Published online Oct 6, 2022. doi: 10.12998/wjcc.v10.i28.10109
Peer-review started: May 30, 2022
First decision: July 14, 2022
Revised: July 20, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: October 6, 2022
Vaccines for coronavirus disease 2019 (COVID-19) include ChAdOx1-SARS-COV-2 (AstraZeneca), Ad26.COV2.S (Janssen), mRNA-1273 (Moderna), BNT162b2 (Pfizer), BBIBP-CorV (Sinopharm), CoronaVac (Sinovac), and Bharat Biotech BBV152 (Covaxin).
To find the association between COVID-19 vaccines and myocardial infarction (MI).
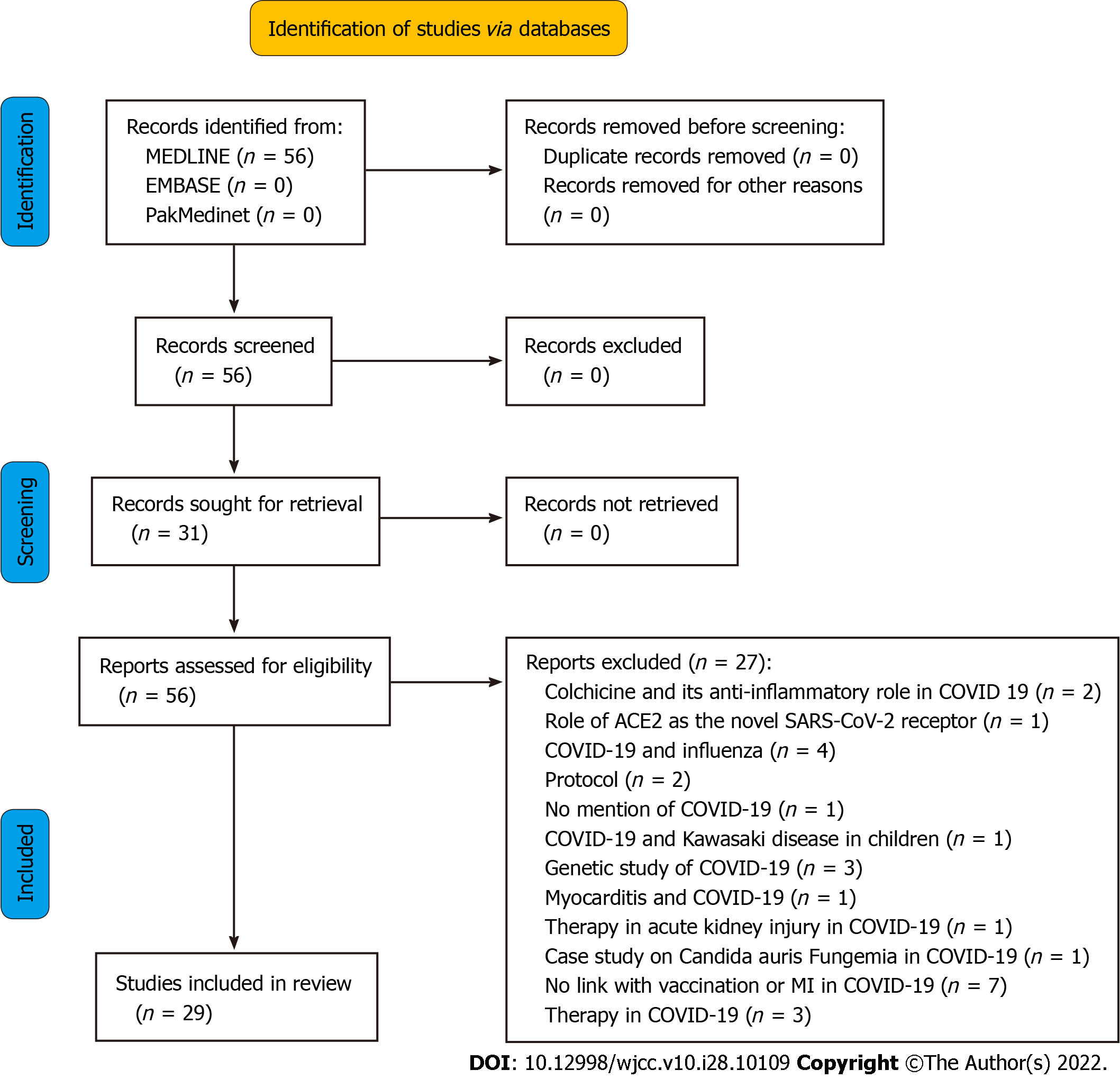
This is a systematic review that involved searching databases such as MEDLINE, EMBASE, and PakMediNet after making a search strategy using MeSH and Emtree terms. Eligibility criteria were set, and studies having no mention of MI as a complication of COVID-19 vaccination, protocols, genetic studies, and animal studies were excluded. Data was extracted using a predesigned extraction table, and 29 studies were selected after screening and applying the eligibility criteria.
