events have also been described in COVID-19, such as acute limb ischemia, which can occur in patients without existing peripheral arterial disease and in those receiving thromboprophylaxis. Acute abdominal-thoracic aortic thrombosis and mesenteric ischemia are less common but associated with significant morbidity and mortality[5]. Several studies have also demonstrated increased rates of deep vein thrombosis and pulmonary embolism in COVID-19 patient[5-7]. Acute cerebrovascular disease, including ischemic stroke, and disseminated intravascular coagulation are also severe thrombotic complications of COVID-19 that must not be forgotten[5].
This article aims to explore the possible mechanisms underlying hepatic and hemocoagulative alterations in COVID-19 through the search of published, readily accessible, peer-reviewed, full articles related to this topic written in English and found on PubMed.
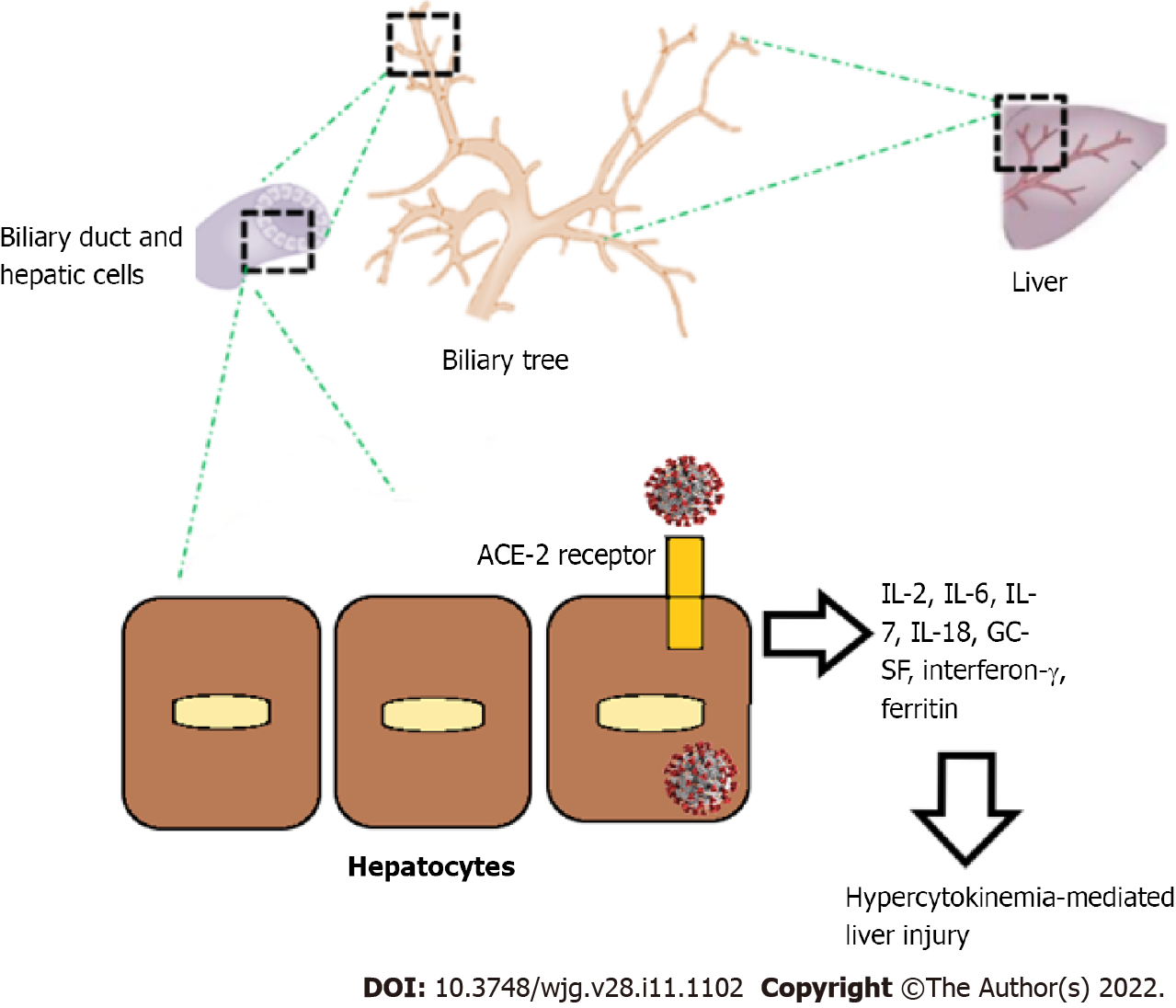
SARS-CoV-2 has the capacity to infect cells through the angiotensin-converting enzyme 2 (ACE2) receptor that is mainly expressed on the type 2 alveolar cells[8]. The ACE2 receptor is mainly present in the liver, lung, heart, renal and gastrointestinal system[9]. Regarding hepatic involvement, the level of ACE2 expression in cholangiocytes (59.7%) is higher than hepatocytes (2.9%)[10]. SARS-CoV-2 cell entry is mediated by its S protein, which specifically interacts with the host cell ACE2 and transmembrane serine protease 2[11]. The major expression of ACE2 in cholangiocytes reveals that SARS-CoV-2 may cause bile duct dysfunction. Cholangiocytes play critical roles in liver regeneration and immune responses, indicating that viral immunologic injury might be important in liver injury in COVID-19[12].
