Effect of Anticoagulant Administration on the Mortality of Hospitalized Patients With COVID-19: An Updated Systematic Review and Meta-Analysis
- 1Department of Nephrology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Nephrology, Jiujiang No. 1 People's Hospital, Jiujiang, China
- 3Disaster Medicine Center, Institute for Disaster Management and Reconstruction, Sichuan University, Chengdu, China
- 4The First People's Hospital of Shuangliu District, Chengdu, China
- 5Med-X Center for Materials, Sichuan University, Chengdu, China
Background: Anticoagulation is generally used in hospitalized patients with coronavirus disease 2019 (COVID-19) as thromboprophylaxis. However, results from different studies comparing the effect of anticoagulation on the mortality of COVID-19 patients with non-anticoagulation are inconclusive.
Methods: Our systematic review included observational trials if they studied anticoagulant therapy in hospitalized patients with COVID-19 for mortality or bleeding events. Dichotomous variables from individual studies were pooled by risk ratio (RR) and their 95% confidence interval (95% CI) using the random-effects model. Grading of Recommendations Assessment, Development and Evaluation was used to assess the quality of evidence.
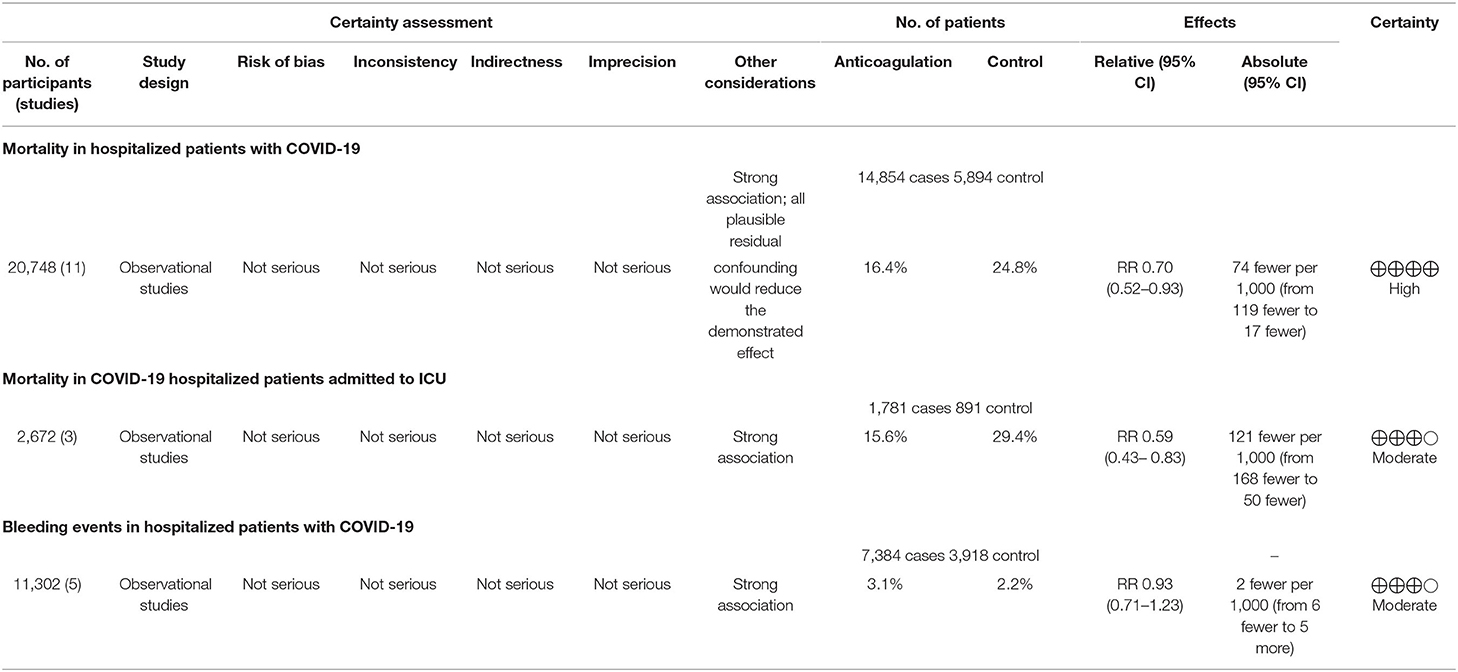
Results: A total of 11 observational studies enrolling 20,748 hospitalized COVID-19 patients overall were included. A pooled meta-analysis of these studies showed that anticoagulation therapy, compared with non-anticoagulation therapy, was associated with lower mortality risk (RR 0.70, 95% CI 0.52–0.93, p = 0.01). The evidence of benefit was stronger among critically ill COVID-19 patients in the intensive care units (RR 0.59, 95% CI 0.43–0.83, p = 0.002). Additionally, severe bleeding events were not associated with the administration of anticoagulants (RR 0.93, 95% CI 0.71–1.23, p = 0.63).