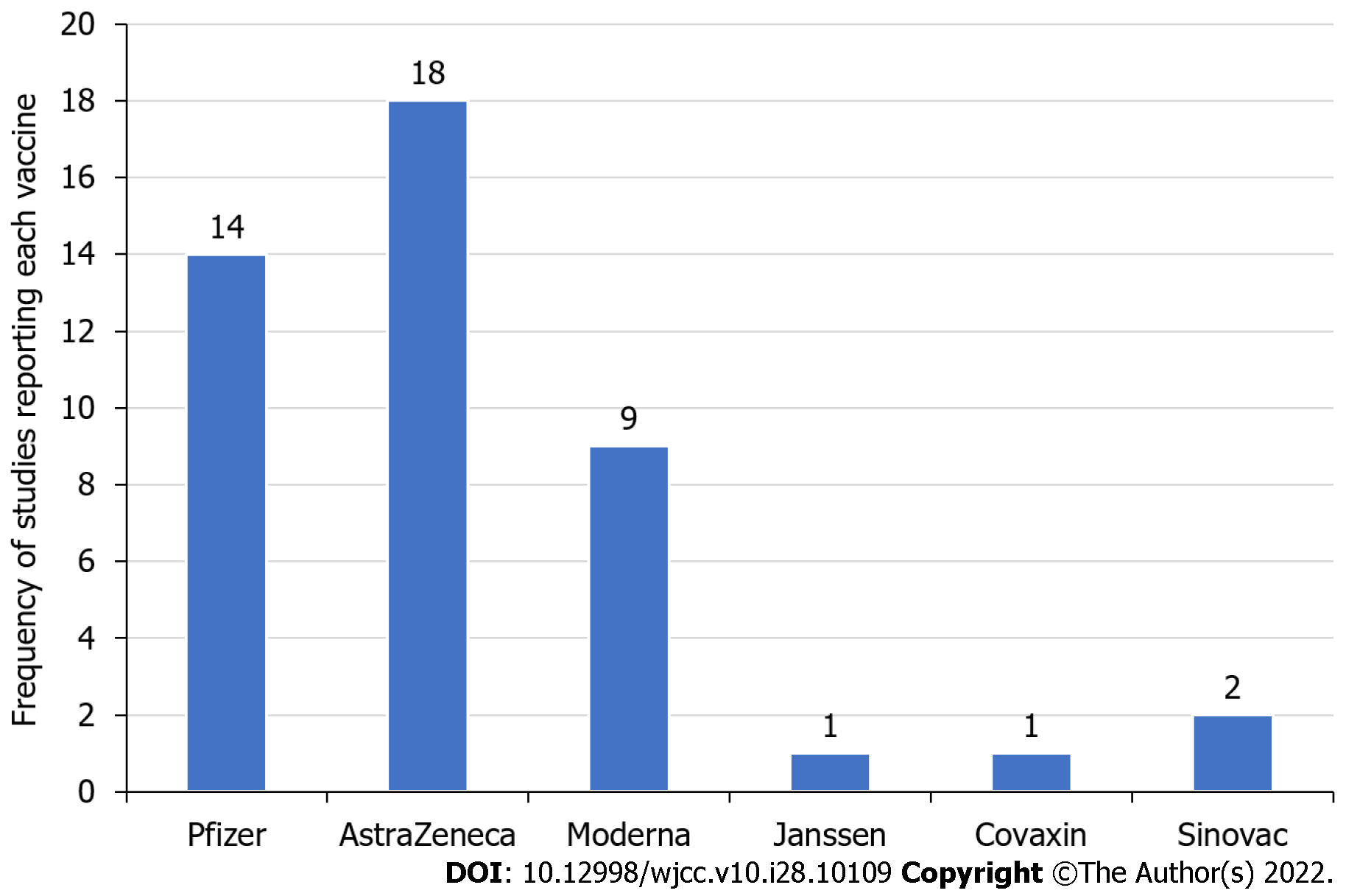
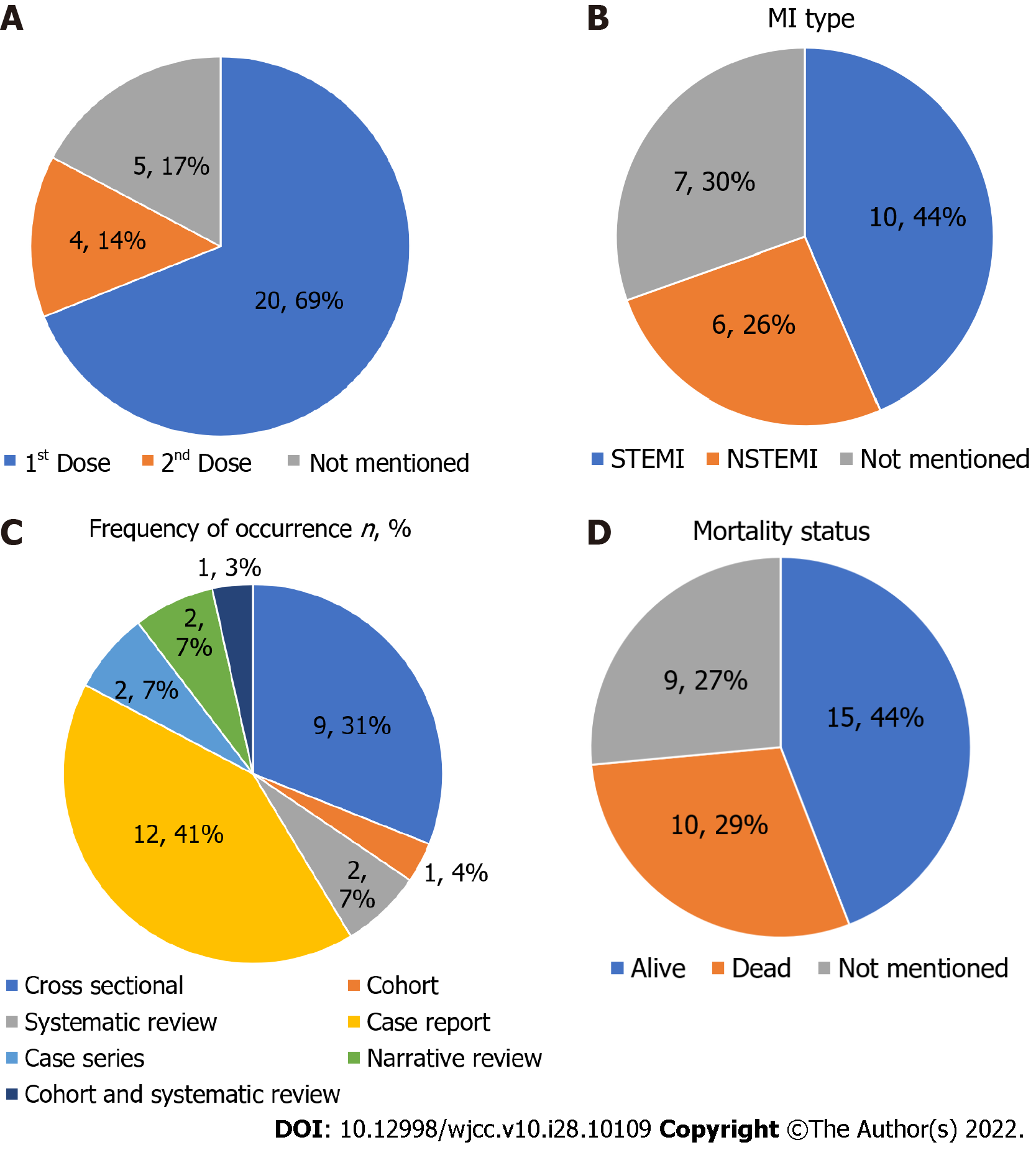
The majority of studies mentioned AstraZeneca (18 studies) followed by Pfizer (14 studies) and Moderna (9 studies) in subjects reporting MI after vaccination. Out of all the studies, 69% reported MI cases after the first COVID-19 vaccination dose and 14% after the second, 44% reported ST-segment elevation MI, and 26% reported non-ST-segment elevation MI. The mortality rate was 29% after MI.