Even if there is a substantial difference in ACE2 receptor expression between the liver and biliary tract, the liver is not unaffected by the SARS-CoV-2 infection. In fact, current data have shown that in patients with COVID-19 aspartate aminotransferase (AST), alanine aminotransferase (ALT) and lactate dehydrogenase levels are increased, while alkaline phosphatase and gamma glutamyl transferase, representatives of bile duct injury, did not increase significantly[12]. Indeed, in the literature many clinical findings and hepatic alterations are described, and there are several reported potential mechanisms used by the virus to cause liver disease.
Most of the available data demonstrate that hepatic injury during SARS-CoV-2 infection is more likely due to systemic inflammation, and it is less likely mediated by a cytopathic effect directed on liver cells. Viral RNA can be detected in liver tissue of patients with COVID-19, but infection of liver cells has not yet been demonstrated[13]. During a viral infection, the innate and the acquired immune systems recognize pathogen associated molecular patterns and specific viral antigens and release inflammatory molecules, such as cytokines and chemokines, that activate macrophages and T cells to clear the virus and kill infected cells. In patients affected by COVID-19, high levels of inflammatory cytokines have been observed, and this context could cause significant liver damage when SARS-CoV-2 infects host hepatocytes. The most involved inflammatory molecules are tumor necrosis factor, interleukin-2 (IL-2), IL-6, IL-7, IL-18, granulocyte-colony stimulating factor, interferon-γ and ferritin[14]. An early hypercytokinemia could lead to multiorgan injuries, including the liver (Figure 1). The literature demonstrates that worse outcomes of COVID-19 are more common in patients with early cytokine elevation[15].
Another potentially harming mechanism is represented by hypoxic injury. The complex vascularization of the liver makes it particularly exposed to circulatory alterations that could be generated in cardiac, circulatory, respiratory failure or in septic shock, causing decreased perfusion of the liver[12]. About 1.1%-20.0% of COVID-19 patients are affected by septic shock, and 23.0% of patients have heart failure[16].
Drugs are also involved with damage mechanisms, particularly causing drug induced liver injury. Antibiotics and non-steroidal anti-inflammatory drugs, which are one of the most common causes of drug induced liver injury in the general population, can contribute to liver damage in COVID-19 patients when used to treat bacterial superinfection, myalgias or fever. Furthermore, drug induced liver injury has been shown in 15.2% of patients receiving remdesivir and in 37.2% of patients in treatment with lopinavir/ritonavir[17]. Finally, dexamethasone, tocilizumab and tofacitinib, used to treat COVID-19, could potentially create hepatic injury via pre-existing chronic liver disease reactivation, especially hepatitis B virus[18].
During SARS-CoV-2 infection, patients can be asymptomatic or present clinical symptoms such as fever, dry cough, headache, dyspnea and fatigue and up to acute respiratory distress syndrome, shock and cardiac failure[19,20]. Hepatic involvement can occur through all the pathophysiological pathways previously investigated and still other studies are needed to better characterize them. In current reports, COVID-19 primary liver injury is less common than secondary liver injury[21,22]. SARS-CoV-2 liver involvement is mainly represented by worsening of underlying chronic liver disease, leading to hepatic decompensation and acute-on-chronic liver failure, with higher mortality. Symptoms mainly reported in the literature associated to liver injury and gastrointestinal involvement are diarrhea, nausea, vomiting and loss of appetite. Abnormal liver function was also observed with the increase of AST, ALT, lactate dehydrogenase and bilirubin and the decrease of albumin[21-25].
According to the current data, hepatic dysfunction is significantly higher in critically ill patients and is associated with a poor outcome, underlining its importance in clinical settings. Elevated liver enzymes are observed predominantly in severe and critical cases of COVID-19. For example, increased AST was observed in 62% of patients in the intensive care unit (ICU) compared to 25% in non-ICU patients[23]. Chen et al[21] reported that AST, ALT, alkaline phosphatase, gamma glutamyl transferase and bilirubin levels were significantly higher in non-survivors than in survivors. Also hypoalbuminemia was found significantly lower in deceased patients rather than in surviving patients. Furthermore, according to Bangash et al[24] the mortality rate in patients with underlying chronic liver disease was 0%-2%. According to the literature data, liver injury is most common in critically ill patients who have diabetes and hypertension[22,25].
The fact that liver injury in COVID-19 is mostly hepatocellular rather than cholestatic is demonstrated by the more frequent elevation of ALT, AST and lactate dehydrogenase than of alkaline phosphatase and gamma glutamyl transferase. The latter two did not increase significantly, and jaundice is uncommon[12,26]. Moreover, the current data demonstrate that AST could represent an important hepatocellular injury marker because its elevation is associated with a major mortality risk[27]. According to a meta-analysis, the pooled prevalence of abnormal liver functions (12 studies, 1267 patients) was 19%, and in subgroup analysis patients with severe COVID-19 had higher rates of abdominal pain and abnormal liver function including increased ALT and AST[28].
In a hospital setting, drugs also have to be considered in liver injury; moreover COVID-19 critically ill patients are treated with multiple drugs, such as antibiotics, immunosuppressants and antiviral and antipyretic agents that are associated to abnormal liver function, especially when used in patients with severe COVID-19[23,29]. In the literature, drug induced liver injury has been shown to result in AST and ALT elevation and reactivation of pre-existing chronic liver disease, above all hepatitis B virus infection[17,18]. Indeed, a retrospective study has shown that patients with chronic hepatitis B virus hepatitis had a worse prognosis for COVID-19 and for a higher mortality and a higher incidence of acute-on-chronic liver failure[30]. In a trial by Goldman et al[31] comparing remdesivir treatment for either 5 d or 10 d, severe but not life-threatening ALT/AST elevations were reported in 4%-6% of patients and life-threatening AST/ALT elevations in 2%-3% of patients.