Conclusion: Among patients with COVID-19 admitted to hospital, the administration of anticoagulants was associated with a decreased mortality without increasing the incidence of bleeding events.
Introduction
Coronavirus disease 2019 (COVID-19), provoked by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, was first reported in December 2019 and is the most serious worldwide public health crisis (1, 2). Venous thromboembolism (VTE) is the most commonly reported thrombotic complication, with a high incidence rate of 27.9% among critically ill COVID-19 patients admitted to intensive care units (ICUs)
(3, 4). The incidence of pulmonary embolism in patients with COVID-19 who underwent pulmonary CT angiography was reported to be between 22 and 30% (2, 5). Accordingly, even though the risk–benefit ratio of anticoagulation has not been established clearly, some guidelines have recommended prophylactic dose anticoagulation for COVID-19 patients who do not have a contraindication to this treatment to reduce the risk of VTE (6–8).
Some retrospective observational studies showed that anticoagulant administration was associated with reduced mortality (9, 10), but others did not confirm these findings and, rather, suggested an elevated risk of bleeding (11–13). Limited evidence exists to guide the prophylactic antithrombotic regimen in COVID-19 patients due to a lack of randomized clinical trials. Up to date, the cumulative number of inpatients with COVID-19 in cohort studies exploring the effect of anticoagulation therapy on the mortality has exceeded 20,000. In this study, we set out to perform an updated meta-analysis of current evidence to further clarify whether hospitalized patients with COVID-19 benefit from anticoagulation therapy (including both therapeutic– and prophylactic–dose anticoagulation therapy).
Methods
This meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (14), with the PRISMA checklist provided in Supplementary Table 1, and was registered in the Open PROSPERO Framework (CRD42021229707). All steps were performed independently by two investigators (JLJ and LYP). Any discrepancies were discussed with the corresponding author (SBH).
Study Selection Criteria
Two reviewers independently screened all relevant articles published on MEDLINE, the Cochrane Library, EMBASE, and the Web of Science databases from inception to March 28, 2021. The search terms used were “COVID-19,” “2019 novel coronavirus infection,” “coronavirus disease-2019,” “2019-nCoV disease,” “2019 novel coronavirus disease,” “severe acute respiratory syndrome coronavirus 2,” “Wuhan coronavirus,” “Wuhan seafood market pneumonia virus,” “SARS-CoV-2,” “SARS2,” “anticoagulant,” “anticoagulation,” “heparin,” “unfractionated heparin,” “UFH,” “fondaparinux,” “enoxaparin,” “low-molecular-weight heparin,” “heparin, low molecular weight,” “LMWH,” “thromboprophylaxis,” “antithrombotic,” and “anti-thrombosis” (see Supplementary Table 2 for the detailed search strategy). The titles and abstracts of the resulting articles were examined to exclude irrelevant studies. The full texts of the remaining articles were read to determine if these articles meet our eligibility criteria. Randomized controlled trials (RCTs) or observational studies that compared the effect of anticoagulation vs. non-anticoagulation on the mortality and/or bleeding events of hospitalized COVID-19 patients were included. The exclusion criteria were as follows: (1) absence of original data; (2) enrollment of non-hospitalized COVID-19 patients; (3) case series/report; (4) absence of a comparator group; and (5) non-human studies.
Data Extraction and Quality Assessment
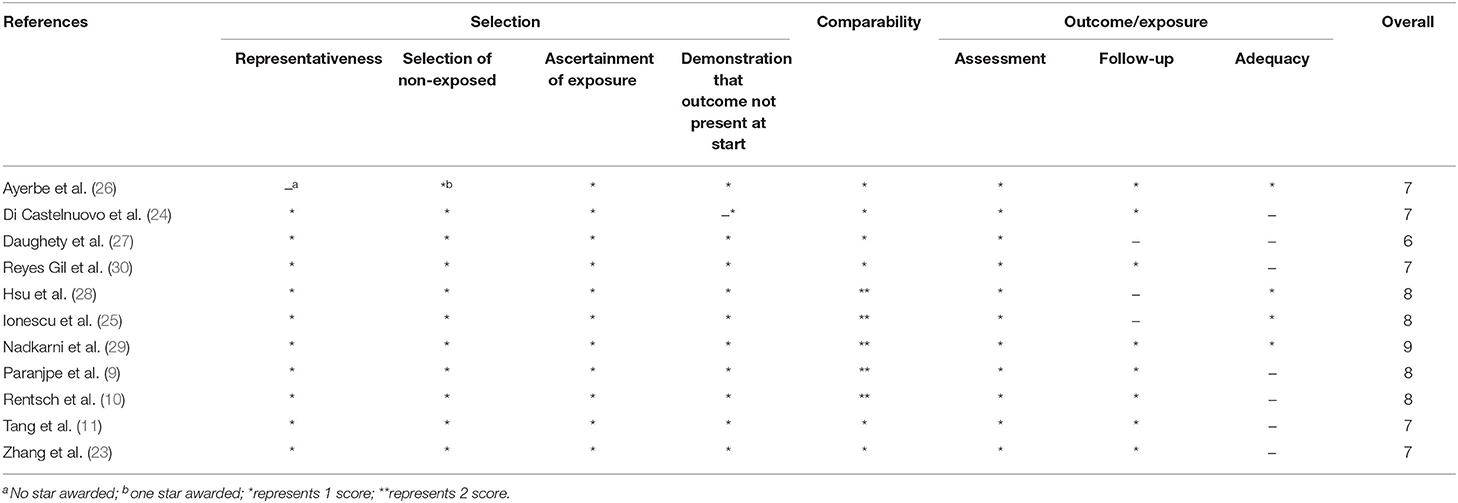
This meta-analysis mainly investigated the impact of anticoagulation on the in-hospital mortality and bleeding risk of inpatients with COVID-19. The Newcastle–Ottawa Scale was used to assess the methodological quality of observational studies (case–control or cohort studies), which has eight criteria and yields scores ranging from 0 to 9. Studies with scores ≥7 were regarded as high quality (15). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework was applied for rating the quality of evidence for each outcome in the pooled analysis, and the quality of evidence was rated as high, moderate, low, and very low quality (16). The credibility of results from subgroup analyses was assessed by specific criteria (17, 18).
Outcomes
The primary outcome was all-cause in-hospital mortality during hospitalization for COVID-19. The secondary outcome was the incidence of bleeding events during hospitalization for COVID-19. Definition of bleeding events was according to definitions in the individual studies, mainly including (1) an active source of bleeding; (2) suspected bleeding without confirmation of an active bleeding source; (3) confirmatory imaging or other evidence (neuroimaging for intracranial bleed); and (4) bleeding necessitating a transfusion of packed red blood cells.
Statistical Analysis
Dichotomous variables such as mortality or bleeding events were expressed as risk ratio (RR) and 95% confidence interval (CI). Heterogeneity was evaluated using the I2 statistic and Cochrane Q test to assess the degree of inter-study variation. I2 values of 0–24.9%, 25–49.9%, 50–74.9%, and 75–100% were considered as having no, mild, moderate, and significant thresholds for statistical heterogeneity, respectively (19, 20). A random-effects model using restricted maximum likelihood (21), which is thought to be better than the conventional DerSimonian–Laird method (22), was performed to pool the data due to potential clinical heterogeneity. Potential publication bias was assessed by inspection of funnel plots when the total number of included studies surpasses 10, with Begg's rank correlation test performed subsequently. Predefined sensitivity analyses were conducted using a random-effects model and a leave-one-out approach. We also conducted two exploratory sensitivity analyses by excluding the clinical trials performed in the USA and by excluding those performed outside the USA in the meta-analysis of mortality, respectively. All statistical analyses were performed using Stata version 12.0 and Review Manager version 5.3.
Results
Eligible Studies
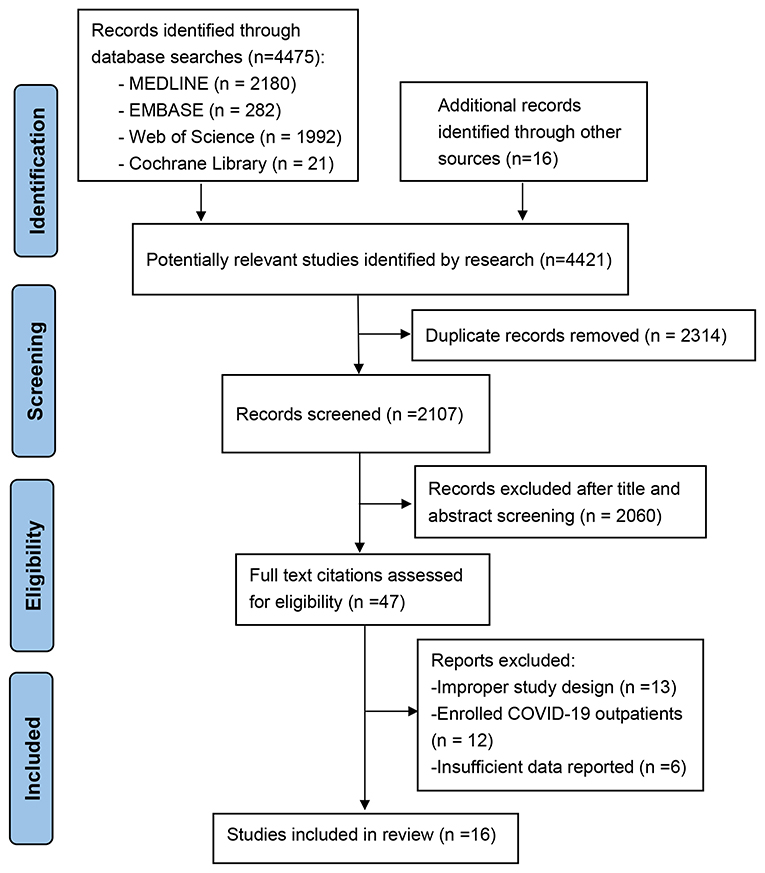
The study selection process is presented in Figure 1. The literature search yielded 4,421 potentially relevant records. We retained 2,107 relevant studies after removing duplicate studies. After we evaluated the abstract and title of each record, 2,060 studies were excluded because they did not meet the inclusion criteria or they met the exclusion criteria. Of the remaining 47 studies, 36 were excluded after reviewing the full-text manuscript (10 used direct oral anticoagulants as a control group, eight did not compare the mortality of COVID patients receiving anticoagulation with those not receiving anticoagulation directly, 12 enrolled outpatients, and six did not have insufficient outcome data or baseline data for further meta-analysis). A total of 11 observational studies reporting the effect of anticoagulants on mortality (9–11, 23–30) (n = 11) and bleeding events (9, 25, 27–29) (n = 5) in hospitalized patients with COVID-19 were included in the review.
This meta-analysis is based on a pooled sample of 20,748 hospitalized COVID-19 patients with reported information related to mortality and a pooled sample of 11,302 patients with reported information related to bleeding events. The sample sizes ranged widely across studies from 81 to 4,389. All studies were retrospective in study design. Among the included studies, seven were conducted in the USA (9, 10, 25, 27–30), two studies were conducted in China (11, 23), one study was conducted in Spain (26), and one study was conducted in Italy (24). Three studies were conducted exclusively in the ICU settings (9, 11, 24). The mean age in most studies was over 60 years. The proportion of male subjects ranged from 47 to 69%. Heparin and enoxaparin were the most used anticoagulants in the included studies. Table 1 further summarizes the detailed characteristics of each study, and anticoagulation doses and routes are listed in Supplementary Table 3.
Quality of Eligible Studies
The Newcastle–Ottawa Scale score for the included studies ranged from 6 to 9, while 10 studies scored above 7 and were of high quality. A full assessment is shown in Table 2.
Meta-Analysis of Mortality
Eleven studies reported the overall mortality of COVID-19 patients receiving prophylactic/therapeutic dose anticoagulation therapy or not receiving anticoagulation, which ranged widely across studies from 8.1 to 27.3% for anticoagulation group and 16.4–29.6% for non-anticoagulation group in mild-to-moderate COVID-19 patients, while it ranged from 29.8 to 40% vs. 62.7 to 78.9% in severe to critical COVID-19 patients, respectively (9–11, 23–30). Of 14,854 hospitalized COVID-19 patients receiving anticoagulant administration, 2,430 patients died during hospitalization, whereas 1,459 of 5,894 patients who did not receive anticoagulant administration died. Figure 2 shows that anticoagulation therapy was significantly associated with reduced in-hospital mortality in inpatients with COVID-19 (RR = 0.70, 95% CI 0.52–0.93, p = 0.01, I2 = 94%). Using the GRADE framework, we rated the quality of evidence in mortality as high (Table 3).
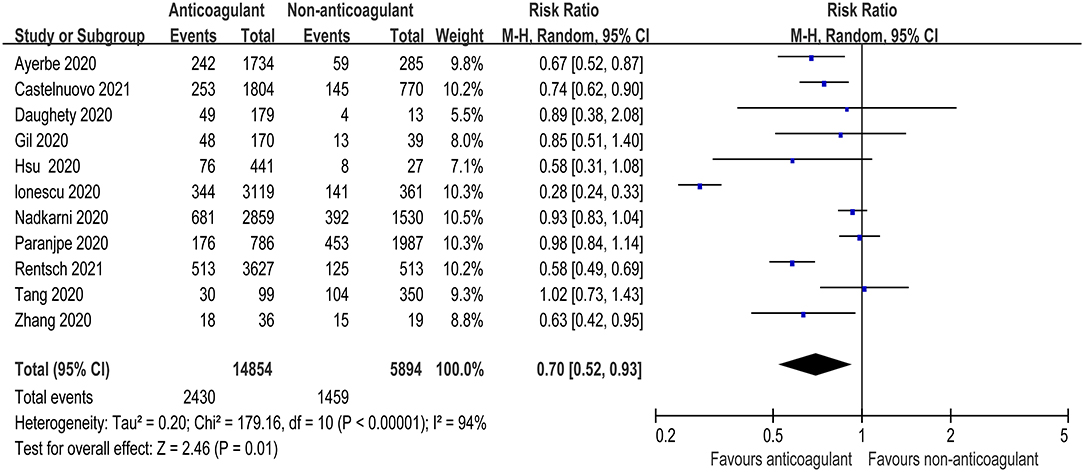
Figure 2. Forest plot of the effect of anticoagulants on risk of mortality in hospitalized COVID-19 patients.
Three studies further reported the mortality in COVID-19 patients admitted to ICU (9, 11, 24). There was also a significant decrease in mortality risk in critically ill COVID patients receiving anticoagulant therapy compared with those not receiving anticoagulant therapy (RR = 0.59, 95% CI 0.43–0.83, p = 0.002, I2 = 76%, Figure 3). The quality of evidence for mortality in hospitalized patients admitted to ICU was rated as moderate because plausible confounding is not considered to reduce or increase demonstrated effect (Table 3).
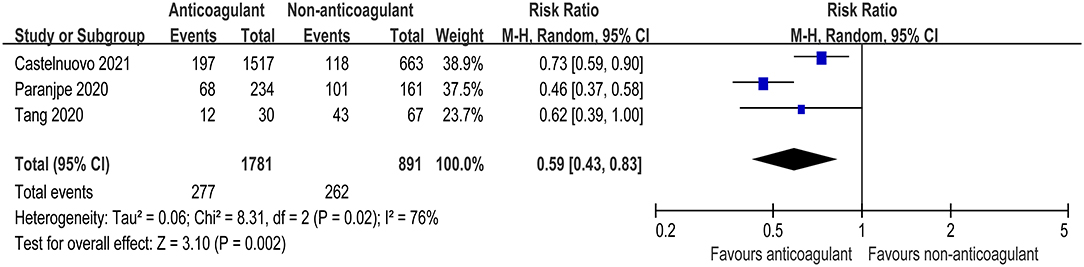
Figure 3. Forest plot of the effect of anticoagulants on risk of mortality in COVID-19 patients admitted to intensive care unit (ICU).
Meta-Analysis of Bleeding Events
There were five studies that specified bleeding events during the anticoagulant therapy for COVID-19 patients (25, 27–29, 31). The meta-analysis with a total of 11,302 patients found that the pooled risk ratio of bleeding risk did not favor either of the two groups (RR = 0.93, 95% CI 0.71–1.23; p = 0.63, I2 = 0%, Figure 4). The quality of evidence for bleeding events was rated as moderate because plausible confounding is not considered to reduce or increase demonstrated effect (Table 3).
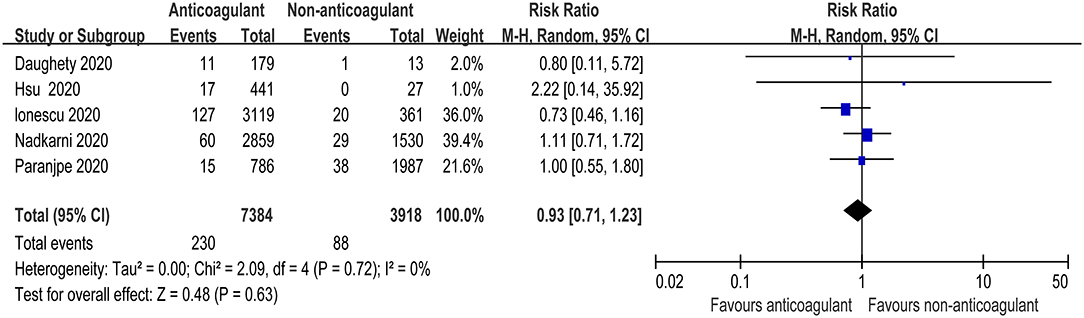
Figure 4. Forest plot of the effect of anticoagulants on bleeding events in hospitalized COVID-19 patients.
Risk of Bias Assessment
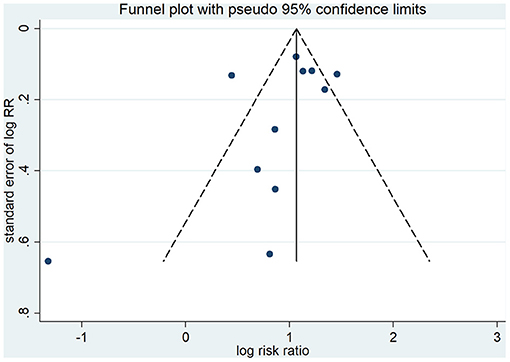
As shown in Figure 5, visual assessment of the funnel plot did show a little substantial asymmetry, but Begg's rank correlation test did not indicate the evidence of publication bias across studies of anticoagulant therapy in COVID-19 patients and mortality (p = 0.213).
Sensitivity Analysis
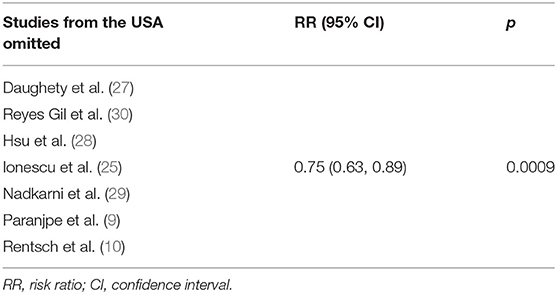
In order to assess the stability of the results of the current meta-analysis, we first performed a one-study-removed sensitivity analysis for mortality. Statistically, similar results were obtained after omitting each of the studies (Table 4), indicating the stability of this meta-analysis. Given that the mortality of in-hospital COVID-19 patients differs significantly among countries due to multiple factors such as reporting bias, transparency, and definition of COVID-19 mortality, we further performed two exploratory sensitivity analyses to test the stability of the results in this meta-analysis. As shown in Table 5, the RR value of anticoagulation therapy was 0.75 (0.63, 0.89) when clinical studies performed in the USA were excluded. However, an insignificantly reduced in-hospital mortality risk was observed when trials conducted outside of the USA were excluded (RR = 0.67, 95% CI 0.43–1.02, p = 0.06, Table 6).
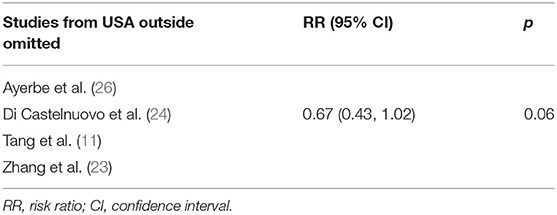
Table 5. Sensitivity analysis by omitting studies performed outside of the USA in random-effects model.
Discussion
This meta-analysis, including 11 observational studies with 20,748 in-hospital patients with COVID-19, demonstrated that anticoagulant administration significantly reduced the relative risk of mortality by 30%, with high-quality evidence as accessed by the GRADE framework and with confirmation using sensitivity analysis. The subgroup analyses showed anticoagulants could potentially reduce greater relative effect on mortality by 41% in trials with severe COVID-19 patients admitted to ICU. Bleeding events as measured by our criteria were relatively rare issues in anticoagulant therapy. These findings demonstrated that anticoagulant therapy could be used to reduce the risk of mortality in hospitalized patients with COVID-19, especially in critically ill patients, which are contrary to a previously published meta-analysis (32).
Results from previous researches investigating the role of anticoagulant administration among inpatients with COVID-19 have varied, which might be derived from different regimens of anticoagulation (e.g., drug type, dosage, and route), or different patient population (e.g., diverse disease severity), or disparate inclusion and exclusion criteria used in each study. For instance, early studies found no significant difference in mortality of COVID-19 patients with anticoagulation therapy (11, 12), and a previous meta-analysis confirmed this finding (32). But these studies were limited in sample size, and only 2,772 patients were included in the previous meta-analysis. In contrast, our review included data from 11 observational studies with more inpatients with COVID-19, which showed a significant association of anticoagulant use with 30% reduced hazard of in-hospital mortality in COVID-19 patients admitted to hospital. Limited evidence from only three studies with 2,672 participants also supported the use of heparin in critically ill COVID-19 patients admitted to ICU. Likewise, in 395 patients who required mechanical ventilation, Paranjpe et al. found that in-hospital mortality was 29.1% with a median survival of 21 days for those treated with anticoagulants as compared with 62.7% with a median survival of 9 days in patients who did not receive anticoagulants (9). Some researchers also argue that heparin, unlike other anticoagulants, may theoretically improve host survival in COVID-19 by exerting its potential anti-inflammatory (33–35) and direct antiviral effects (36–39).
Our sensitivity analyses further demonstrated that the overall effect of anticoagulant use was affected by the location of studies as evidenced by an insignificant beneficial effect found in the pooled analysis of the studies conducted in the USA. However, the results should be cautiously interpreted because this beneficial effect might be underestimated since we only extracted data of crude in-hospital mortality in each study for comparison during the data extraction process. For instance, the studies performed in the USA by Nadkarni et al. (n = 4,389) with significantly different baseline characteristics showed higher crude in-hospital mortality in anticoagulation group (28.6%) vs. non-anticoagulation group (25.6%), but a following analysis using inverse probability treatment weighted models for confounder adjustment inversely suggested that anticoagulation was associated with a lower adjusted risk of mortality vs. no anticoagulation (29).
Bleeding remains as a major safety concern when anticoagulant is administrated to COVID-19 patients with abnormal coagulation function. It is widely accepted that the potential benefits of systemic anticoagulation with heparin need to be weighed against the risk of bleeding and therefore should be individualized. In the current meta-analysis, bleeding events as measured by requirement for blood transfusions were not associated with the use of anticoagulants in COVID-19 patients, and major bleeding events were less common. Billett and colleagues did not find any anticoagulant regimen (prophylactic/therapeutic dose of apixaban, enoxaparin, and heparin) with a likelihood of transfusion greater than for patients on non-anticoagulation regimen either (40). Likewise, no signs were found of more subclinical bleeding in critically ill COVID-19 patients with higher doses of anticoagulation, perhaps because COVID-19 patients are hypercoagulable and might not bleed easily despite high-dose thromboprophylaxis (41).
Our study also has several limitations. First, the mean age of COVID-19 patients including our studies is almost over 60 years. Given that the mortality is significantly higher in the elderly hospitalized COVID-19 patients with more comorbid diseases, it will be better to include more studies with a broader age scope. Second, we extracted the raw data from the included studies even though the median survival days, sample sizes, and baseline characteristics were significant variants between different groups. Third, our meta-analysis was largely based on observational studies and might have been affected by allocation or selection bias.
Conclusions
Among patients with COVID-19 admitted to hospital, the administration of anticoagulants was significantly associated with decreased in-hospital mortality without increasing the incidence of bleeding events. However, pragmatic trials and well-designed RCT studies with longer follow-up duration and larger sample size are warranted to further investigate the effect of anticoagulant therapy on the mortality of hospitalized COVID-19 patients in the real-world practice.
Data Availability Statement
The original contributions generated for the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
LJ and BS conceived the study and planned the content. LJ and YL drafted the manuscript and contributed equally to this work. BS reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially sponsored by the National Natural Science Foundation of China (Grant No. 82000702), the Science and Technology Achievement Transformation Fund of West China Hospital of Sichuan University (Grant No. CGZH19006), and Med+ Biomaterial Institute of West China Hospital/West China School of Medicine.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.698935/full#supplementary-material
References
1. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. (2020) 382:727–33. doi: 10.1056/NEJMoa2001017
2. Poyiadji N, Cormier P, Patel PY, Hadied MO, Bhargava P, Khanna K, et al. Acute pulmonary embolism and COVID-19. Radiology. (2020) 297:E335–8. doi: 10.1148/radiol.2020201955
3. Jiménez D, García-Sanchez A, Rali P, Muriel A, Bikdeli B, Ruiz-Artacho P, et al. Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and meta-analysis. Chest. (2021) 159:1182–96. doi: 10.1016/j.chest.2020.11.005
4. Al-Ani F, Chehade S, Lazo-Langner A. Thrombosis risk associated with COVID-19 infection. A scoping review. Thromb Res. (2020) 192:152–60. doi: 10.1016/j.thromres.2020.05.039
5. Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. (2020) 296:E186–8. doi: 10.1148/radiol.2020201544
6. Spyropoulos AC, Levy JH, Ageno W, Connors JM, Hunt BJ, Iba T, et al. Scientific and Standardization Committee communication: clinical guidance on the diagnosis, prevention, and treatment of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18:1859–65. doi: 10.1111/jth.14929
7. Moores LK, Tritschler T, Brosnahan S, Carrier M, Collen JF, Doerschug K, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. (2020) 158:1143–63. doi: 10.1016/j.chest.2020.05.559
8. Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A, et al. Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis. (2020) 50:72–81. doi: 10.1007/s11239-020-02138-z
9. Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. (2020) 76:122–4. doi: 10.1016/j.jacc.2020.05.001
10. Rentsch CT, Beckman JA, Tomlinson L, Gellad WF, Alcorn C, Kidwai-Khan F, et al. Early initiation of prophylactic anticoagulation for prevention of coronavirus disease 2019 mortality in patients admitted to hospital in the United States: cohort study. BMJ. (2021) 372:n311. doi: 10.1136/bmj.n311
11. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. (2020) 18:1094–9. doi: 10.1111/jth.14817
12. Rivera-Izquierdo M, Valero-Ubierna MDC, JL Rd, Fernández-García M, Martínez-Diz S, Tahery-Mahmoud A, et al. Therapeutic agents tested in 238 COVID-19 hospitalized patients and their relationship with mortality. Med Clin. (2020) 155:375–81. doi: 10.1016/j.medcle.2020.06.024
13. Al-Samkari H, Gupta S, Leaf RK, Wang W, Rosovsky RP, Brenner SK, et al. Thrombosis, bleeding, and the observational effect of early therapeutic anticoagulation on survival in critically ill patients with COVID-19. Ann Intern Med. (2021) 174:622–32. doi: 10.7326/L21-0148
14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
16. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. doi: 10.1136/bmj.39489.470347.AD
17. Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. (2010) 340:c117. doi: 10.1136/bmj.c117
18. Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ. (2012) 344:e1553. doi: 10.1136/bmj.e1553
19. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
20. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
21. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. (2011) 342:d549. doi: 10.1136/bmj.d549
22. Cornell JE, Mulrow CD, Localio R, Stack CB, Meibohm AR, Guallar E, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. (2014) 160:267–70. doi: 10.7326/M13-2886
23. Zhang P, Qu Y, Tu J, Cao W, Hai N, Li S, et al. Applicability of bedside ultrasonography for the diagnosis of deep venous thrombosis in patients with COVID-19 and treatment with low molecular weight heparin. J Clin Ultrasound. (2020) 48:522–6. doi: 10.1002/jcu.22898
24. Di Castelnuovo A, Costanzo S, Antinori A, Berselli N, Blandi L, Bonaccio M, et al. Heparin in COVID-19 patients is associated with reduced in-hospital mortality: the multicenter Italian CORIST Study. Thromb Haemost. (2021). doi: 10.1055/a-1347-6070. [Epub ahead of print].
25. Ionescu F, Jaiyesimi I, Petrescu I, Lawler PR, Castillo E, Munoz-Maldonado Y, et al. Association of anticoagulation dose and survival in hospitalized COVID-19 patients: a retrospective propensity score-weighted analysis. Eur J Haematol. (2021) 106:165–74. doi: 10.1111/ejh.13533
26. Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. J Thromb Thrombolysis. (2020) 50:298–301. doi: 10.1007/s11239-020-02162-z
27. Daughety MM, Morgan A, Frost E, Kao C, Hwang J, Tobin R, et al. COVID-19 associated coagulopathy: thrombosis, hemorrhage and mortality rates with an escalated-dose thromboprophylaxis strategy. Thromb Res. (2020) 196:483–5. doi: 10.1016/j.thromres.2020.10.004
28. Hsu A, Liu Y, Zayac AS, Olszewski AJ, Reagan JL. Intensity of anticoagulation and survival in patients hospitalized with COVID-19 pneumonia. Thromb Res. (2020) 196:375–8. doi: 10.1016/j.thromres.2020.09.030
29. Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. (2020) 76:1815–26. doi: 10.1016/j.jacc.2020.08.041
30. Reyes Gil M, Gonzalez-Lugo JD, Rahman S, Barouqa M, Szymanski J, Ikemura K, et al. Correlation of coagulation parameters with clinical outcomes during the coronavirus-19 surge in New York: observational cohort. Front Physiol. (2021) 12:618929. doi: 10.3389/fphys.2021.618929
31. Paolisso P, Bergamaschi L, D'Angelo EC, Donati F, Giannella M, Tedeschi S, et al. Preliminary experience with low molecular weight heparin strategy in COVID-19 patients. Front Pharmacol. (2020) 11:1124. doi: 10.3389/fphar.2020.01124
32. Abdel-Maboud M, Menshawy A, Elgebaly A, Bahbah EI, El Ashal G, Negida A. Should we consider heparin prophylaxis in COVID-19 patients? a systematic review and meta-analysis. J Thromb Thrombolysis. (2021) 51:830–2. doi: 10.1007/s11239-020-02253-x
33. Shi C, Wang C, Wang H, Yang C, Cai F, Zeng F, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective cohort study. Clin Transl Sci. (2020) 13:1087–95. doi: 10.1111/cts.12880
34. Yan Y, Ji Y, Su N, Mei X, Wang Y, Du S, et al. Non-anticoagulant effects of low molecular weight heparins in inflammatory disorders: a review. Carbohydr Polym. (2017) 160:71–81. doi: 10.1016/j.carbpol.2016.12.037
35. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
36. Kim SY, Jin W, Sood A, Montgomery DW, Grant OC, Fuster MM, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. (2020) 181:104873. doi: 10.1016/j.antiviral.2020.104873
37. Mycroft-West CJ, Su D, Pagani I, Rudd TR, Elli S, Gandhi NS, et al. Heparin inhibits cellular invasion by SARS-CoV-2: structural dependence of the interaction of the spike S1 receptor-binding domain with heparin. Thromb Haemost. (2020) 120:1700–15. doi: 10.1101/2020.04.28.066761
38. Hadigal SR, Agelidis AM, Karasneh GA, Antoine TE, Yakoub AM, Ramani VC, et al. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nat Commun. (2015) 6:6985. doi: 10.1038/ncomms7985
39. Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. (1999) 99:13–22. doi: 10.1016/S0092-8674(00)80058-6
40. Billett HH, Reyes-Gil M, Szymanski J, Ikemura K, Stahl LR, Lo Y, et al. Anticoagulation in COVID-19: effect of enoxaparin, heparin, and apixaban on mortality. Thromb Haemost. (2020) 120:1691–1699. doi: 10.1055/s-0040-1720978
Keywords: anticoagulation, coronavirus disease 2019, heparin, mortality, bleeding
Citation: Jiang L, Li Y, Du H, Qin Z and Su B (2021) Effect of Anticoagulant Administration on the Mortality of Hospitalized Patients With COVID-19: An Updated Systematic Review and Meta-Analysis. Front. Med. 8:698935. doi: 10.3389/fmed.2021.698935
Received: 22 April 2021; Accepted: 05 July 2021;
Published: 04 August 2021.
Edited by:
Murat Akova, Hacettepe University, TurkeyReviewed by:
Mohammad Shehab, Mubarak Al Kabeer Hospital, KuwaitOana Sandulescu, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2021 Jiang, Li, Du, Qin and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baihai Su, subaihai@scu.edu.cn
†These authors have contributed equally to this work
 Luojia Jiang1,2†
Luojia Jiang1,2†  Yupei Li
Yupei Li Baihai Su
Baihai Su