In conclusion, many studies linked MI to COVID-19 vaccinations, but no definitive association could be found.
Core Tip: Mechanisms like vaccine-induced thrombotic thrombocytopenia and myocarditis complication have been identified in the literature linking myocardial infarction with coronavirus disease 2019 (COVID-19) vaccination. The majority of myocardial infarction cases reported were after vaccination with the vaccine from AstraZeneca, were ST-segment elevation, and were reported after first dose of vaccine. Although there are reports of myocardial infarction after COVID-19 vaccination, no definitive link was found in the previous literature linking the two.
- Citation: Zafar U, Zafar H, Ahmed MS, Khattak M. Link between COVID-19 vaccines and myocardial infarction. World J Clin Cases 2022; 10(28): 10109-10119
- URL: https://www.wjgnet.com/2307-8960/full/v10/i28/10109.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i28.10109
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2. The first case of COVID-19 was reported in Wuhan, China and rapidly spread to the rest of the world[1]. The COVID-19 outbreak was declared a pandemic on March 11, 2020[2]. Although severe acute respiratory syndrome coronavirus 2 primarily affects the respiratory tract, it frequently causes cardiac, gastrointestinal, hepatic, nephrological, and central nervous system distress[3]. It mainly causes nonspecific symptoms like fever, cough, and myalgia; however, in severe cases, it can lead to respiratory failure, septic shock, multiorgan dysfunction, and even death[4].
COVID-19 treatment is most often general supportive care and nutritional support in addition to respiratory care. However, due to the lack of clinically approved interventions for COVID-19 treatment, attempts have been made for vaccine development to prevent the disease[3]. As a result, the safety and efficacy of several vaccines have been approved by the World Health Organization. Among these are AstraZeneca/Oxford, Johnson and Johnson/Janssen, Moderna, Pfizer/BioNTech, Sinopharm, Sinovac, and the Bharat Biotech BBV152 Covaxin vaccine[5]. Vaccines for severe acute respiratory syndrome coronavirus 2 have several different mechanisms of action, including: (1) RNA and DNA vaccines that are genetically engineered to produce a protein that warrants an immune response; (2) Vector vaccines that introduce a type of virus that cannot produce disease but can effectively generate an immune response; (3) Vaccines that use genetically modified viruses to become weaker or inactivated in order not to cause virulence but retain their antigenicity; and (4) Harmless fragments of proteins or protein shells with similar antigenicity to the COVID-19 virus that are used in protein-based vaccines to produce immunity[5].
It is not uncommon to experience side effects following a COVID-19 vaccine. Fatigue, headache, muscle pain, joint pain, chills, fever, generalized body pain, and local reaction at the injection site are some of the side effects that have been reported after COVID-19 vaccination[6]. Several cardiovascular adverse effects have also been reported after COVID-19 vaccination, including myocarditis, pericarditis, and thrombotic events. Additionally, rare cardiovascular events such as hypertension, acute coronary syndrome, stress cardiomyopathy, arrhythmias, and cardiac arrest have also been reported. Although the relationship between these rare events and the vaccination is doubtful, the incidence of these rare side effects post vaccination in the absence of any other obvious cause in otherwise healthy individuals may suggest a causal relationship between the two[7].
When only focusing on myocardial infarction (MI), it is observed that after a person experiences an MI, there is a 20% chance of mortality in the 1st year and 10% thereafter[8]. According to Johansson et al[9], the relative risk of cardiovascular morbidity and mortality was 30% higher in those who suffered from MI compared to the general population. The aims of this review were to identify the pathophysiological links between MI and COVID-19 vaccination and investigate the doses and frequencies of COVID-19 vaccines, the types of MI, and mortality statistics linked with the vaccine administration in the available literature.
This systematic review was conducted over a period of 6 mo. The databases searched were MEDLINE, EMBASE, and PakMediNet. MeSH and Emtree terms were used as well as free text words (Table 1) to develop a search strategy. Various concepts were identified and then searched using Boolean operators. “AND” was used to separate various concepts, and synonyms for a single concept were separated by “OR.” The data was searched, and studies from the first delivery of COVID-19 vaccines in December 2020 until May 1, 2022 were included.
| Key concepts | Concept 1 | Concept 2 | Concept 3 | Concept 4 |
| COVID-19 | Vaccines | Myocardial infarction | Link | |
| Free text terms | Vaccination | Heart attack | Relation | |
| Shot | Interrelation | |||
| Jab | ||||
| Dose | ||||
| Booster | ||||
| Controlled vocabulary terms (MeSH terms, Emtree terms) | 2019 novel coronavirus | Viral Vaccines | Anterior wall myocardial infarction | Association |
| Immunization | ||||
| 2019-nCoV disease | ||||
| 2019-nCoV infection | Inferior wall myocardial infarction | |||
| COVID-19 pandemic | ||||
| Non-ST elevated myocardial infarction | ||||
| COVID-19 virus disease | ||||
| ST elevation myocardial infarction | ||||
| COVID-19 virus infection | ||||
| SARS-CoV-2 infection |
Articles that mentioned COVID-19 vaccination along with MI as a vaccine complication were included. Case reports, case series, original research, and systematic reviews were also included.