SARS-CoV-2 infection can induce multiple vascular district atherothrombosis by simultaneously affecting cerebral, coronary and peripheral vascular beds. Data in the literature highlight how the virus can trigger an exaggerated immune response, which added to the cytopathic effect of the virus can induce endothelial damage and a prothrombotic dysregulation of hemostasis[32-34]. Nowadays, there are several reports and original papers on cases of venous thromboembolism and pulmonary embolism related to COVID-19[35-40].
Incidence of symptomatic and confirmed venous thrombosis in COVID-19 patients hospitalized in the ICU can reach 30%-40%[41]. Data from a study performed in China demonstrated that 25% of COVID-19 patients developed lower extremity deep vein thrombosis without venous thromboembolism prophylaxis[42]. A work by Klok et al[36] described pulmonary embolisms in 25 of 184 ICU patients with COVID-19 (13.6%), 72% of which were in central, lobar or segmental pulmonary arteries, despite standard dose pharmacological prophylaxis[36,43]. In Italy, Lodigiani et al[35] showed thromboembolic events (venous and arterial) in 7.7% of patients admitted with COVID-19, corresponding to a cumulative rate of 21.0%.
There are currently fewer data for arterial thrombosis. A study from Wuhan showed that about 12% of patients displayed Hs-troponin I above the threshold of 28 pg/mL[14]. Other data revealed that myocardial injury was diagnosed in 7%-17% of patients hospitalized with SARS-CoV-2 infection and 22%-31% in the ICU setting[16,25]. Dysregulated inflammatory response enhances atherosclerotic plaque disruption[21,44-46]. Previous studies demonstrated that influenza and community-acquired pneumonia are related to an increased risk of myocardial infarction, within the first 7 d of diagnosis and even after hospitalization[47]. Regarding cerebrovascular disease, occurrence of stroke in COVID-19 patients ranges between 2.7% and 3.8%, and these subgroup of patients often displayed comorbidities such as hypertension and were older on average[48,49]. Moreover, there are some reports documenting more serious forms of peripheral arterial disease in patients with SARS-CoV-2 infection[50].
Hemostasis dysregulation has been described as an early pathological change in the COVID-19 course. Lower platelet count, mildly increased prothrombin time and increased D-dimer are typical laboratory features of patients with severe SARS-CoV-2 infection, and they are correlated to poor outcomes[51]. Those changes have been described as “COVID-19 associated coagulopathy”[52-54]. Moreover, patients with severe SARS-CoV-2 infection are characterized by high levels of IL-6, thus leading to a subsequent increase in proteins such as fibrinogen and von Willebrand factor (vWF)[55]. High lactate dehydrogenase and ferritin values are other laboratory findings of patients with severe COVID-19 infection, resembling a thrombotic microangiopathy[51,55,56]. Furthermore, complement components C5b-9, C4d and mannose-binding lectin-associated serine protease 2 have been detected in the small vessels of the lung due to complement-associated microvascular injury[57]. High levels of vWF and subsequent ADAMTS13 deficiency seem to be other typical findings of severe COVID-19 infection[52,58,59]. Decreased levels of ADAMTS13 can induce increased platelet-endothelial interaction generating a thrombotic microangiopathy-like state[52,60-63].
Recent data demonstrate that fibrinolysis is impaired in patients with severe COVID-19[64,65]. Critical COVID-19 patients are characterized by low levels of plasminogen, as for a consumptive state[65]. Nougier et al[66] reported elevated levels of plasminogen activator inhibitor 1 and low levels of tissue plasminogen activator, along with high thrombin generation, thus demonstrating a significant imbalance between inhibitor and activator factors of fibrinolysis.
Platelet hyperreactivity, hypercoagulability and hypofibrinolysis induce a pathological state that has been named “immuno-thromboinflammation” during SARS-CoV-2 infection[67]. SARS-CoV-2 binds to the ACE2 receptor on the surface of endothelial and arterial smooth muscle cells, inducing a cytopathic effect and a subsequent endothelial injury[68]. As it is well known, endothelial damage triggers platelet activation, adhesion to the subendothelial matrix and aggregation, thus generating a platelet plug[69]. Moreover, a release of vWF, inefficient cleavage of ultralarge vWF catalyzed by ADAMTS13, direct contact with activating surfaces in the subendothelial matrix, loss of heparan sulfates at the surface of injured blood vessels, disrupted generation of nitric oxide, prostaglandin E2 and prostaglandin I2 and loss of surface expression of ectonucleotidases occur[70,71]. Platelet activation may represent a consequence of the production of a consistent level of thrombin after initiation of coagulation[64].
Furthermore, high IL-6 levels can induce megakaryocytopoiesis generation and platelet formation, which could play a role to generate a hypercoagulability state, in particular within the lung. Intriguing, SARS-CoV-2 can bind directly to platelets since they express both ACE2 and transmembrane serine protease 2 on their surface[72]; this binding can favor platelet activation and the release of clotting factors, inflammatory molecules and leukocyte-platelet aggregates[72].
In patients affected by severe COVID-19 pneumonia, levels of tissue factor (TF) on monocytes are higher than normal, together with P-selectin expression and the amount of platelet-neutrophil and platelet-monocyte aggregates[2]. Therefore, platelet activation due to adenosine diphosphate, thrombin and collagen stimulation is enhanced in these patients. Platelet hyperactivation induced by SARS-CoV-2 infection induce the release of inflammatory molecules such as cytokines, chemokines, growth factors and even procoagulant factors like fibrinogen and vWF[73]. This mechanism seems to generate a vicious cycle since inflammatory molecules worsen endothelial injury by decreasing nitric oxide availability and enhancing oxidative stress and/or favoring leukocyte-endothelial interaction[74,75].
