Abstract
Coronavirus disease 2019 (COVID-19) typically presents with pulmonary symptoms. Extra-pulmonary symptomatology of COVID-19 has drawn significant attention. However, information about the incidence, course and outcomes of acute pancreatitis in these patients is still limited.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) typically presents with pulmonary symptoms. Extra-pulmonary symptomatology of COVID-19 has drawn significant attention. Meta-analysis of 47 studies including 10,890 COVID-19 patients showed that gastrointestinal symptoms are present in less than 10% of cases with a pooled prevalence of 7.8% for nausea/vomiting, 7.7% for diarrhoea and 2.7% for abdominal pain and 15% for liver enzyme abnormalities (transaminitis) [1]. However, presentation with isolated digestive symptom is rare [1].
Moreover, severe COVID-19 patients are prone to more serious gastrointestinal complications. In a study by Kaafarani et al., 74% (104/141) of the critically ill patients developed at least one gastrointestinal complication including hepatic necrosis, bowel ischemia requiring emergent surgery and bowel resection and Ogilvie-like syndrome with a very high (40%) postoperative mortality [2].
Besides these digestive presentations of COVID-19, another clinical entity that deserves discussion is acute pancreatitis (AP). Surgeons involved in the management of AP should be aware of its existence in the context of COVID-19 as with the increasing prevalence of COVID-19 it is expected that we would encounter more of its atypical presentations including AP. Information about the incidence, course, and outcomes of acute pancreatitis in these patients is still limited.
Clinical Spectrum of Pancreatic Injury in COVID-19
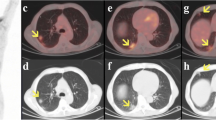
With reference to the clinical presentation of AP, we are yet to know the full spectrum of the pancreatic injury in COVID-19. Based on the currently available reports, there can be an isolated rise in the pancreatic enzymes without any clinical or radiological evidence of AP, subclinical disease with only serological and/or radiological evidence of AP, “pancreatitis-like clinical presentation”, and finally, it can be an overt episode of AP (Table 1) [3,4,5,6,7].
Wang et al. earlier reported 17% incidence of pancreatic injury among 52 patients with COVID-19 pneumonia. Serum markers were mildly elevated (mean serum amylase 115 ± 25 U/L and serum lipase 71 ± 34 U/L). However, none had abdominal pain or clinically severe pancreatitis [3]. When compared with the patients without pancreatic injury, those with pancreatic injury had a higher incidence of anorexia and diarrhoea, severer illness on admission, lower level of CD3+ T cell and CD4+ T cell counts, and higher level of serum aspartate aminotransferase, gamma glutamyl transpetidase, creatinine and lactate dehydrogenase.
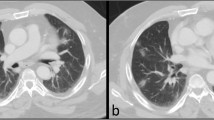
Liu et al. also showed 17% incidence of pancreatic injury in 67 severe COVID-19 cases although injury was evident on computed tomography (CT) scan in only 7.46% cases, mainly as focal pancreatic enlargement or pancreatic ductal dilatation [8]. Incidence of pancreatic injury was low (1.85%) in patients with mild disease [8]. However, none of the patients with pancreatic injury presented with abdominal pain or had pancreatic necrosis [8].
On the contrary, severe AP has also been reported (Table 1). Hadi et al. reported familial clustering of COVID-19 cases with two of three family members with COVID-19 had AP [6]. One of these cases had no abdominal symptoms but had rising pancreas specific serum amylase levels that warranted further investigation, and diagnosis of AP was made on ultrasonography that showed inflamed edematous pancreas without gallstones [6]. Anand et al. reported a case of COVID-19 who initially presented with fever, cough, sore throat and myalgia [7]. Patient recovered from this illness but presented again with abdominal symptoms 5 days after the discharge. Adhesive intestinal obstruction was suspected, but later, AP was diagnosed on CT scan that showed diffusely edematous pancreatitis. However, pancreatic enzyme assay was not done either at admission or subsequently. Although authors considered AP idiopathic in nature in this case, they did suspect causal relationship between COVID-19 and AP due to their temporal association [7]. None of the cases reported so far had necrotising pancreatitis even though some were classified as severe, and none required any intervention for pancreatitis-related local complications.
Mechanisms of Pancreatic Injury in COVID-19
Various mechanisms might be involved in the pancreatic injury in COVID-19. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) seems to have an affinity for the pancreas as angiotensin-converting enzyme 2 (ACE2) receptor is expressed in the pancreas, both in the exocrine glands and islet cells [8]. Such expression has been shown to be higher in the pancreas than in the lungs [8]. Thus, pancreatic injury can result from the direct cytopathic effect of SARS-CoV-2 mediated by its local replication [3]. Another putative mechanism involves dysregulated immune response induced by SARS-CoV-2 that targets pancreas in addition to the lungs and kidneys causing AP besides organ failure [3]. However, organ failure can also occur in AP secondary to other causes (e.g. biliary pancreatitis) in a patient with COVID-19 who might otherwise be asymptomatic or mildly symptomatic. Such presentation can be encountered more commonly in near future with the increasing prevalence of COVID-19. Lastly, AP can also result from drug-induced injury either directly (e.g. use of nonsteroidal anti-inflammatory drugs (NSAID) or glucocorticoids) [3, 8] or indirectly (through tocilizumab-induced hypertriglyceridemia) [9].
Diagnosis of Acute Pancreatitis in COVID-19
It is important to make the correct diagnosis of AP in COVID-19 patients although it can be difficult. Isolated rise in the pancreatic enzymes in a patient with COVID-19 should not be attributed to AP without clinico-radiological correlation as such rise can be non-pancreatic in origin and can be seen in gastroenteritis and lung injury also, especially in the presence of renal or respiratory failure; conditions commonly present in severe COVID-19 [10]. Rather, criteria as per the revised Atlanta classification should be used to make the correct diagnosis which requires at least two of the following three criteria: (1) typical abdominal pain, (2) serum amylase or lipase > 3 times the upper normal limit, and (3) characteristic findings on diagnostic imaging; the latter might play more significant role in COVID-19 patients where clinical evaluation for abdominal symptoms might not be feasible in severe case requiring intensive care unit care with ventilatory support.
What Could Be the Surgical Implications?
As target organs are same in severe AP and severe COVID-19, acute respiratory distress syndrome or acute renal failure resulting from the latter can lead to inaccurate severity assessment of AP and response assessment when “step-up” approach is adopted to deal with local complications. In addition, occurrence of AP can aggravate the inflammatory response already induced by SARS-CoV-2 leading to accelerated organ failure.
Possible presence of SARS-CoV-2 in the pancreatic tissue is also a concern for the surgical team. As this virus has been detected in the peritoneal fluid with a load higher than in the respiratory tract [11], there is a possibility that this virus could also be present in the (peri) pancreatic fluid and necrotic tissues. In addition, viremia may not be directly related to the severity of symptoms, and all the patients, irrespective of the symptom’s severity, could have viral load in the peritoneal fluid [11]. Although the possibility of viral load in pancreatic necrotic tissue and fluid is uncertain, interventions required to manage local complications (acute necrotic collections or walled off necrosis), whether percutaneous, endoscopic, or minimally invasive (retroperitoneal/transperitoneal), might expose the health care workers to SARS-CoV-2, more so with high-risk aerosol generating procedures like endoscopic or minimally invasive drainage / necrosectomy including video-assisted retroperitoneal debridement (VARD). In addition, it is unknown how long virus might persist in the (peri)pancreatic fluid or tissues that becomes relevant while managing symptomatic pseudocyst or walled-off necrosis once acute episode is over.
Key Points to Remember
Surgeons should be aware of that (1) pancreas is not immune to COVID-19 though its involvement seems to be uncommon and much less as compared to digestive tract involvement; (2) in patients with COVID-19, elevation of the pancreatic enzymes seems to be common but cannot be attributed to acute pancreatitis in all the cases; (3) diagnosis of AP should follow standard criteria (revised Atlanta) in COVID-19 patients, although accurate severity assessment can be a problem; (4) development of severe AP in a patient with COVID-19 is a double-trouble for both the patient and physician alike because of the diagnostic dilemma, possibility of common target organs(s) involvement and accelerated clinical course; and (5) those involved in the interventional management of moderate / severe necrotising pancreatitis needs to be more careful given the unknown possibility of exposure to SARS-CoV-2 through drained fluid or necrotic pancreatic tissues during procedure.
Conclusions
Our knowledge and understanding about the interaction between SARS-CoV-2 and pancreas are limited at present, but are expected to evolve rapidly given the increasing prevalence of COVID-19 in the population that would allow us to formulate appropriate management strategies. At present, it is imperative for the surgeons to keep in mind the possible association between COVID-19 and pancreas as they might be involved in the management of AP at some point of time during its course.
References
Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK et al (2020) AGA Institute Rapid Review of the GI and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology S0016–5085(20):30593-X. https://doi.org/10.1053/j.gastro.2020.05.001
Kaafarani HMA, Moheb ME, Hwabejire JO, Naar L, Christensen MA, Breen K, Gaitanidis A, Alser O, Mashbari H, Bankhead-Kendall B, Mokhtari A, Maurer L, Kapoen C, Langeveld K, el Hechi MW, Lee J, Mendoza AE, Saillant NN, Parks J, Fawley J, King DR, Fagenholz PJ, Velmahos GC (2020) Gastrointestinal complications in critically ill patients with COVID-19. Ann Surg Publish Ahead of Print(May 1). https://doi.org/10.1097/SLA.0000000000004004
Wang F, Wang H, Fan J, Zhang Y, Wang H, Zhao Q (2020) Pancreatic injury patterns in patients with COVID-19 pneumonia. Gastroenterology. (Apr 1). https://doi.org/10.1053/j.gastro.2020.03.055
Spinelli A, Pellino G (2020) COVID-19 pandemic: perspectives on an unfolding crisis. Br J Surg https:// bjssjournals.onlinelibrary.wiley.com/ doi/https://doi.org/10.1002/bjs.11627
Aloysius MM, Thatti A, Gupta A, Sharma N, Bansal P, Goyal H (2020) COVID-19 presenting as acute pancreatitis. Pancreatology. https://doi.org/10.1016/.pan.2020.05.003
Hadi A, Werge M, Kristiansen KT, Pedersen UG, Karstensen JG, Novovic S, et al (2020) Coronavirus Disease-19 (COVID-19) associated with severe acute pancreatitis: case report on three family members Pancreatology May 5:S1424-3903(20)30147-2. doi: https://doi.org/10.1016/j.pan.2020.04.021
Anand ER, Major C, Pickering O, Nelson M (2020) Acute pancreatitis in a COVID-19 patient. Br J Surg 107(Apr 27):e182. https://doi.org/10.1002/bjs.11657
Liu F, Long X, Zhang B, Zhang W, Chen X, Zhang Z (2020) ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol Apr 22:S1542-3565(20):30537–30531. https://doi.org/10.1016/j.cgh.2020.04.040
Morrison AR, Johnson JM, Ramesh M, Bradley P, Jennings J, Smith ZR (2020) Letter to the editor: acute hypertriglyceridemia in patients with COVID-19 receiving tocilizumab J Med Virol Apr 21. doi: https://doi.org/10.1002/jmv.25907
de-Madaria E, Siau K, Cárdenas-Jaén K (2020) Increased amylase and lipase in patients with COVID-19 pneumonia: don’t blame the pancreas just yet! Gastroenterology. Apr 21:S0016–5085(20):30561–30568. https://doi.org/10.1053/j.gastro.2020.04.044
Coccolini F, Tartaglia D, Puglisi A, Giordano C, Pistello M, Lodato M, et al (2020) SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann Surg (in press). Retrieved from https://journals.lww.com/annalsofsurgery/Documents/SARS-CoV-2%20is%20present%20in%20peritoneal%20fluid%20in%20COVID-19%20patients.pdf
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gupta, V. COVID-19 and Acute Pancreatitis: What Do Surgeons Need to Know?. Indian J Surg 82, 301–304 (2020). https://doi.org/10.1007/s12262-020-02447-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12262-020-02447-w