All studies that included COVID-19 cases but had no mention of vaccinations were excluded. Studies with no mention of MI as a complication of a COVID-19 vaccination were also excluded. Protocols and research based on animals and studies mentioning vaccinations for similar diseases, like influenza, were also excluded. Genetic studies involving COVID-19 without mention of vaccination and narrative reviews not identifying a link between MI and COVID-19 vaccination were further excluded (Figure 1).
The data was then extracted from the eligible studies by a structured data extraction form. Two independent researchers were engaged in both screening and data extraction, and data was analyzed for frequencies and percentages.
A total of 56 studies were identified, but only 29 were selected after applying the eligibility criteria (Table 2)[5-7,10-35]. The majority of these were case reports that reported the incidence of MI after COVID-19 vaccination but could not prove any causative relationship between the two. They also could not prove whether MI was due to vaccination-related changes in the body. The majority of side effects reported were from AstraZeneca followed by Pfizer and Moderna vaccines (Figure 2).
| Ref. | Study design | No. of cases reported | Type of vaccine given | Time during symptoms and vaccination | Dose of vaccine, 1st or 2nd | Type of MI | Patient outcome, dead or alive |
| Jabagi et al[10] | Cross sectional | 6510/11113 (58.6%) after first dose, 4843/11113 (43.6%) after second dose of vaccine | Pfizer | Not given | 1st and 2nd both | Not given | NA |
| Li et al[11] | Cohort study | Ages 35-74 incidence = between 1/100 and 1/1000 ages 75 and above incidence = between 1/10 and 1/100 patients | NA | Not given | NA | Not given | NA |
| Aye et al[12] | Cohort and systematic review | 29 in cohort, 35 in systematic review | Pfizer 30/35 (86%), Moderna 1/35 (3%), AstraZeneca 4/35 (11%), Janssen 0% | Median of 1 d | 2nd | STEMI 20/35 (57%) NSTEMI 15/35 (43%) | 1 dead, remaining were alive |
| Maadarani et al[13] | Case report | 1 | AstraZeneca | 1.5 h | 1st | STEMI and NSTEMI | Alive |
| Fazlollahi et al[14] | Systematic review | 3 | 2 Pfizer, 1 Moderna | Case 1: 6 h, case 2: 1 h, case 3: 30 min | 1st | Case 1: NSTEMI, case 2: STEMI, case 3: STEMI | Case 1: alive, case 2: alive, case 3: dead |
| Şancı et al[15] | Case report | 1 | Pfizer | 15 min | 1st | STEMI | Alive |
| Chatterjee et al[16] | Case report | 1 | AstraZeneca | 2 d | 1st | STEMI | Alive |
| Jeet Kaur et al[17] | Cross sectional | Pfizer: 44, AstraZeneca: 1, Moderna: 3 | Pfizer, AstraZeneca, and Moderna | NA | NA | NA | NA |
| Iqbal et al[18] | Case report | 1 | Moderna | 90 min | 1st | NSTEMI | Alive |
| Cari et al[19] | Cross sectional | Pfizer: 0.00044%, AstraZeneca: 0.00168%, Janssen: 0.00217% | Pfizer, AstraZeneca, Janssen | Not given | NA | NA | Dead Pfizer: 0.00013%, AstraZeneca: 0.0004%, Janssen: 0.00048% |
| Tajstra et al[20] | Case report | 1 | Pfizer | 30 min | 1st | STEMI | Dead |
| Flower et al[21] | Case report | 1 | AstraZeneca | 8 d | 1st | STEMI | Alive |
| Chiang et al[22] | Case report | 1 | AstraZeneca | 8 d | 1st | NSTEMI | Alive |
| Fialho et al[23] | Case report | 1 | AstraZeneca | 20 min | 1st | STEMI | Alive |
| Hsu et al[24] | Case report | 1 | AstraZeneca | 9 d | 1st | STEMI | Dead |
| Showkathali et al[25] | Cross sectional | 37 (42%) | AstraZeneca was the most used vaccine-28 (76%), while 9 (24%) had Covaxin | < 1 wk: 9 (24%), 1-4 wk: 19 (52%), and > 4 wk: 9 (24%) | 1st (65%), 2nd dose (35%) | STEMI and NSTEMI | NA |
| Whiteley et al[26] | Cross sectional | Post vaccination < 28 d 3814/13787 (27.66%). Post vaccination > 28 d 2050/7758 (26.42%) | AstraZeneca and Pfizer | NA | 1st | NA | Dead, < 28 d 16192/43766 (37.00%), > 28 d 11738/19496 (60.21%) |
| Shiravi et al[5] | Narrative review | Not given | Pfizer, AstraZeneca, and Sinovac vaccines | 15 min to 2 d | NA | NA | NA |
| Barda et al[27] | Cross sectional | 59/892785 = 0.006% | Pfizer | Not given | NA | NA | NA |
| Barsha et al[6] | Case report | 1 | Moderna | 12 h | 1st | NSTEMI | Alive |
| Boivin et al[28] | Case report | 1 | Moderna | 1 h | 1st | STEMI | Alive |
| Hippisley-Cox et al[29] | Cross sectional | AstraZeneca: 0-7 d 1983/22079 (8.98%), 8-21 d 4028/22079 (18.23%), 22-28 d 1889/22.79 (8.55%). Pfizer: 0-7 d 1578/15124 (10.43%), 8-21 d 3457/15124 (22.83%), 22-28 d 1510/15124 (10.00%) | AstraZeneca and Pfizer | AstraZeneca: 0-7 d 1983, 8-21 d 4028, 22-28 d 1889, Pfizer: 0-7 d 1578, 8-21 d 3457, 22-28 d 1510 | 1st | Not given | Not given |
| Edler et al[30] | Case series | 1 | Pfizer | 2 d | 1st | Not given | Dead |
| Hana et al[7] | Systematic review | 1644 cases total | AstraZeneca, Pfizer and Moderna | NA | NA | Not given | NA |
| Ho et al[31] | Systematic review | 10 | Pfizer, Moderna, AstraZeneca, Sinovac | NA | 1st | STEMI and NSTEMI | Some dead, some alive |
| Sung et al[32] | Case series | 2 | Moderna | Case 1 < 24 h, case 2 < 12 h | 1st | Case 1 STEMI, case 2 Not given | Alive |
| Pottegård et al[33] | Cross sectional | Incidence rate 1.04/1.21, no of cases 20 | AstraZeneca | Not given | 1st | Not given | NA |
| Agostino et al[34] | Case report | 1 | AstraZeneca | 12 d | 1st | Not given | Dead |
| Bardenheier et al[35] | Cross sectional | 1 | Pfizer | 2 d | 2nd | Not given | NA |
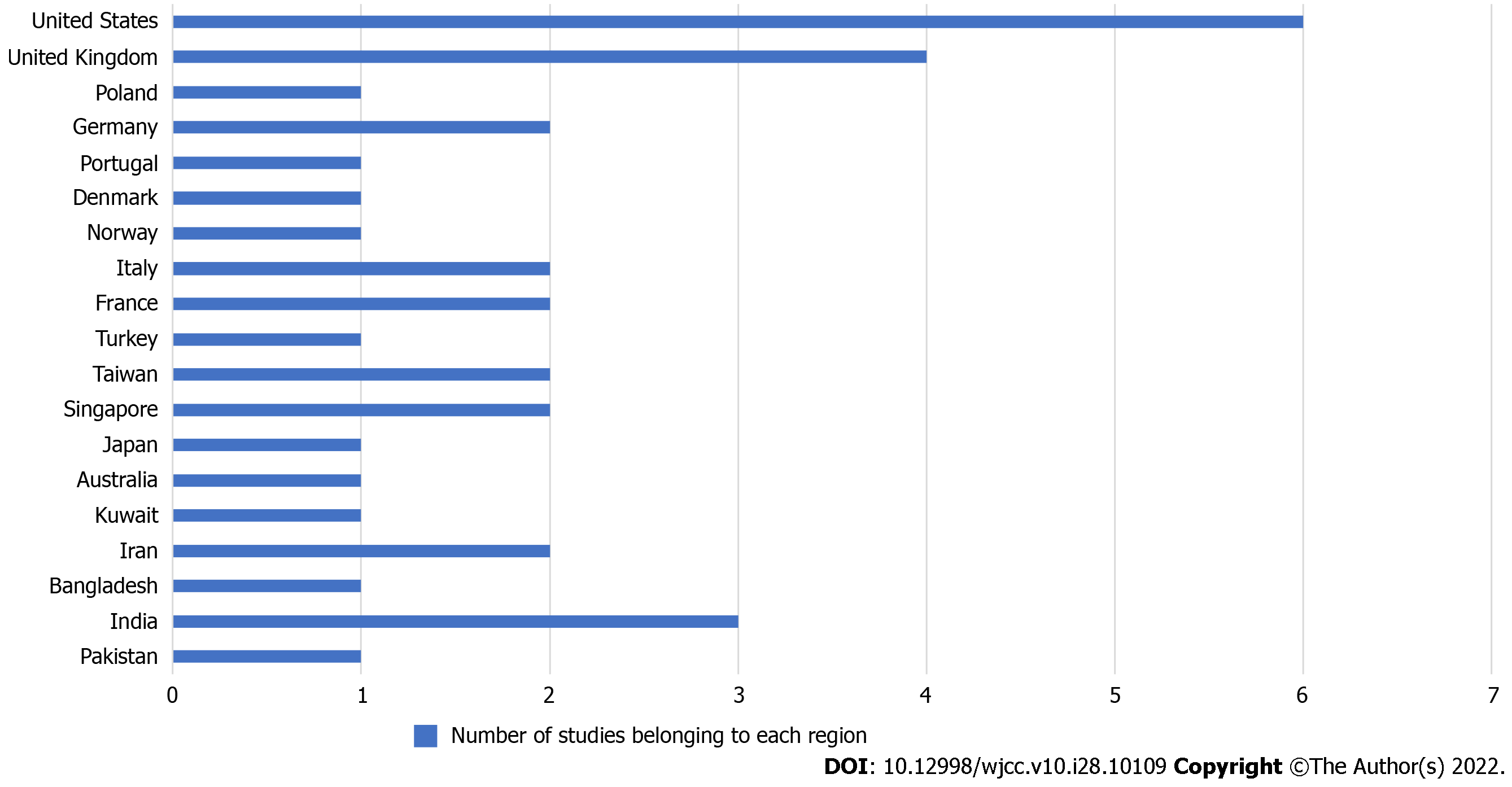
Based on the studies included in this report, patients usually suffered from MI after the first dose of the vaccine (69%). Of these studies, 53.3% of case reports reported MI within a few hours of vaccine administration and the rest within 10 d. Out of these patients, 44% of cases reported were of ST-segment elevation MI (STEMI), and 26% were non-ST-segment elevation MI (NSTEMI). The mortality rate was 29% (Figure 3). The most studies reported were from the United States, the United Kingdom, and India (Figure 4).
Possible mechanisms behind MI that have been identified after the literature review are as follows: (1) Kounis syndrome, which can cause MI through different mechanisms like allergic vasospasm and stent occlusion with a thrombus infiltrated by eosinophils or mast cells; (2) Vaccine-induced thrombotic thrombocytopenia; (3) Acute coronary syndrome after influenza vaccine administration as both influenza and COVID-19 vaccines share a common excipient (polysorbate 80); (4) Demand–supply mismatch ischemia, which can be caused by the stress of getting the vaccine in elderly people with other associated comorbidities; and (5) Myocarditis.