Endothelial injury triggers the release of TF in the blood stream. Moreover, TF can derive from macrophage/monocyte cells and through their microparticles, as a consequence of macrophage activation syndrome shown in patients with severe COVID-19 infection[76]. SARS-CoV-2 can directly induce macrophage activation, which can even occur as a consequence of the inflammatory hyperactivation (cytokine storm), as demonstrated by high levels of interferon-g, C-C motif chemokine ligand 2 and C-X-C motif chemokine 9 and 10 detected in critical COVID-19 patients[77,78].
Neutrophils can also be activated both directly by SARS-CoV-2 and by other inflammatory cells, thus generating neutrophil extracellular traps that may activate Factor XII and the intrinsic pathway of coagulation[79]. Hemostasis dysregulation in SARS-CoV-2 infection is also characterized by a decreased activity of endogenous anticoagulants like antithrombin, TF pathway inhibitor and anticoagulation proteins C and S[78,80]. Endothelial injury and platelet hyper-activation in severe COVID-19 patients enhance the release of plasminogen activator inhibitor 1 (PAI-1)[81]. High levels of PAI-1 can further inhibit fibrinolysis, thus worsening the thrombotic burden[82,83]. Therefore, as the pulmonary inflammation progresses, there is consumption of plasminogen, along with high levels of PAI-1 and depletion of tPA, thus inducing a state of hypofibrinolysis and allowing perpetuation of prothrombotic state[84].
In patients with SARS-CoV-2 infection, the presence of antiphospholipid antibodies has been demonstrated, which may directly induce endothelial cell activation and enhance TF expression by monocytes, thus contributing to the procoagulant and prothrombotic state[85-87]. Particularly, antiphospholipid antibodies can bind to the platelets and trigger their activation decreasing levels of inhibitors such as activated protein C and antithrombin and increasing clotting factors such as FXa and thrombin[88-90].
SARS-CoV-2 can dysregulate the ACE pathway by its binding to the ACE2 receptor on tissues; high levels of angiotensin (Ang) II favor PAI-1 and TF expression, thus promoting hypercoagulability and impairing fibrinolysis[60]. Furthermore, Ang II receptors on platelets can induce platelet activation and aggregation[91]. Ang 1,7 levels are lower in COVID-19 patients who develop severe disease[92]. Ang 1,7 is a vasoprotective molecule by mediating vasodilation and blocking platelet aggregation through nitric oxide release[93-95]. Therefore, low Ang 1,7 levels can contribute to the procoagulant state in COVID-19 infection. Finally, obesity is a main risk factor for thrombosis due to adipocytokine-mediated mechanisms, increased inflammatory molecules, Ang II/Ang 1,7 imbalance, reactive oxygen species-mediated endothelial dysfunction and lipid and glucose metabolism changes[96-98].
Liver dysfunction and coagulopathy are often observed in patients with COVID-19. Japanese researchers found that patients with high ALT had higher levels of D-dimer and fibrin/fibrinogen degradation products. In particular elevation of ALT and D-dimer were identified simultaneously[99]. Moreover D-dimer was independently associated to ALT elevation[99].
This study suggests the hypothesis that liver dysfunction could be mediated by microvascular thrombosis, and intrahepatic microvascular thrombosis could theoretically play a role in this physiopathological context. This hypothesis is supported by post-mortem findings by Sonzogni et al[100] who found marked derangement of intrahepatic blood vessels with aspects of intravascular thrombosis, suggesting a possible liver damage linked to thrombotic processes. Other evidence has shown a correlation between coagulation and liver damage in COVID-19. In fact, a Chinese study on COVID-19 patients demonstrated the association between AST and ALT values with coagulopathy, identified through laboratory markers such as prothrombin time, international normalized ratio, fibrinogen, D-dimer and platelet count[101]. Pathological findings are consistent with a vascular-related damage caused by impaired blood flow, with lesions similar to histological characteristics of hepatopulmonary syndrome and in obliterative portal venopathy[100]. Indeed it has been shown a diffuse network of sinusoids decorated by CD34 suggesting an abnormal hepatic circulation of blood[100].
A likely explanation could be related to increased hepatic blood flow: this aspect may be linked to heart distress or to thrombotic phenomena in portal and sinusoidal vessels[100]. The abnormal high levels of transaminases in some patients whose liver sample were analyzed post-mortem could be explained by extensive vascular portal and sinusoidal thrombosis, leading to confluent parenchymal necrosis and hepatic cells accelerating apoptosis[100]. Attention must be paid to the evidence of massive pericyte activation in liver samples obtained post-mortem[100]. Pericytes are involved in the recruitment of inflammatory cells in liver injury, and their transformation in myofibroblast-like cells leads to the production of abundant amounts of extracellular matrix proteins and to the consequent vessel wall fibrosis[100].
Liver abnormalities and coagulopathy are physiopathological characteristics of COVID-19 that represent the most relevant global health problem. Hypercoagulability, hypofibrinolysis and platelet alterations during SARS-CoV-2 infection induce a sort of “immuno-thromboinflammation,” while mildly increased prothrombin time and increased D-dimer are typical laboratory features of patients with severe SARS-CoV-2 infection, described as “COVID-19 associated coagulopathy.” These phenomena are clinically relevant with manifestations of thrombosis in a large variety of anatomic districts. Moreover, hepatic alterations are mainly represented by worsening of underlying chronic liver disease leading to hepatic decompensation and liver failure with higher mortality, and they appear to be mediated more by systemic inflammation, direct cytopathic effect on liver cells, hypoxic injury and drugs. Liver dysfunction and coagulopathy are also observed at the same time in patients with COVID-19, probably mediated by microvascular thrombosis, immunological mechanisms and pericyte activation. More data are needed to better investigate the relevant relationship between coagulation, liver dysfunction and COVID-19 with the aim to understand more deeply the physiopathological mechanisms of SARS-CoV-2 and to evaluate new therapeutic strategies to prevent mortality.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Delgado-Gallegos JL, Moreira TMM S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | D'Ardes D, Boccatonda A, Rossi I, Pontolillo M, Cocco G, Schiavone C, Santilli F, Guagnano MT, Bucci M, Cipollone F. Long-term Positivity to SARS-CoV-2: A Clinical Case of COVID-19 with Persistent Evidence of Infection. Eur J Case Rep Intern Med. 2020;7:001707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Manne BK, Denorme F, Middleton EA, Portier I, Rowley JW, Stubben C, Petrey AC, Tolley ND, Guo L, Cody M, Weyrich AS, Yost CC, Rondina MT, Campbell RA. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317-1329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 628] [Article Influence: 157.0] [Reference Citation Analysis (0)] |
| 3. | D'Ardes D, Pontolillo M, Esposito L, Masciarelli M, Boccatonda A, Rossi I, Bucci M, Guagnano MT, Ucciferri C, Santilli F, Di Nicola M, Falasca K, Vecchiet J, Schael T, Cipollone F. Duration of COVID-19: Data from an Italian Cohort and Potential Role for Steroids. Microorganisms. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, Bikdeli B, Ahluwalia N, Ausiello JC, Wan EY, Freedberg DE, Kirtane AJ, Parikh SA, Maurer MS, Nordvig AS, Accili D, Bathon JM, Mohan S, Bauer KA, Leon MB, Krumholz HM, Uriel N, Mehra MR, Elkind MSV, Stone GW, Schwartz A, Ho DD, Bilezikian JP, Landry DW. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2019] [Cited by in F6Publishing: 1810] [Article Influence: 452.5] [Reference Citation Analysis (2)] |
| 5. | Avila J, Long B, Holladay D, Gottlieb M. Thrombotic complications of COVID-19. Am J Emerg Med. 2021;39:213-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 80] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 6. | Boccatonda A, Ianniello E, D'Ardes D, Cocco G, Giostra F, Borghi C, Schiavone C. Can Lung Ultrasound be Used to Screen for Pulmonary Embolism in Patients with SARS-CoV-2 Pneumonia? Eur J Case Rep Intern Med. 2020;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Cook DJ, Crowther MA, Meade MO, Douketis J; VTE in the ICU Workshop Participants. Prevalence, incidence, and risk factors for venous thromboembolism in medical-surgical intensive care unit patients. J Crit Care. 2005;20:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11946] [Cited by in F6Publishing: 13059] [Article Influence: 3264.8] [Reference Citation Analysis (0)] |
| 9. | Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1286] [Cited by in F6Publishing: 1443] [Article Influence: 360.8] [Reference Citation Analysis (0)] |
| 10. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 315] [Article Influence: 78.8] [Reference Citation Analysis (1)] |
| 11. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 203] [Article Influence: 67.7] [Reference Citation Analysis (2)] |
| 12. | Yang RX, Zheng RD, Fan JG. Etiology and management of liver injury in patients with COVID-19. World J Gastroenterol. 2020;26:4753-4762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 47] [Cited by in F6Publishing: 41] [Article Influence: 10.3] [Reference Citation Analysis (2)] |
| 13. | Saviano A, Wrensch F, Ghany MG, Baumert TF. Liver Disease and Coronavirus Disease 2019: From Pathogenesis to Clinical Care. Hepatology. 2021;74:1088-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 14. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28482] [Article Influence: 7120.5] [Reference Citation Analysis (3)] |
| 15. | Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A; Yale IMPACT Team, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463-469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1544] [Cited by in F6Publishing: 1420] [Article Influence: 355.0] [Reference Citation Analysis (0)] |
| 16. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17476] [Cited by in F6Publishing: 17231] [Article Influence: 4307.8] [Reference Citation Analysis (0)] |
| 17. | Kulkarni AV, Kumar P, Tevethia HV, Premkumar M, Arab JP, Candia R, Talukdar R, Sharma M, Qi X, Rao PN, Reddy DN. Systematic review with meta-analysis: liver manifestations and outcomes in COVID-19. Aliment Pharmacol Ther. 2020;52:584-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 155] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 18. | Hammond A, Ramersdorfer C, Palitzsch KD, Schölmerich J, Lock G. [Fatal liver failure after corticosteroid treatment of a hepatitis B virus carrier]. Dtsch Med Wochenschr. 1999;124:687-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1169] [Cited by in F6Publishing: 1069] [Article Influence: 267.3] [Reference Citation Analysis (0)] |
| 20. | Jamil S, Mark N, Carlos G, Cruz CSD, Gross JE, Pasnick S. Diagnosis and Management of COVID-19 Disease. Am J Respir Crit Care Med. 2020;201:P19-P20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 22. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13871] [Cited by in F6Publishing: 12369] [Article Influence: 3092.3] [Reference Citation Analysis (1)] |
| 23. | Cravioto J, Matsubara M, Arrieta R. [Low birth weight and the functioning of the central nervous system in the first years of life]. Bol Med Hosp Infant Mex. 1988;45:718-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1184] [Cited by in F6Publishing: 1219] [Article Influence: 304.8] [Reference Citation Analysis (4)] |
| 24. | Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5:529-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 338] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 25. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14113] [Cited by in F6Publishing: 14140] [Article Influence: 3535.0] [Reference Citation Analysis (0)] |
| 26. | Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: A retrospective study. Liver Int. 2020;40:1321-1326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 212] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 27. | Lei F, Liu YM, Zhou F, Qin JJ, Zhang P, Zhu L, Zhang XJ, Cai J, Lin L, Ouyang S, Wang X, Yang C, Cheng X, Liu W, Li H, Xie J, Wu B, Luo H, Xiao F, Chen J, Tao L, Cheng G, She ZG, Zhou J, Wang H, Lin J, Luo P, Fu S, Ye P, Xiao B, Mao W, Liu L, Yan Y, Chen G, Huang X, Zhang BH, Yuan Y. Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China. Hepatology. 2020;72:389-398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 288] [Article Influence: 72.0] [Reference Citation Analysis (0)] |
| 28. | Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:667-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 692] [Article Influence: 173.0] [Reference Citation Analysis (0)] |
| 29. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19202] [Cited by in F6Publishing: 17996] [Article Influence: 4499.0] [Reference Citation Analysis (5)] |
| 30. | Zou X, Fang M, Li S, Wu L, Gao B, Gao H, Ran X, Bian Y, Li R, ShanshanYu, Ling J, Li D, Tian D, Huang J. Characteristics of Liver Function in Patients With SARS-CoV-2 and Chronic HBV Coinfection. Clin Gastroenterol Hepatol. 2021;19:597-603. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 31. | Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn MY, Nahass RG, Chen YS, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A; GS-US-540-5773 Investigators. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med. 2020;383:1827-1837. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 987] [Cited by in F6Publishing: 915] [Article Influence: 228.8] [Reference Citation Analysis (0)] |
| 32. | D'Ardes D, Boccatonda A, Rossi I, Guagnano MT, Santilli F, Cipollone F, Bucci M. COVID-19 and RAS: Unravelling an Unclear Relationship. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 33. | Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential Effects of Coronaviruses on the Cardiovascular System: A Review. JAMA Cardiol. 2020;5:831-840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1095] [Cited by in F6Publishing: 1174] [Article Influence: 293.5] [Reference Citation Analysis (1)] |
| 34. | Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;382:e102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 683] [Cited by in F6Publishing: 685] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 35. | Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Bertuzzi A, Sandri MT, Barco S; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1298] [Cited by in F6Publishing: 1475] [Article Influence: 368.8] [Reference Citation Analysis (0)] |
| 36. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2925] [Cited by in F6Publishing: 3225] [Article Influence: 806.3] [Reference Citation Analysis (0)] |
| 37. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1017] [Cited by in F6Publishing: 1132] [Article Influence: 283.0] [Reference Citation Analysis (0)] |
| 38. | Sofia S, Boccatonda A, Montanari M, Spampinato M, D'ardes D, Cocco G, Accogli E, Cipollone F, Schiavone C. Thoracic ultrasound and SARS-COVID-19: a pictorial essay. J Ultrasound. 2020;23:217-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 39. | Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F; CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1669] [Cited by in F6Publishing: 1931] [Article Influence: 482.8] [Reference Citation Analysis (0)] |
| 40. | Lessiani G, Boccatonda A, D'Ardes D, Cocco G, Di Marco G, Schiavone C. Mondor's Disease in SARS-CoV-2 Infection: A Case of Superficial Vein Thrombosis in the Era of COVID-19. Eur J Case Rep Intern Med. 2020;7:001803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, Dager WE, Deitelzweig SB, Ellsworth S, Garcia D, Kaatz S, Minichiello T. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. 2020;50:72-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 301] [Article Influence: 75.3] [Reference Citation Analysis (1)] |
| 42. | Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421-1424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1298] [Cited by in F6Publishing: 1277] [Article Influence: 319.3] [Reference Citation Analysis (0)] |
| 43. | Boccatonda A, Ianniello E, D'Ardes D, Cocco G, Giostra F, Borghi C, Schiavone C. Can Lung Ultrasound be Used to Screen for Pulmonary Embolism in Patients with SARS-CoV-2 Pneumonia? Eur J Case Rep Intern Med. 2020;7:001748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2516] [Cited by in F6Publishing: 2688] [Article Influence: 672.0] [Reference Citation Analysis (0)] |
| 45. | Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, Newman A, Loehr L, Folsom AR, Elkind MS, Lyles MF, Kronmal RA, Yende S. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264-274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 46. | Welt FGP, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, Kirtane AJ; American College of Cardiology’s Interventional Council and the Society for Cardiovascular Angiography and Interventions. Catheterization Laboratory Considerations During the Coronavirus (COVID-19) Pandemic: From the ACC's Interventional Council and SCAI. J Am Coll Cardiol. 2020;75:2372-2375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 360] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 47. | Santilli F, Boccatonda A, Davì G. Coagulation at the crossroads of the communicable/non-communicable disease dyad: The case of pneumonia. Respirology. 2016;21:1344-1346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382:e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1553] [Cited by in F6Publishing: 1514] [Article Influence: 378.5] [Reference Citation Analysis (0)] |
| 49. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683-690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4761] [Cited by in F6Publishing: 4395] [Article Influence: 1098.8] [Reference Citation Analysis (0)] |
| 50. | Farhan S, Kamran H, Vogel B, Garg K, Rao A, Narula N, Jacobowitz G, Tarricone A, Kapur V, Faries P, Marin M, Narula J, Lookstein R, Olin JW, Krishnan P. Considerations for Patients With Peripheral Artery Disease During the COVID-19 Pandemic. Clin Appl Thromb Hemost. 2021;27:1076029620986877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58:1021-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 1066] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 52. | Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438-e440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 878] [Cited by in F6Publishing: 986] [Article Influence: 246.5] [Reference Citation Analysis (0)] |
| 53. | Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin Chim Acta. 2020;506:145-148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 946] [Cited by in F6Publishing: 1046] [Article Influence: 261.5] [Reference Citation Analysis (0)] |
| 54. | Lippi G, Favaloro EJ. D-dimer is Associated with Severity of Coronavirus Disease 2019: A Pooled Analysis. Thromb Haemost. 2020;120:876-878. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 369] [Article Influence: 92.3] [Reference Citation Analysis (0)] |
| 55. | Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, Pesenti A, Peyvandi F, Tripodi A. Hypercoagulability of COVID-19 patients in intensive care unit: A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18:1738-1742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 926] [Cited by in F6Publishing: 908] [Article Influence: 227.0] [Reference Citation Analysis (0)] |
| 56. | Henry BM, Aggarwal G, Wong J, Benoit S, Vikse J, Plebani M, Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis. Am J Emerg Med. 2020;38:1722-1726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 316] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 57. | Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, Baxter-Stoltzfus A, Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl Res. 2020;220:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1635] [Cited by in F6Publishing: 1543] [Article Influence: 385.8] [Reference Citation Analysis (1)] |
| 58. | Ladikou EE, Sivaloganathan H, Milne KM, Arter WE, Ramasamy R, Saad R, Stoneham SM, Philips B, Eziefula AC, Chevassut T. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond). 2020;20:e178-e182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 59. | Huisman A, Beun R, Sikma M, Westerink J, Kusadasi N. Involvement of ADAMTS13 and von Willebrand factor in thromboembolic events in patients infected with SARS-CoV-2. Int J Lab Hematol. 2020;42:e211-e212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 60. | Henry BM, Vikse J, Benoit S, Favaloro EJ, Lippi G. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: A novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020;507:167-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 254] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 61. | Bazzan M, Montaruli B, Sciascia S, Cosseddu D, Norbiato C, Roccatello D. Low ADAMTS 13 plasma levels are predictors of mortality in COVID-19 patients. Intern Emerg Med. 2020;15:861-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 62. | Martinelli N, Montagnana M, Pizzolo F, Friso S, Salvagno GL, Forni GL, Gianesin B, Morandi M, Lunardi C, Lippi G, Polati E, Olivieri O, De Franceschi L. A relative ADAMTS13 deficiency supports the presence of a secondary microangiopathy in COVID 19. Thromb Res. 2020;193:170-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 63. | Tiscia GL, Favuzzi G, De Laurenzo A, Cappucci F, Fischetti L, di Mauro L, Miscio G, Mirijello A, Chinni E, Grandone E; CSS COVID-19 Group. Reduction of ADAMTS13 Levels Predicts Mortality in SARS-CoV-2 Patients. TH Open. 2020;4:e203-e206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 64. | Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, Nydam TL, Moore PK, McIntyre RC Jr. Fibrinolysis Shutdown Correlation with Thromboembolic Events in Severe COVID-19 Infection. J Am Coll Surg. 2020;231:193-203.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 277] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 65. | Henry BM, Benoit SW, Hoehn J, Lippi G, Favaloro EJ, Benoit JL. Circulating Plasminogen Concentration at Admission in Patients with Coronavirus Disease 2019 (COVID-19). Semin Thromb Hemost. 2020;46:859-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 66. | Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, David JS, Bonnet A, Negrier C, Dargaud Y. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thromb Haemost. 2020;18:2215-2219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 194] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 67. | Lippi G, Sanchis-Gomar F, Henry BM. Coronavirus disease 2019 (COVID-19): the portrait of a perfect storm. Ann Transl Med. 2020;8:497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 68. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4227] [Cited by in F6Publishing: 4259] [Article Influence: 1064.8] [Reference Citation Analysis (0)] |
| 69. | Lippi G, Franchini M, Targher G. Arterial thrombus formation in cardiovascular disease. Nat Rev Cardiol. 2011;8:502-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 70. | van Hinsbergh VW. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol. 2012;34:93-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 327] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 71. | Yau JW, Teoh H, Verma S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 2015;15:130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 406] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 72. | Zhang S, Liu Y, Wang X, Yang L, Li H, Wang Y, Liu M, Zhao X, Xie Y, Yang Y, Zhang S, Fan Z, Dong J, Yuan Z, Ding Z, Zhang Y, Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J Hematol Oncol. 2020;13:120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 426] [Article Influence: 106.5] [Reference Citation Analysis (0)] |
| 73. | Taus F, Salvagno G, Canè S, Fava C, Mazzaferri F, Carrara E, Petrova V, Barouni RM, Dima F, Dalbeni A, Romano S, Poli G, Benati M, De Nitto S, Mansueto G, Iezzi M, Tacconelli E, Lippi G, Bronte V, Minuz P. Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia. Arterioscler Thromb Vasc Biol. 2020;40:2975-2989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 74. | Dixon JT, Gozal E, Roberts AM. Platelet-mediated vascular dysfunction during acute lung injury. Arch Physiol Biochem. 2012;118:72-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med. 2018;56:1035-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 76. | Grover SP, Mackman N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler Thromb Vasc Biol. 2018;38:709-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 285] [Cited by in F6Publishing: 393] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 77. | Park MD. Macrophages: a Trojan horse in COVID-19? Nat Rev Immunol. 2020;20:351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 78. | Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1356] [Cited by in F6Publishing: 1648] [Article Influence: 412.0] [Reference Citation Analysis (0)] |
| 79. | Gould TJ, Vu TT, Swystun LL, Dwivedi DJ, Mai SH, Weitz JI, Liaw PC. Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. 2014;34:1977-1984. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 299] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 80. | Lippi G, Henry BM, Sanchis-Gomar F. Plasma Antithrombin Values Are Significantly Decreased in Coronavirus Disease 2019 (COVID-19) Patients with Severe Illness. Semin Thromb Hemost. 2021;47:460-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | Morrow GB, Whyte CS, Mutch NJ. Functional plasminogen activator inhibitor 1 is retained on the activated platelet membrane following platelet activation. Haematologica. 2020;105:2824-2833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 82. | Ozolina A, Sarkele M, Sabelnikovs O, Skesters A, Jaunalksne I, Serova J, Ievins T, Bjertnaes LJ, Vanags I. Activation of Coagulation and Fibrinolysis in Acute Respiratory Distress Syndrome: A Prospective Pilot Study. Front Med (Lausanne). 2016;3:64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 83. | Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, Ragazzi R, Ruggeri P, Hansel TT, Caramori G, Volta CA. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J Inflamm (Lond). 2019;16:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 84. | Lippi G, Sanchis-Gomar F, Favaloro EJ, Lavie CJ, Henry BM. Coronavirus Disease 2019-Associated Coagulopathy. Mayo Clin Proc. 2021;96:203-217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 85. | Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1532] [Cited by in F6Publishing: 1549] [Article Influence: 387.3] [Reference Citation Analysis (0)] |
| 86. | Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18:2064-2065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 203] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 87. | Pierangeli SS, Chen PP, Raschi E, Scurati S, Grossi C, Borghi MO, Palomo I, Harris EN, Meroni PL. Antiphospholipid antibodies and the antiphospholipid syndrome: pathogenic mechanisms. Semin Thromb Hemost. 2008;34:236-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 88. | Amengual O, Atsumi T, Khamashta MA, Hughes GR. The role of the tissue factor pathway in the hypercoagulable state in patients with the antiphospholipid syndrome. Thromb Haemost. 1998;79:276-281. [PubMed] [Cited in This Article: ] |
| 89. | Forastiero RR, Martinuzzo ME, Lu L, Broze GJ. Autoimmune antiphospholipid antibodies impair the inhibition of activated factor X by protein Z/protein Z-dependent protease inhibitor. J Thromb Haemost. 2003;1:1764-1770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Yang YH, Chang CJ, Chuang YH, Hsu HY, Chen PP, Chiang BL. Identification of anti-prothrombin antibodies in the anti-phospholipid syndrome that display the prothrombinase activity. Rheumatology (Oxford). 2010;49:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 91. | Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension. 2002;40:521-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 110] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Henry BM, Benoit JL, Berger BA, Pulvino C, Lavie CJ, Lippi G, Benoit SW. Coronavirus disease 2019 is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J Med Virol. 2021;93:678-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Durand MJ, Zinkevich NS, Riedel M, Gutterman DD, Nasci VL, Salato VK, Hijjawi JB, Reuben CF, North PE, Beyer AM. Vascular Actions of Angiotensin 1-7 in the Human Microcirculation: Novel Role for Telomerase. Arterioscler Thromb Vasc Biol. 2016;36:1254-1262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Fraga-Silva RA, Pinheiro SV, Gonçalves AC, Alenina N, Bader M, Santos RA. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14:28-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Fang C, Stavrou E, Schmaier AA, Grobe N, Morris M, Chen A, Nieman MT, Adams GN, LaRusch G, Zhou Y, Bilodeau ML, Mahdi F, Warnock M, Schmaier AH. Angiotensin 1-7 and Mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121:3023-3032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Reaven GM, Scott EM, Grant PJ, Lowe GD, Rumley A, Wannamethee SG, Stratmann B, Tschoepe D, Blann A, Juhan-Vague I, Alessi MC, Bailey C. Hemostatic abnormalities associated with obesity and the metabolic syndrome. J Thromb Haemost. 2005;3:1074-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 97. | Mertens I, Van Gaal LF. Obesity, haemostasis and the fibrinolytic system. Obes Rev. 2002;3:85-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 98. | Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood. 2013;122:3415-3422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 99. | Tsutsumi T, Saito M, Nagai H, Yamamoto S, Ikeuchi K, Lim LA, Adachi E, Koga M, Okushin K, Akai H, Kunimatsu A, Yotsuyanagi H. Association of coagulopathy with liver dysfunction in patients with COVID-19. Hepatol Res. 2021;51:227-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | Sonzogni A, Previtali G, Seghezzi M, Grazia Alessio M, Gianatti A, Licini L, Morotti D, Zerbi P, Carsana L, Rossi R, Lauri E, Pellegrinelli A, Nebuloni M. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020;40:2110-2116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 189] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 101. | Chen S, Liu H, Li T, Huang R, Gui R, Zhang J. Correlation analysis of coagulation dysfunction and liver damage in patients with novel coronavirus pneumonia: a single-center, retrospective, observational study. Ups J Med Sci. 2020;125:293-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |