
Clinical findings in a group of COVID-19 patients: a single-center retrospective study
Introduction
Since the first officially reported case of the coronavirus disease 2019 (COVID-19) in late December, 2019 in Wuhan, China, the outbreak has evolved into a global public health crisis with an extensive number of infected patients, causing devastating death toll all over the world (1). So far, the COVID-19 pandemic is still ongoing and the causing virus SARS-CoV-2 is under extensive investigation in terms of its transmission routes (2-4), infection mechanisms (5), genomic evolution (6), and environmental viability (7). According to the real-time data released by the Coronavirus Resource Center at the Johns Hopkins University and Medicine (https://coronavirus.jhu.edu/map.html), it seems that the outbreak in China has been contained, with rarely new local infections, which, though still disputable, may indicate that the lockdown and mask-wearing policies have positive effects on slowing down and blocking the spread of the virus. In addition, the New Coronavirus Pneumonia Diagnosis and Treatment Plan by the China National Health Commission (CNHC) also plays vital roles in the control and prevention of COVID-19 in China, which is revised regularly based on clinical experience and findings. Procedures for COVID-19 detection and medical therapy are also described in detail. Thus, Chinese experience in battling with COVID-19 disease could be generalizable to patients in other countries. Although there have been tens of millions of confirmed cases, investigations of the disease are still not adequate and more clinical reports are urgently needed to share with medical staffs all over the world.
Xuzhou is a prefecture-level city with around 10 million residents and is around 550 km away from Wuhan. During the outbreak, there were 54 fever clinics for screening COVID-19 patients through the established steps (8) and 13 designated hospitals for medical therapies of SARS-CoV-2 infections in Xuzhou (9). According to Xuzhou Center for Disease Control and Prevention, a total of 79 cases were confirmed in Xuzhou. After hospitalization, all of the infected patients have been discharged home and are recovering from the disease. However, asymptomatic infection and imported cases have been emphasized by the central and local governments as the potential risks for the second wave of outbreak. In this study, we performed a complete single-center retrospective analysis of a group of 25 patients with COVID-19 disease in terms of epidemiological data, laboratory tests, clinical outcomes, radiological features, and medical treatments. This descriptive study gives an overall clinical understanding of the COVID-19 patients in a prefecture-level city and provides a valuable experience in the prevention and treatment of COVID-19 disease in China. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-3333).
Methods
Patients
During the COVID-19 outbreak, a total of 79 patients were confirmed in all 10 administrative divisions in Xuzhou, China. Case definitions of confirmed human infection with SARS-CoV-2 are in accordance with the COVID-19 Diagnosis and Treatment Plan of the CNHC (9). Only patients with a laboratory confirmed infection were enrolled in this study. Twenty-five patients were admitted to the affiliated hospital of Xuzhou Medical University from January 26, 2020 to February 13, 2020. These patients were retrospectively and consecutively analysed in this study. Epidemiological characteristics of 25 patients with COVID-19 before admission into the Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu Province, China were recorded and are available in online table (Table S1). Standardised case report form was used to collect clinical data such as laboratory tests, clinical outcomes, chest CT, and medical treatments. If information was not clear, medical staff in the hospital contacted patients for clarification. The present study was performed in accordance with the Helsinki Declaration (as revised in 2013) and was approved by the Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (No. XYFY2020-KL016-01). Written informed consent was obtained from participants or their families, retrospectively.
Detection of coronavirus
All cases were tested for SARS-CoV-2 via fluorescent real-time reverse transcription PCR (RT-PCR) on throat swab samples. Confirmed cases were those with positive results. One hundred and fifty µL of sample from throat swab of each patient was used to extract total RNA. Nucleocapsid (N) gene and open reading frame lab (ORF1ab) gene were amplified for detection of the virus.
Laboratory tests and chest CT
Laboratory diagnoses including routine blood test (RBT), comprehensive metabolic panel (CMP), infection test, and coagulation factors were performed for the clustered cases at the Department of Laboratory Medicine, the Affiliated Hospital of Xuzhou Medical University. Details of test dates and values are recorded in online table (Table S2). Chest CT scanning were performed for all patients except for case No. XYFY-002 due to pregnancy.
Medical treatments
Medical treatment during hospitalization include: antiviral medicines lopinavir/ritonavir (400 mg/100 mg bid po), Umifenovir (200 mg tid po), and interferon alfa-2b (5 MIU, aerosolized inhalation); antibacterial drugs moxifloxacin hydrochloride (400 mg, ivgtt, qd), biapenem (300 mg, ivgtt, q8h), and/or linezolid (600 mg, ivgtt, q12h). Immunoglobulin (20 g/day) was given to index patient only. Drugs prescribed for glucocorticoid therapy include methylprednisolone (20–60 mg bid ivgtt), ketotifen fumarate (1 mg, qd, qn) and/or budesonide inhalation (1 mg, qd). Traditional Chinese medicine (TCM) Lianhuaqingwen (LH) capsule was also used when necessary. Treatment schemes for all patients were detailed in online table (Table S3) and illustrated in Figure S1 except for case No. XYFY-002 due to pregnancy.
Discharge standards
Patients were discharged home by following the COVID-19 Diagnosis and Treatment Plan by China National Health Commission (9). This is, patients were discharged when their body temperatures returned to normal for more than 3 consecutive days with improved respiratory symptoms, and pulmonary imaging shows significant resolution of inflammation. Meanwhile, nucleic acid detection for the pathogen SARS-CoV-2 need to be negative for two consecutive tests with at least 1 day apart.
Data visualization and statistical analysis
Data visualization and statistical analyses were performed with R package. All the continuous measurement is present as an average with standard deviations when comparing the indices inn different groups while swarm plot and time series curves were used for data visualization. Classification variable is presented in percentage. Laboratory parameters outside the normal range were marked out in plots. Two-tailed unequal variance Student’s t-test was used for statistical analysis (P value <0.05).
Patient and public involvement
This is a retrospective study and no patients were involved in the study design, setting the research questions, or the outcome measures directly. No patients were asked to advise on interpretation or writing up of results.
Results
Epidemiological characteristics
In this study, we performed a single-center retrospective analysis of 25 COVID-19 patients admitted to the affiliated hospital of Xuzhou Medical University. Professions of these patients are diverse, including farmers, teachers, workers, and hospital cleaners, etc. Fifteen patients are male while 10 patients are female. Age distribution ranges from 21 to 80 years old with the average age at 45 years old and the standard deviation of 17.4 years old. Three cases were imported, and 6 clustered cases were identified. From symptom onset to confirmed infection, the average time is 6.6 days. Among the 25 patients, 3 patients had very mild conditions, 2 patients were in severe conditions while other patients were in regular conditions. After hospitalization and medical treatments, all the patients were discharged home. Interestingly, two discharged patients were tested positive again during recovering period and re-admitted to the hospital until nucleic acids tested negative. No patient is dead due to COVID-19 in this study. Screening of underlying diseases shows that all the patients do not have any of the diseases such as autoimmune liver disease, non-alcoholic fatty liver disease, alcoholic fatty liver disease, chronic liver disease, liver failure, acute heart failure, shock, chronic lung diseases, renal insufficiency, immunodeficiency, and hepatitis C. However, 7 patients (28%) have hypertension, 7 (28%) having diabetes, 1 (4%) having malignant tumor (cervical cancer and breast cancer), 2 having chronic hepatitis B (8%), 2 (8%) smoking, 1 (4%) drinking, 1 (4%) having coronary heart disease, and 1 (4%) having cerebrovascular disease. For details, please refer to online table (Table S1).
Basic description of symptoms
Before hospitalization, symptoms of the 25 patients include fever (76%), dry cough (56%), expectoration (48%), shortness of breath (36%), sore throat (28%), fatigue (28%), breath difficulty (12%), vomit (8%), diarrhea (8%), and headache (4%). For the 19 patients with fever, peak temperature ranges from 37.2 to 39.5 °C, with the average at 38.4 °C. When admitted to hospital, body temperature ranges from 36.6 to 39.3 °C, with the average at 36 °C. Respiratory frequency ranges from 15 to 32 times/minute. Blood pressure of most patients are at normal range below 120/80 mmHg and above 90/60 mmHg while several patients have stage I and stage II hypertensions. Heart rate (times/minute) ranges from 60 to 123 and has an average of 84. In addition, blood oxygen saturation SpO2 (%) ranges from 92% to 100% and is averaged at 97.4%. For detailed information of each patient, please refer to online table (Table S4). During hospitalization, 60% of patients showed recurrence of fever symptoms with peak body temperature ranging from 37.5 to 39.2 °C. Sixty-four percent of patients had dry cough while 52% having sputum. Breath of shortness and breath difficulty happened in 44% and 20% of patients, respectively, while 36% of patients felt fatigue. In addition, 16% of patients had diarrhoea. Other symptoms include vomit (12%), sore throat (8%), sore muscle (4%), and headache (4%). Clinical characteristics such as breath frequency, blood pressure, heart rate, and blood oxygen saturation were all monitored and recorded. For detailed information of each patient, please refer to online table (Table S5).
Comparison of clinical features
In order to get a better understanding of the clinical features of COVID-19, we analyzed all the available laboratory test data of the 25 patients comparatively in terms of course of treatment. Four main categories of laboratory test results involving 42 different indicators were studies, which include route blood test (RBT), CMP, infection tests, and coagulation tests. 6 clusters were identified among the patients while other people had no clear contact history. A timeline of events for hospital admission, hospital discharge, PCR test, chest CT scanning, and all the laboratory tests was constructed so as to provide an overview of the whole clinical diagnosis procedures (Figure 1).
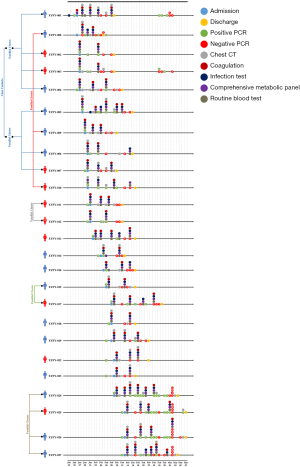
We compared all the available laboratory test results for patients at the stages of hospital admission and discharge. According to the analysis, it was found that indicators such as white blood cells [admission (5.13±1.78) ×109/L vs. discharge (6.39±1.87) ×109/L], platelet count [admission (213.72±108.45) ×109/L vs. discharge (268.08±64.12) ×109/L], lactate dehydrogenase [admission 212.76±72.40 U/L vs. discharge 174.36±31.81 U/L], triglyceride (admission 1.20±0.55 mmol/L vs. discharge 3.03±1.93 mmol/L), sodium (admission 139.73±4.15 mmol/L vs. discharge 142.85±3.56 mmol/L), C-reactive protein (admission 34.78±46.98 mg/L vs. discharge 3.55±4.37 mg/L), international normalized ratio (INR) (admission 1.18±0.11 vs. discharge 1.10±0.11), and prothrombin time (PT) (admission 12.74±1.19 s vs. discharge 11.84±1.22 s) were significantly different during admission and discharge. In addition, no statistical differences were identified for indicators such as red blood cell [admission (4.24±1.06) ×1012/L vs. discharge (4.29±0.61) ×1012/L], haematocrit (admission 40.40%±5.98% vs. 39.25%±5.70%), albumin (admission 41.08±5.35 g/L vs. discharge 39.09±4.72 g/L), eGFR (admission 118.86±8.05 mL/min/1.73 m2vs. discharge 123.78±2.99 mL/min/1.73 m2), erythrocyte sedimentation rate (admission 22.00±16.52 mm/h vs. discharge 37.33±24.23 mm/h), and ferritin (admission 384.61±253.51 µg/L vs. discharge 444.46±344.26 µg/L), at admission and discharge stage. However, their average values were out of normal range. As for other indicators, they were in normal range with no significant difference at both admission and discharge times. For details, please refer to Table 1.
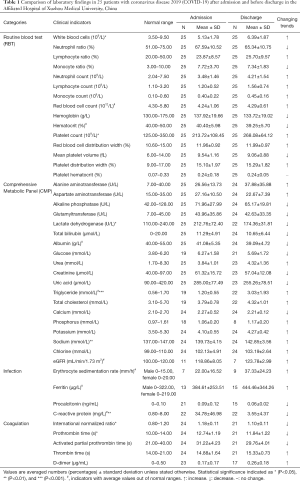
Full table
Discussion
COVID-19 is a novel infectious disease caused by SARS-CoV-2 in human population. Currently, there is no vaccine or cure for the disease. The transmission modes of COVID-19 and infection mechanisms of SARS-CoV-2 are still not completely solved due to its jump from bats to human beings via unknown intermediate host(s) (10). Since the outbreak of COVID-19 disease from late December, 2019, many studies swiftly reported the epidemiological features and clinical findings of the infected patients in different regions of China (11-13), which greatly facilitate the disease diagnosis and the prevention of the virus spread. In a recent epidemiological study of 44,672 confirmed cases of the COVID-19 disease, which was conducted by Chinese Center for Disease Control and Prevention (China CDC), it was found that 80% of the cases were in mild conditions (14). In addition, the sex ratio analysis showed that male-to-female ratio was 0.99:1 in Wuhan, 1.04:1 in Hubei, and 1.06:1 in China overall (14). Thus, there seems to be roughly equal numbers of cases between men and women so far, though sex differences in mortality and vulnerability were observed, that is, men having higher mortality than women with unclear reasons (15). In this study, we summarized the epidemiological features of 25 patients in a single hospital, together with clinical findings in terms of laboratory tests.
According to the 7th edition of the New Coronavirus Pneumonia Diagnosis and Treatment Plan, at the early stage of the disease onset, the total number of white blood cells in the peripheral blood was normal or decreased, and the lymphocyte count was decreased. Some patients had increased levels of liver enzymes, lactate dehydrogenase, muscle enzymes, and myoglobin. Some critically ill patients saw increased troponin. In addition, most patients had elevated C-reactive protein and erythrocyte sedimentation rate and normal procalcitonin. In severe cases, D-dimer may increase, and peripheral blood lymphocytes progressively decrease. Severe and critically ill patients often have elevated inflammatory factors. It was suggested that decrease of lymphocytes could be due to the functional exhaustion (16). However, the specific reasons were still under investigation (17).
In this study, we confirmed that white blood cells, lymphocytes, monocytes, and neutrophils were in normal range during infection, which were all increased after medical treatment, suggesting enhanced immunity and the effects of medical therapy. It was also noticed that liver dysfunction was associated with SARS-CoV-2 infection with elevated level of lactate dehydrogenase and creatinine, which was more prevalent in severe cases than in mild cases (18). In this study, we observed that the significant decrease of lactate dehydrogenase for medical treatments, which indicated patients in recovering mode. As for the significantly raised triglyceride and sodium levels at discharge, it could be due to the diet change and long-term best rest without exercise during hospitalization. This might also explain the apparent increase of total cholesterol for the patients. As for the C-reactive protein, it was reported to be positively correlated with lung lesions and could reflect disease severity at the early stage of COVID-19 (19). Abnormally high level of C-reactive protein (34.78±46.98 mg/L) was observed at admission and returned to normal range at 3.55±4.37 mg/L. In terms of the coagulation test, two indicators, both INR and PT were significantly reduced, which was consistent with recent findings that the two indicators were lower in normal group than COVID-19 patients (20). In terms of ferritin, it is a major intracellular iron storage protein in all organisms, which binds free ions of the trace element, neutralizing its toxic properties and increasing its solubility. High level of ferritin has been associated with increased illness severity and adverse outcomes, including COVID-19, which might lead to cytokine storm. In this study, we observed a slightly higher ferritin level on average for patients from admission to discharge. The possible explanations for this abnormality include (I) the ferritin data for patients from admission to discharge is not complete, which might not reflect the real trend of the indictor and (II) when patients discharging from hospital, they only need to meet the criteria of no fever, two negative PCR test, and well-adsorbed lung lesions. Thus, these patients are still in recovering stage and there might still be some indicators out of normal range. In general, the indicators identified in this study with significant alterations could be used as assessment of medical therapy during patient recovery. However, it is rather hard to draw any clear correlation with patient recovery time and lab values or treatment strategies. In fact, given the wide variation in treatments, the lab parameters would be uninterpretable without larger cohort sizes.
It is noteworthy that some of the cured patients also received the treatment of LH capsules, a TCM. According to several clinical trials in terms of its efficacy and safety toward SARS-CoV-2 infections, LH capsules could be considered to ameliorate clinical symptoms of Covid-19 and shorten the duration of viral shedding (21-23). Previously, a bioinformatic analysis constructed an influenza-related protein-protein interaction (PPI) network, which revealed that there were 15 main effective components in the medicine while 7 of them were further experimentally validated to have antivirus efficacy in vitro (24). As for SARS-CoV-2 infection, it was postulated that key components in LH capsules could block the binding of SARS-CoV-2 with the angiotensin converting enzyme and ameliorate lung injury via the suppression of oxidative stress and apoptosis, though more experimental evidences were required (21).
Conclusions
In this retrospective study, a total of 25 patients with COVID-19 from the affiliated hospital of Xuzhou Medical University were investigated. Epidemiological characterization and clinical findings were reported. In particular, temporal changes in laboratory markers during hospitalization of patients were reported, though the small sample size might not be sufficient to draw generalized conclusions and require further studies. Patients received medical treatments by following the official guide of the New Coronavirus Pneumonia Diagnosis and Treatment Plan and were all discharged home for recovering. In addition, chest CT scanning showed continuing resolution of lung lesions for these patients. In sum, this study provided a clinical overview of COVID-19 disease and identified some significantly altered laboratory markers during SARS-CoV-2 infection through comparative analysis of a small group of patients in a single hospital, which might facilitate clinicians to prevent the transmission of the virus and help diagnose COVID-19 patients at an early stage.
Acknowledgments
We express our special appreciation and thanks to Professor Minglong Chen, Professor Peisheng Jin, and Professor Xianliang Yan for their advices and guidance on this study.
Funding: This work was supported by National Key Research and Development Project (grant number 2020YFC0848100). Dr. LW greatly appreciated the financial support by Jiangsu Qinglan Project (2020) and Young Science and Technology Team of Xuzhou Medical University (TD202001).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-3333
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-3333
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-3333
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-3333). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The present study was performed in accordance with the Helsinki Declaration (as revised in 2013) and was approved by the Ethics Committee of the affiliated hospital of Xuzhou Medical University (No. XYFY2020-KL016-01). Written informed consent was obtained from participants or their families, retrospectively.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-62. [Crossref] [PubMed]
- Li Q, Guan X, Wu P, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020;382:1199-207. [Crossref] [PubMed]
- Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med 2020;382:970-1. [Crossref] [PubMed]
- Li C, Ji F, Wang L, et al. Asymptomatic and Human-to-Human Transmission of SARS-CoV-2 in a 2-Family Cluster, Xuzhou, China. Emerg Infect Dis 2020;26:1626-8. [Crossref] [PubMed]
- Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444-8. [Crossref] [PubMed]
- Forster P, Forster L, Renfrew C, et al. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A 2020;117:9241-3. [Crossref] [PubMed]
- Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infect 2020;104:246-51. [Crossref] [PubMed]
- Zhang J, Zhou L, Yang Y, et al. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med 2020;8:e11-e12. [Crossref] [PubMed]
- National Health Commission of the People's Republic of China. Diagnosis and treatment of 2019-nCoV pneumonia in China. 2020. Available online: http://www.nhc.gov.cn/
- Zhang T, Wu Q, Zhang Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr Biol 2020;30:1346-1351.e2. [Crossref] [PubMed]
- Wu J, Liu J, Zhao X, et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin Infect Dis 2020;71:706-12. [Crossref] [PubMed]
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020;382:1708-20. [Crossref] [PubMed]
- Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m792. [PubMed]
- Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:145-51. [PubMed]
- Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet 2020;395:846-8. [Crossref] [PubMed]
- Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020;17:533-5. [Crossref] [PubMed]
- Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther 2020;5:33. [Crossref] [PubMed]
- Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428-30. [Crossref] [PubMed]
- Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 2020;50:332-4. [Crossref] [PubMed]
- Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med 2020;58:1116-20. [Crossref] [PubMed]
- Hu K, Guan WJ, Bi Y, et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine 2020. Epub ahead of print. [Crossref] [PubMed]
- Wang J, Qi F. Traditional Chinese medicine to treat COVID-19: the importance of evidence-based research. Drug Discov Ther 2020;14:149-50. [Crossref] [PubMed]
- Zhang D, Zhang B, Lv JT, et al. The clinical benefits of Chinese patent medicines against COVID-19 based on current evidence. Pharmacol Res 2020;157:104882.
- Wang CH, Zhong Y, Zhang Y, et al. A network analysis of the Chinese medicine Lianhua-Qingwen formula to identify its main effective components. Mol Biosyst 2016;12:606-13. [Crossref] [PubMed]