Globally, 65.8% of people have received at least one dose of a COVID-19 vaccine, and 11.79 billion doses have been administered globally[36]. The doses of various vaccines administered through May 25, 2022 are as follows: Pfizer, 613 million; Moderna, 147 million; AstraZeneca, 67 million; Janssen, 19 million; Sputnik V, 1.85 million; Sinopharm, 2.31 million; Sinovac, 3737; and Covaxin, 44. Pakistan has admi
MI remains one of the rare complications of a COVID-19 vaccination[7]. In this review, nearly 69% of the studies reported MI occurrence after the first dose, and 14% reported it after the second. It is a known fact that a subsequent or a booster dose of a vaccine is given to increase the antibody titer. This is especially crucial in those with a weakened immune response due to comorbidities[38]. A theory based on this finding is that since MI occurrence is higher after the first vaccine dose, immune response probably plays no role or only a minimal role in MI pathophysiology. If MI was due to overstimulation of the immune system, then the frequency of MI after the second dose would be higher.
A study showed that COVID-19 vaccination produces an optimum immune response in chronic inflammatory disease patients on immunosuppressive therapy, and the risk of adverse consequences is not higher than in the normal population[39]. No single inflammatory biomarker of systemic manifestations of vaccines has been identified but rather a battery of biomarkers, specifically, interleukin-6, C-reactive protein, and the interferon signaling pathway. A vaccine starts its action within minutes, starting from the injection site and spreading throughout the body. The factors known to affect the reactogenicity of various COVID-19 vaccines are age, gender, psychological/physical stressors, obesity, pre-existing immunity, vaccine characteristics (route, site, and method of vaccine administration), vaccine composition, antigen type, combination, and dose[40].
According to McManus et al[41], the incidence of STEMI compared to NSTEMI has reduced over the past few years. In the present research, patients with STEMI were present in 44% of the studies compared to 26% with NSTEMI after COVID-19 vaccination[41]. A recent case reported STEMI in a post-COVID-19-vaccinated patient in April 2022, and the patient also had a history of myocarditis. A possible explanation was the transient atrial fibrillation or plaque rupture after the myocarditis[42]. Therefore, myocarditis on the one hand is a primary complication of COVID-19 vaccination and on the other hand is a secondary cause of MI development after COVID-19 vaccination.
In his blog, Labos wrote a critique in which he pointed out that in order to state the link between inflammation and MI after COVID-19 vaccination, the inflammatory biomarkers need to be measured before, soon after, and a month after vaccination[43]. Jabagi et al[10] proved that there was no association between increased risk of MI post Pfizer vaccination for COVID-19. He did a serial analysis 1 d, 1 wk, and 2 wk post vaccination[10].
Kounis syndrome is one of the mechanisms identified for the occurrence of MI post COVID-19 vaccination as it has been observed in various patients who received a COVID-19 vaccination (Pfizer, AstraZeneca, and Sinovac vaccines)[5,15]. It manifests as an acute coronary syndrome accompanied by mast cell activation due to hypersensitivity or an allergic or anaphylactic reaction[44]. It is more commonly linked with STEMI, which probably explains the higher percentage of STEMI cases after COVID-19 vaccination compared to NSTEMI in the present research.
Vaccine-induced thrombotic thrombocytopenia is another mechanism linking MI with COVID-19 vaccines. In vaccine-induced thrombotic thrombocytopenia, tetramers of platelet factor 4 crosslink with vaccine proteins to form multimolecular aggregates. The ethylenediamine tetraacetic acid in the vaccine possibly causes capillary leakage and dissemination of components in the blood. The multimolecular aggregates are recognized by immunoglobulin G antibodies, which activate platelets, neutrophils, and the complement system, resulting in massive coagulation system activation[45]. This can lead to MI and eventually mortality or long-term morbidity.
Polysorbate 80, an excipient in the AstraZeneca vaccine, is seen to trigger allergic reactions in those allergic to the compound. Similarly, polyethylene glycol is the excipient found in Pfizer and is a cause of severe anaphylaxis in those allergic to polyethylene glycol. However, polyethylene glycol allergy is very rare compared to polysorbate 80 allergy[46], which probably explains the increased number of MI cases reported in patients vaccinated with AstraZeneca compared to other vaccines.
The literature searched and analyzed in this study was quite diverse. Therefore, one of the challenges faced while compiling this review was to bring the results under one umbrella. It was observed that robust original research needs to be conducted to probe the mechanisms of and the association between MI and COVID-19 vaccination. Considering the amount of clinical evidence available linking MI with COVID-19 vaccination, it is a bit early to make definitive deductions regarding their link.
It can be concluded that there is a link between COVID-19 vaccinations and MI in temporal terms of occurrence. However, there is also a possibility that the link was found purely by chance as a majority of cases were reported within 1 d, which is too short a time for a build-up of definitive causative mechanisms for MI. This is due to the diversity of risk factors for MI, such as age, gender, body mass index, ethnicity, and physical/mental stressors. This link can be definitively proven by conducting research while considering the confounders. AstraZeneca was seen to induce the greatest amount of coronary artery disease, with STEMI as the leading type, and this primarily followed the first vaccination dose.
Coronavirus disease 2019 (COVID-19) was first identified November 2019 and subsequently caused a world pandemic. The development of vaccines was quickly achieved with the first vaccinations occurring in December 2020. From then on, a global campaign commenced to vaccinate the majority of the world’s population. The implications of these vaccines have been researched abundantly.
The side effects of COVID-19 vaccines, particularly serious cardiovascular side effects, mention myocarditis. However, myocardial infarction (MI) and its link with COVID-19 vaccines remain unclear.
To investigate and pinpoint if any link exists between COVID-19 vaccination and MI amongst the vaccinated individuals.
We devised a search strategy to search MEDLINE, EMBASE, and PakMediNet. All studies that reported MI after COVID-19 vaccine delivery were included in this research. A structured data extraction form was developed to extract the data from the eligible studies.
The majority of MI cases reported were after vaccination with the AstraZeneca vaccine. Out of all the studies, 69% reported MI cases after the first COVID-19 vaccination dose and 14% after the second. The majority (44%) of MI reported was STEMI.
Many studies linked MI to COVID-19 vaccinations, but no definitive association could be found.
More robust clinical trials in this regard could clarify the link between COVID-19 vaccinations and MI.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: American Physiological Society, No. 00199119; and Pakistan Biological Safety Association, No. 1032.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kirkik D, Turkey; Krishnamoorthy Y, India; Munteanu C, Romania S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Sultana J, Mazzaglia G, Luxi N, Cancellieri A, Capuano A, Ferrajolo C, de Waure C, Ferlazzo G, Trifirò G. Potential effects of vaccinations on the prevention of COVID-19: rationale, clinical evidence, risks, and public health considerations. Expert Rev Vaccines. 2020;19:919-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Yuan P, Ai P, Liu Y, Ai Z, Wang Y, Cao W, Xia X, Zheng JC. Safety, Tolerability, and Immunogenicity of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. medRxiv. 2020;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Majumder J, Minko T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021;23:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 213] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 4. | Umakanthan S, Sahu P, Ranade AV, Bukelo MM, Rao JS, Abrahao-Machado LF, Dahal S, Kumar H, Kv D. Origin, transmission, diagnosis and management of coronavirus disease 2019 (COVID-19). Postgrad Med J. 2020;96:753-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 150] [Reference Citation Analysis (0)] |
| 5. | Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K. Cardiovascular Complications of SARS-CoV-2 Vaccines: An Overview. Cardiol Ther. 2022;11:13-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Barsha SY, Akiful Haque MM, Rashid MU, Rahman ML, Hossain MA, Zaman S, Bhuiyan E, Sultana R, Hossian M, Nabi MH, Hawlader MDH. A case of acute encephalopathy and non-ST segment elevation myocardial infarction following mRNA-1273 vaccination: possible adverse effect? Clin Exp Vaccine Res. 2021;10:293-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hana D, Patel K, Roman S, Gattas B, Sofka S. Clinical Cardiovascular Adverse Events Reported Post-COVID-19 Vaccination: Are They a Real Risk? Curr Probl Cardiol. 2022;47:101077. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Law MR, Watt HC, Wald NJ. The underlying risk of death after myocardial infarction in the absence of treatment. Arch Intern Med. 2002;162:2405-2410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 138] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Johansson S, Rosengren A, Young K, Jennings E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: a systematic review. BMC Cardiovasc Disord. 2017;17:53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 160] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 10. | Jabagi MJ, Botton J, Bertrand M, Weill A, Farrington P, Zureik M, Dray-Spira R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA. 2022;327:80-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 11. | Li X, Ostropolets A, Makadia R, Shaoibi A, Rao G, Sena AG, Martinez-Hernandez E, Delmestri A, Verhamme K, Rijnbeek PR, Duarte-Salles T, Suchard M, Ryan P, Hripcsak G, Prieto-Alhambra D. Characterizing the incidence of adverse events of special interest for COVID-19 vaccines across eight countries: a multinational network cohort study. medRxiv. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | Aye YN, Mai AS, Zhang A, Lim OZH, Lin N, Ng CH, Chan MY, Yip J, Loh PH, Chew NWS. Acute Myocardial Infarction and Myocarditis following COVID-19 Vaccination. QJM. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 13. | Maadarani O, Bitar Z, Elzoueiry M, Nader M, Abdelfatah M, Zaalouk T, Mohsen M, Elhabibi M. Myocardial infarction post COVID-19 vaccine - coincidence, Kounis syndrome or other explanation - time will tell. JRSM Open. 2021;12:20542704211025259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Fazlollahi A, Zahmatyar M, Noori M, Nejadghaderi SA, Sullman MJM, Shekarriz-Foumani R, Kolahi AA, Singh K, Safiri S. Cardiac complications following mRNA COVID-19 vaccines: A systematic review of case reports and case series. Rev Med Virol. 2022;32:e2318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Şancı E, Örçen C, Çelik OM, Özen MT, Bozyel S. Kounis syndrome associated with BNT162b2 mRNA COVID-19 vaccine presenting as ST-elevation acute myocardial infarction. Anatol J Cardiol. 2022;26:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Chatterjee S, Ojha UK, Vardhan B, Tiwari A. Myocardial infarction after COVID-19 vaccination-casual or causal? Diabetes Metab Syndr. 2021;15:1055-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Jeet Kaur R, Dutta S, Charan J, Bhardwaj P, Tandon A, Yadav D, Islam S, Haque M. Cardiovascular Adverse Events Reported from COVID-19 Vaccines: A Study Based on WHO Database. Int J Gen Med. 2021;14:3909-3927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Iqbal S, Adnan G, Farhad A, Ahmed I, Rahman MN. Acute Myocardial Infarction After Coronavirus Vaccine: A Rare Adverse Effect. Cureus. 2022;14:e21544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Cari L, Alhosseini MN, Fiore P, Pierno S, Pacor S, Bergamo A, Sava G, Nocentini G. Cardiovascular, neurological, and pulmonary events following vaccination with the BNT162b2, ChAdOx1 nCoV-19, and Ad26.COV2.S vaccines: An analysis of European data. J Autoimmun. 2021;125:102742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Tajstra M, Jaroszewicz J, Gąsior M. Acute Coronary Tree Thrombosis After Vaccination for COVID-19. JACC Cardiovasc Interv. 2021;14:e103-e104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 21. | Flower L, Bares Z, Santiapillai G, Harris S. Acute ST-segment elevation myocardial infarction secondary to vaccine-induced immune thrombosis with thrombocytopaenia (VITT). BMJ Case Rep. 2021;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Chiang CY, Chen CY, Yu WL, Kan WC, Feng YH. Myocardial Infarction and Azygos Vein Thrombosis After ChAdOx1 nCoV-19 Vaccination in a Hemodialysis Patient. Cureus. 2021;13:e18390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Fialho I, Mateus C, Martins-Dos-Santos G, Pita J, Cabanelas N, Baptista SB, Roque D. Recurrent Kounis syndrome - a life-threatening event after COVID-19 vaccine administration. J Cardiol Cases. 2022;25:400-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Hsu MH, Lee CP, Huang YC. Acute ST-Segment Elevation Myocardial Infarction After ChAdOx1 nCoV-19 Vaccination in a 33-Year-Old Man. Ann Emerg Med. 2022;79:220-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Showkathali R, Yalamanchi R, Narra L, Vinayagamoorthy N, Gunasekaran S, Nayak R, Vijayachandra Reddy Y, Mahilmaran A, Srinivasan KN, Oomman A, Kaliyamoorthy D. Coronary thrombo-embolic events after Covid-19 vaccination- a single centre study. Indian Heart J. 2022;74:131-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Whiteley WN, Ip S, Cooper JA, Bolton T, Keene S, Walker V, Denholm R, Akbari A, Omigie E, Hollings S, Di Angelantonio E, Denaxas S, Wood A, Sterne JAC, Sudlow C; CVD-COVID-UK consortium. Association of COVID-19 vaccines ChAdOx1 and BNT162b2 with major venous, arterial, or thrombocytopenic events: A population-based cohort study of 46 million adults in England. PLoS Med. 2022;19:e1003926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 36] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 27. | Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 669] [Cited by in F6Publishing: 616] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 28. | Boivin Z, Martin J. Untimely Myocardial Infarction or COVID-19 Vaccine Side Effect. Cureus. 2021;13:e13651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Hippisley-Cox J, Patone M, Mei XW, Saatci D, Dixon S, Khunti K, Zaccardi F, Watkinson P, Shankar-Hari M, Doidge J, Harrison DA, Griffin SJ, Sheikh A, Coupland CAC. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. BMJ. 2021;374:n1931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 170] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 30. | Edler C, Klein A, Schröder AS, Sperhake JP, Ondruschka B. Deaths associated with newly launched SARS-CoV-2 vaccination (Comirnaty®). Leg Med (Tokyo). 2021;51:101895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Ho JS, Sia CH, Ngiam JN, Loh PH, Chew NW, Kong WK, Poh KK. A review of COVID-19 vaccination and the reported cardiac manifestations. Singapore Med J. 2021;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Sung JG, Sobieszczyk PS, Bhatt DL. Acute Myocardial Infarction Within 24 Hours After COVID-19 Vaccination. Am J Cardiol. 2021;156:129-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 33. | Pottegård A, Lund LC, Karlstad Ø, Dahl J, Andersen M, Hallas J, Lidegaard Ø, Tapia G, Gulseth HL, Ruiz PL, Watle SV, Mikkelsen AP, Pedersen L, Sørensen HT, Thomsen RW, Hviid A. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: population based cohort study. BMJ. 2021;373:n1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 34. | D'Agostino V, Caranci F, Negro A, Piscitelli V, Tuccillo B, Fasano F, Sirabella G, Marano I, Granata V, Grassi R, Pupo D. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J Pers Med. 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Bardenheier BH, Gravenstein S, Blackman C, Gutman R, Sarkar IN, Feifer RA, White EM, McConeghy K, Nanda A, Mor V. Adverse events following mRNA SARS-CoV-2 vaccination among U.S. nursing home residents. Vaccine. 2021;39:3844-3851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Our World in Data. Coronavirus (COVID-19) Vaccinations. 2022. Available from: https://ourworldindata.org/covid-vaccinations#which-vaccines-have-been-administered-in-each-country. [Cited in This Article: ] |
| 37. | Pakistan: the latest coronavirus counts, charts and maps [Internet]. Reuters. 2022 Available from: https://graphics.reuters.com/world-coronavirus-tracker-and-maps/countries-and-territories/pakistan/. [Cited in This Article: ] |
| 38. | UW–Madison. What is the difference between an additional dose and a booster shot ? COVID-19 Response. 2022. Available from: https://covidresponse.wisc.edu/faq/what-is-the-difference-between-a-third-dose-and-a-booster-shot/. [Cited in This Article: ] |
| 39. | Leigh S. COVID-19 Vaccines Produce Immune Responses in Patients With Chronic Inflammatory Diseases COVID-19 Vaccine ‘Clearly a Benefit’ Despite. 2021. Available from: https://www.ucsf.edu/news/2021/08/421346/covid-19-vaccines-produce-immune-responses-patients-chronic-inflammatory. [Cited in This Article: ] |
| 40. | Hervé C, Laupèze B, Del Giudice G, Didierlaurent AM, Tavares Da Silva F. The how's and what's of vaccine reactogenicity. NPJ Vaccines. 2019;4:39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 41. | McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 436] [Cited by in F6Publishing: 446] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 42. | Botros MB, Narvaez-Guerra O, Aurigemma GP, Harrington C. STEMI following MRNA COVID-19 vaccination. J Am Coll Cardiol. 2022;79 (9_Suppl):2341. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Labos C. Do the COVID-19 Vaccines Really Increase MI What to Read Next on Medscape. Medscape 2022. Available from: https://www.medscape.com/viewarticle/964358. [Cited in This Article: ] |
| 44. | Memon S, Chhabra L, Masrur S, Parker MW. Allergic acute coronary syndrome (Kounis syndrome). Proc (Bayl Univ Med Cent). 2015;28:358-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | John CV, Kumar R, Sivan AK, Jithin S, Abraham R, Philip CC. Vaccine-induced thrombotic thrombocytopenia (VITT): first report from India. Thromb J. 2022;20:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 46. | Sellaturay P, Gurugama P, Harper V, Dymond T, Ewan P, Nasser S. The Polysorbate containing AstraZeneca COVID-19 vaccine is tolerated by polyethylene glycol (PEG) allergic patients. Clin Exp Allergy. 2022;52:12-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |