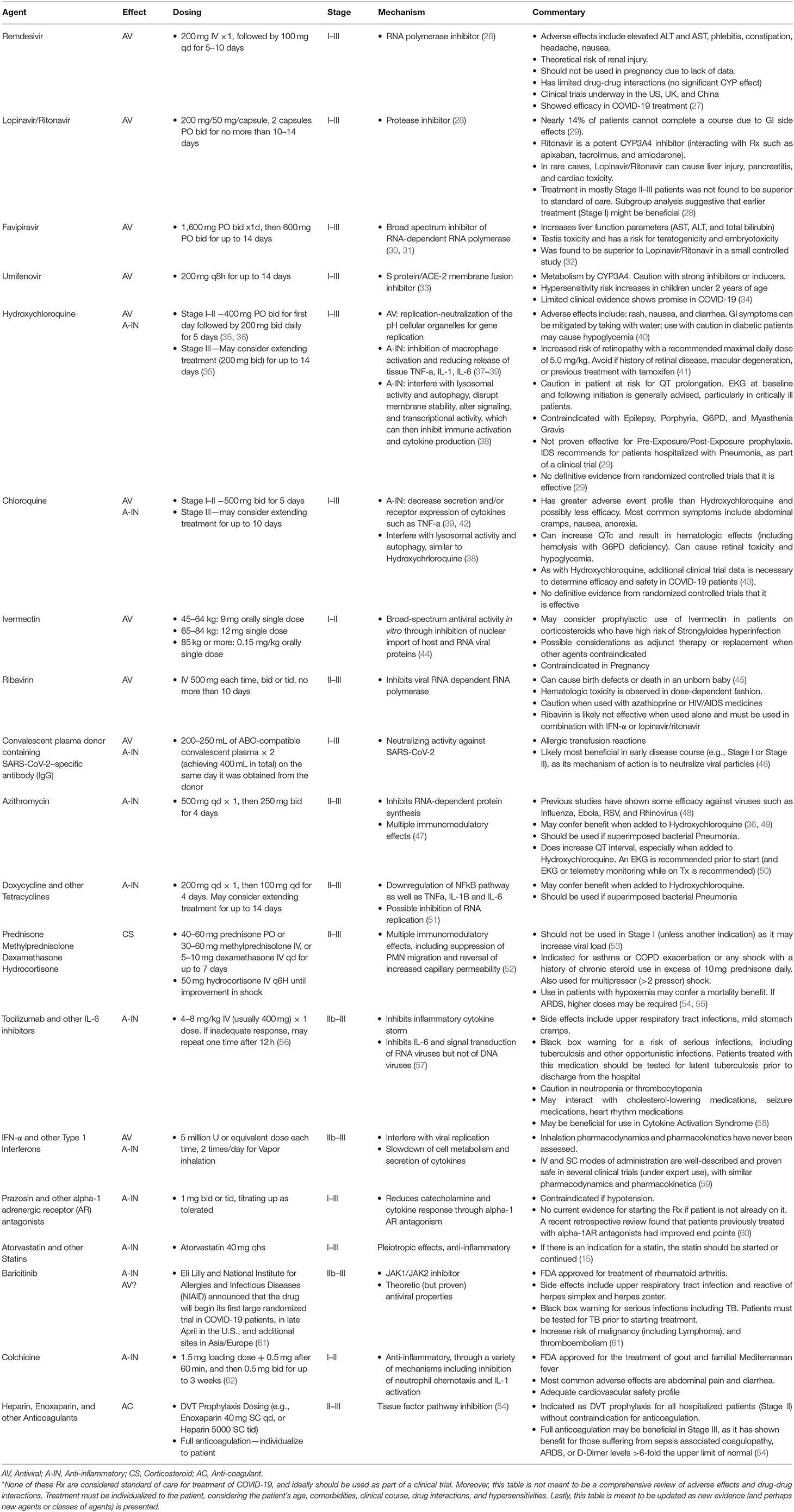
Current Understanding of COVID-19 Clinical Course and Investigational Treatments
- 1Cano Health, Miami, FL, United States
- 2Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, United States
- 3Dr. Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University (NSU), Fort Lauderdale, FL, United States
- 4Internal Medicine, Cleveland Clinic Florida, Weston, FL, United States
- 5Cleveland Clinic Florida, Weston, FL, United States
- 6Health Professions Division, Nova Southeastern University (NSU), Fort Lauderdale, FL, United States
Importance: Currently, there is no unified framework linking disease progression to established viral levels, clinical tests, inflammatory markers, and investigational treatment options.
Objective: It may take many weeks or months to establish a standard treatment approach. Given the growing morbidity and mortality with respect to COVID-19, this systemic review presents a treatment approach based on a thorough review of scholarly articles and clinical reports. Our focus is on staged progression, clinical algorithms, and individualized treatment.
Evidence Review: We followed the protocol for a quality review article proposed by Heyn et al. (1). A literature search was conducted to find all relevant studies related to COVID-19. The search was conducted between April 1, 2020, and April 13, 2020, using the following electronic databases: PubMed (1809 to present); Google Scholar (1900 to present); MEDLINE (1946 to present), CINAHL (1937 to present); and Embase (1980 to present). The keywords used included COVID-19, 2019-nCov, SARS-CoV-2, SARS-CoV, and MERS-CoV, with terms such as efficacy, seroconversion, microbiology, pathophysiology, viral levels, inflammation, survivability, and treatment and pharmacology. No language restriction was placed on the search. Reference lists were manually scanned for additional studies.
Findings: Of the articles found in the literature search, 70 were selected for inclusion in this study (67 cited in the body of the manuscript and 3 additional unique references in the Figures). The articles represent work from China, Japan, Taiwan, Vietnam, Rwanda, Israel, France, the United Kingdom, the Netherlands, Canada, and the United States. Most of the articles were cohort or case studies, but we also drew upon other information, including guidelines from hospitals and clinics instructing their staff on procedures to follow. In addition, we based some decisions on data collected by organizations such as the CDC, FDA, IHME, IDSA, and Worldometer. None of the case studies or cohort studies used a large number of participants. The largest group of participants numbered <500 and some case studies had fewer than 30 patients. However, the review of the literature revealed the need for individualized treatment protocols due to the variability of patient clinical presentation and survivability. A number of factors appear to influence mortality: the stage at which the patient first presented for care, pre-existing health conditions, age, and the viral load the patient carried.
Conclusion and Relevance: COVID-19 can be divided into three distinct stages, beginning at the time of infection (Stage I), sometimes progressing to pulmonary involvement (Stage II, with or without hypoxemia), and less frequently to systemic inflammation (Stage III). In addition to modeling the stages of disease progression along with diagnostic testing, we have also created a treatment algorithm that considers age, comorbidities, clinical presentation, and disease progression to suggest drug classes or treatment modalities. This paper presents the first evidence-based recommendations for individualized treatment for COVID-19.
Highlights
- Question: What are the most effective treatment recommendations for COVID-19?
- Findings: COVID-19 can be divided into three distinct Stages, beginning at the time of infection (Stage I), sometimes progressing to pulmonary involvement (Stage II, with or without hypoxemia) and less frequently to systemic inflammation (Stage III). In addition to modeling the stages of disease progression, we also created a treatment algorithm which considers age, comorbidities, clinical presentation, and disease progression to suggest drug classes or treatment modalities.
- Meaning: This paper presents the first evidence-based recommendations for individualized treatment for COVID-19.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has spread throughout the globe. According to the Centers for Disease Control and Prevention (CDC), in the United States alone there were 5,460,429 cases along with 171,012 deaths, as of August 19, 2020 (2). A mathematical model created by The Institute for Health Metrics and Evaluation (IHME) predicts that in the United States the number of deaths may climb to over 295,000 by December 1, 2020 (3). This creates a critical and immediate need for medical treatment and resources.
Preliminary data in the US suggests that COVID-19 may be more infectious and lethal than Influenza H1N1. To place this in context, Figure 1 provides a comparison of the reproduction rate and case-fatality rates for major respiratory virus pandemics (4–6). In the general population, case-fatality rates for COVID-19 are about 1.4% (7). Data strongly emphasizes early intervention to reduce case-fatality and inhibit reproductive rates.
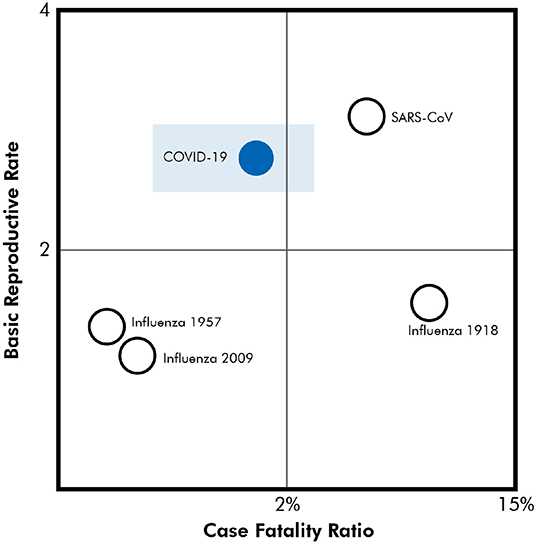
Figure 1. Reproduction rate and case-fatality rates for major respiratory virus pandemics (4, 5). Rectangle donates case fatality range from multiple publications.
To date, a number of articles have been published on the clinical course and treatment of the disease (8–10). The majority of patients present with more than one symptom on admission, although the combination of fever, cough, and shortness of breath is rare. Siddiqi and Mehra proposed a staged progression model based on observed clinical courses in published studies (11). In Stage 1, or the mild phase, the virus multiplies and establishes residence in the host, predominantly in the respiratory tract. In Stage 2, there is viral multiplication and localized inflammation in the lungs. Stage 3 is marked by extra-pulmonary systemic hyperinflammation syndrome. The prognosis and recovery from Stage 3 is generally poor. Rapid recognition of which stage the patient is in and the deployment of appropriate therapy may have the greatest yield.
Common correlating factors that tend to lead to poorer outcomes include age, hypertension, diabetes, coronary artery disease, chronic lung disease, and malignancies (12). Research also finds variations in outcomes due to a dysregulated and exuberant immune response. Patients requiring intensive care have significantly higher levels of IL-6, CRP, ferritin, and D-Dimer. An important therapeutic modality may be to downregulate the cytokine storm, particularly in severe illness (13). The literature also suggests that disease progression can be predicted. During the severe acute respiratory syndrome (SARS) pandemic, a retrospective analysis revealed that 2-week cumulative case data could help estimate the total case numbers with accuracy—well before the date of the last reported case (14).
As we have found, there is no unified framework linking disease progression to established viral levels, clinical tests, inflammatory markers, and investigational treatment options. Given that it may take many weeks or months to establish a standard treatment approach and that rates of morbidity and mortality are increasing, we present an initial treatment approach based on a thorough review of currently available scholarly articles and clinical reports. Our focus is on staged progression, clinical algorithms, and individualized treatment.
Methods
We followed the protocol for a quality review article proposed by Heyn et al. (1). A literature search was conducted to find all relevant studies related to COVID-19. The search was conducted between April 1, 2020, and April 13, 2020, using the following electronic databases: PubMed (1809 to present); Google Scholar (1900 to present); MEDLINE (1946 to present), CINAHL (1937 to present); and Embase (1980 to present). The keywords used in this search included COVID-19, 2019-nCoV, SARS-CoV-2, SARS-CoV, and MERS-CoV, with terms such as efficacy, seroconversion, microbiology, pathophysiology, viral levels, inflammation, survivability, and treatment and pharmacology. No language restriction was placed on the search. Reference lists were manually scanned for additional studies. From this systematic review, a model was created that incorporated clinical course, diagnostics, disease management, and treatment.
Our results focus on recommendations for individualized treatment, by selecting the most appropriate drug or modality for the patient, carefully weighing risks and benefits. Clinicians and patients should understand the staged progression of COVID-19 (Figure 2). As such, we present a treatment algorithm that recommends no treatment for some and specific treatment for others, depending on age, comorbidities, and symptom severity (Figure 3).
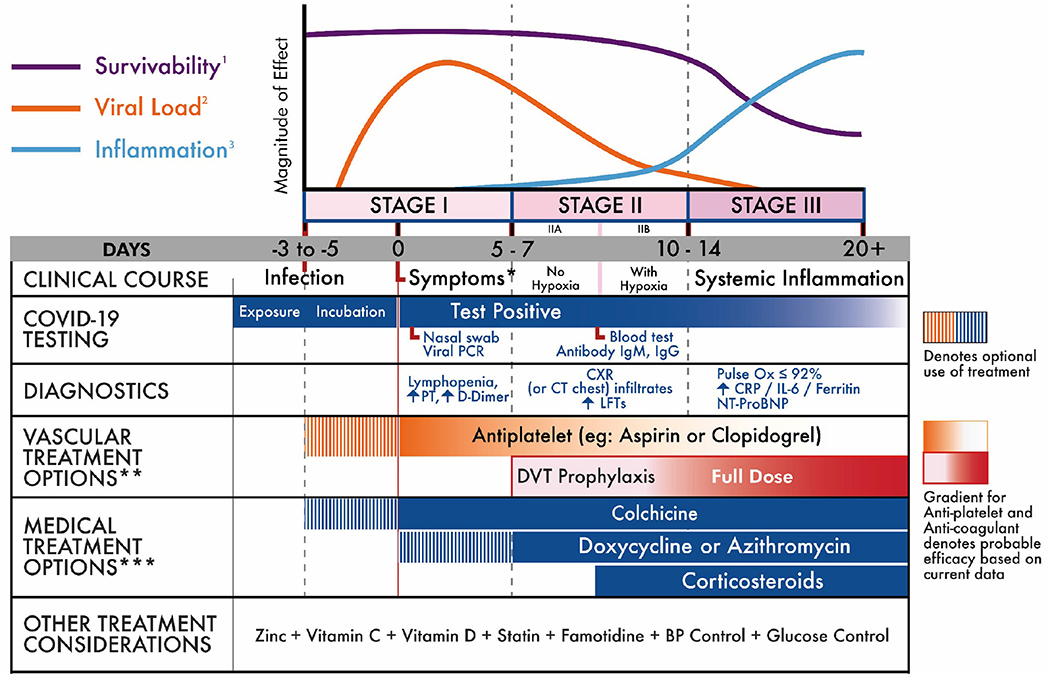
Figure 2. COVID-19 clinical stages and management strategy (15–17). *Initially mild in stage I (fever, cough, myalgia, other non-specific). May progress in stage II-III to severe dyspnea and respiratory distress (16, 18–20). **As with all treatment options, risks, and benefits should be carefully reviewed with the patient. ***No treatments are currently FDA approved for COVID-19 treatment. The FDA has approved remdesivir and convalescent plasma for inpatient use.
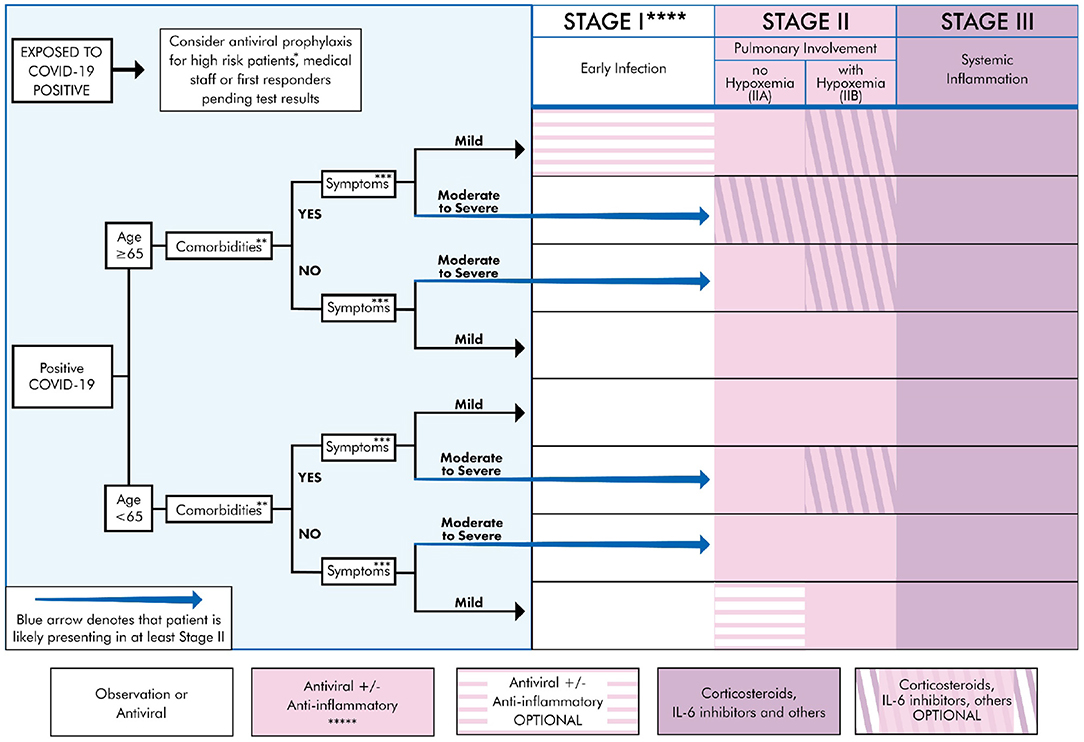
Figure 3. Treatment algorithm for COVID-19+ patients based on clinical presentation and therapeutic staging. *High risk patient: Anyone that is ≥65 y/o or meets comorbidities criteria as defined below. **Comorbidities: Defined as any two of the following: HTN, DM, CVD, CKD, Pre-existing lung disease, CHF, diabetes >7.6%, use of biologicals, HIV+, history of transplant, morbid obesity (BMI ≥ 40) (21, 22). ***Symptoms Mild: Fever, cough, fatigue, myalgia, headache, anosmia. Rarely, patients may also present with diarrhea, nausea, and vomiting (8, 21, 23). Moderate: Symptomatic viral pneumonia with possible hypoxemia (PaO2/FiO2 < 300). Confirmed by chest imaging (CXR or CT) which demonstrate bilateral infiltrates or ground glass opacities (21). Severe symptoms: Systemic (extra-pulmonary) hyperinflammation with one of the following: respiratory rate > 30 or SpO2 < 92% on room air (11, 17). Will also include abnormal chest imaging (CXR, CT scan, or lung ultrasound) characterized by bilateral opacities that are not primarily due to volume overload or lung collapse (partial or full). Echocardiogram can be used rule out of primary cardiac causes (24, 25). ****See Table 1 for appropriate Rx for stage. Treatment must be individualized to the patient by considering risks, benefits, and contraindications of the particular Rx. Note: there may be a potential for combining multiple agents if no drug interaction exists, as there are pleural mechanisms of actions. *****Convalescent plasma can be used during any stage, though likely more beneficial earlier in the disease course (63).
Results
Based on our thorough review of the literature, we correlated the disease course to COVID-19 testing, diagnostic options, and treatment strategies (see Figure 2). COVID-19 can be divided into three distinct stages, beginning at the time of infection (Stage I), sometimes progressing to pulmonary involvement (Stage II, with or without hypoxemia), and less frequently to systemic inflammation (Stage III). We also created a treatment algorithm that considers age, comorbidities, clinical presentation, and disease progression to suggest drug classes or treatment modalities (see Figure 3). The specific treatments are summarized in Table 1 (15, 21–62, 64).
Comorbidity
Data exists for early identification of cases at high risk of progression to severe COVID-19. One promising model created in China found that patients who developed severe COVID-19 possessed one of the following diseases: hypertension, diabetes, coronary heart disease, chronic respiratory disease, or tuberculosis. The same model cited age and various serological indicators [such as C-reactive protein (CRP), lactate dehydrogenase (LDH), bilirubin, and others] as factors associated with worse outcomes (65). Additional research confirmed, in a case-control study, that subjects with high Sequential Organ Failure Assessment (SOFA) scores, with age >65, with hypertension, diabetes, and/or coronary heart disease were at greatest risk (66). Lastly, research focusing on viral load and survival found that higher initial viral load is independently associated with worse prognosis (2).
Disease Progression
The most common presenting symptoms are fever and cough, followed by myalgia and fatigue. Less commonly, patients may present with sputum production, headache, or abdominal symptoms like diarrhea (21). In terms of disease progression, a case study of the first five patients diagnosed with COVID-19 in Europe points the way to two different clinical evolutions of the disease: 1. Presenting few symptoms, but showing high viral load from the respiratory tract; 2. A two-step disease process, with worsening of symptoms around 10 days of symptom onset despite decreased viral load in respiratory samples. In our model, we plot the disease progression as a function of infection, survivability, and inflammation (Figure 2).
We identify the inflection point where survival decreases as inflammation increases—approximately day 10 from symptom onset. Support for this is found in research by Chen et al. published in The Journal of Infection (37). Their research found that sepsis and ARDS in hospitalized patients start at around days 10 and 11, respectively. They also found temporal changes in inflammatory laboratory markers beginning at day 4 of illness onset. These included temporal changes in D-dimer, IL-6, serum ferritin, high-sensitivity cardiac troponin I, and lactate dehydrogenase. The differences were statistically significant between survivors and non-survivors for all time points. Figure 4 provides the percent change between survivors and non-survivors from day 4. In addition, Yang et al. found that the patients admitted to the ICU with severe hypoxemia had a 50% probability of survival at day 7 of ICU admission (corresponding to Day ~17 in Figure 2) (16).
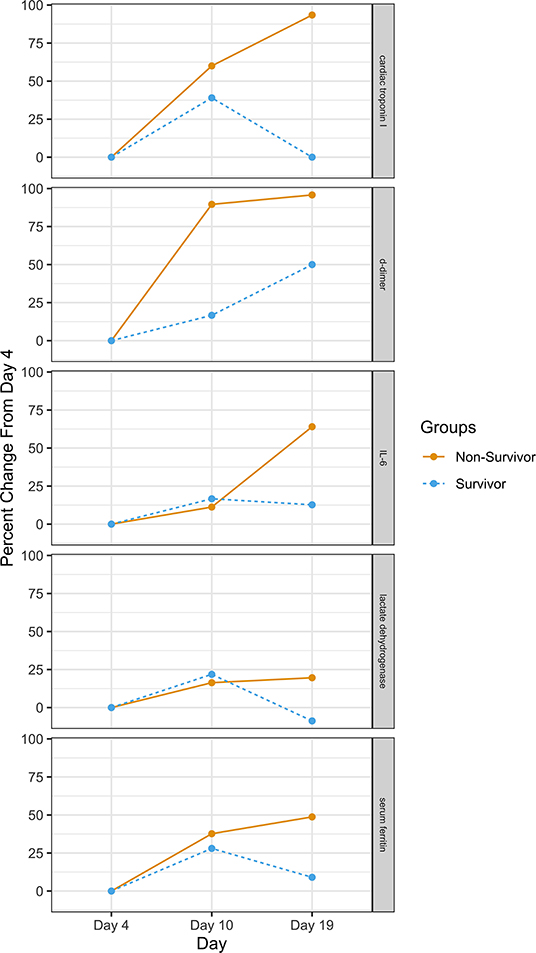
Figure 4. Percent change in clinical measures between survivors and non-survivors. Source: (21).
Stage I
The incubation period is on average 5 days. In most patients, initial presenting symptoms are mild (though a small number of patients can be asymptomatic throughout the disease). Stage I symptoms include fever, cough, fatigue, and body aches. In a minority of cases, symptoms may also include headache, abdominal symptoms, anosmia, as well as others. The duration of initial symptoms is 5–7 days, correlating with a peak in viral load (21). During this time, the appropriate diagnostic test is a nasopharyngeal PCR. Laboratory studies may include an elevated D-Dimer and prothrombin time, as well as lymphopenia (see Figure 2). Given that symptoms in this stage are mild, and correlated with viremia, the appropriate treatment modality is supportive care or antiviral medication. Nevertheless, treatment must be individualized, based on a patient's age, comorbidities, presenting symptoms, and drug interactions (see Figure 3 and Table 1).
Stage II
Some patients progress into Stage II, which is characterized by a decrease in viral levels and an increase in inflammation that initially localizes to the lungs. Infiltrates are typically seen on chest x-ray (CXR) or computed tomography (CT). Similar to symptom duration in Stage I, the typical symptom course in Stage II is also 5–7 days. Treatment with antivirals is still indicated, but given an average decrease in viral levels during this stage, that treatment is theoretically less effective than in Stage I. Moreover, Stage II is divided into two sub-stages (IIA and IIB), depending on whether a patient is hypoxemic or not. This distinction is important for management (see Figure 2). In Stage IIB, the patient is significantly dyspneic and may benefit, depending on age and comorbidities, from the use of corticosteroids or other anti-inflammatory treatments (see Figure 3).
Stage III
Although only a minority of patients (estimated at 10–15%) progress to Stage III, mortality within this stage is considerable (estimated at 20–30%). The morbidity and mortality are generally due to uncontrolled inflammation, which at this point is systemic. The most important symptom is respiratory distress (correlating in a typical patient to a Pulse Ox ≤ 92%). Laboratory markers include significantly increased CRP and IL-6 levels (16, 67). As in Stage II, treatment may include antivirals (if the patient is still viremic), but agents to counteract inflammation and its effects (such as microthrombi) must be considered (see Figure 2). A summary of investigational therapies can be found in Table 1. It should be noted that many ongoing clinical trials will more clearly define COVID-19 specific treatment risks and benefits.
Pre-exposure and Post-exposure Prophylaxis
A number of clinical trials are exploring pre-exposure and post-exposure prophylaxis. There is no definitive evidence that any particular treatment modality is effective but antivirals, anti-parasitics, and convalescent plasma have been proposed. Antivirals, like Remdesivir, may prove beneficial at any stage of disease (26, 27). Convalescent plasma provides the antibody support needed to envelop and destroy the virus while preventing the exuberant immune response or cytokine release that leads to significant pathology, particularly in Stages IIb and III (46).
Limitations
This review has several limitations. First, the incredible volume and speed at which data is published about the treatment of COVID-19 indicates that research findings and recommendations may change. Second, the research used to create this review came from small studies, often-times with very few controls. Third, the articles were limited to English-language publications or translations, so relevant international data could be lacking.
Conclusion
This paper presents the first evidence-based recommendations for individualized treatment for COVID-19. Based upon the observed transmission and mortality rates, health professionals urgently need to align patient baseline risk to disease stage and investigational treatment options. The COVID-19 pandemic represents the greatest public health crisis in three generations: the need for comprehensive management cannot be overstated.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
MH and RA revised the project, the main conceptual ideas and proof outline. BM, DS, JP, JPM, JD, YS, AC, and LA worked out almost all of the technical details with MH and RA assistance. PH, KH performed the numerical calculations and verified the numerical results. All authors contributed to writing and editing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Heyn PC, Meeks S, Pruchno R. Methodological guidance for a quality review article. Gerontologist. (2019) 59:197–201. doi: 10.1093/geront/gny123
2. Centers for Disease Control and Prevention. Cases in U.S. 2020, August 19. Available online at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html#2019coronavirus-summary (accessed August 19, 2020).
3. The Institute for Health Metrics and Evaluation. COVID-19. Available online at: https://covid19.healthdata.org/united-states-of-america (accessed August 20, 2020).
4. Craven M, Liu L, Mysore M, Wilson M. COVID-19: Briefing Note. McKinsey and Company (2020). Available online at: https://www.mckinsey.com/~/media/McKinsey/Business%20Functions/Risk/Our%20Insights/COVID%2019%20Implications%20for%20business/COVID%2019%20March%209/COVID-19-Briefing-note-March-9-2020-v5.ashx (accessed April 6, 2020).
5. Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. (2016) 24:490–502. doi: 10.1016/j.tim.2016.03.003
6. Biggerstaff M, Cauchemez S, Reed C, Gambhir M, Finelli L. Estimates of the reproduction number for seasonal, pandemic, and zoonotic influenza: a systematic review of the literature. BMC Infect Dis. (2014) 14:480. doi: 10.1186/1471-2334-14-480
7. Worldometers.info. COVID-19 Coronavirus Pandemic. Available online at: https://www.worldometers.info/coronavirus/ (accessed August 20, 2020).
8. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. doi: 10.1016/S0140-6736(20)30211-7
9. Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA. (2020) 323:1612–4. doi: 10.1001/jama.2020.4326
10. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
11. Siddiqi HS, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical therapeutic staging proposal. J Heart Lung Transplant. (2020) 39:405–407. doi: 10.1016/j.healun.2020.03.012
12. Parker BS, Walker KH. Clinical Course, Prognosis, and Epidemiology. Brigham and Women's Hospital COVID-19 Clinical Guidelines. Available online at: https://covidprotocols.org/protocols/01-clinical-course-prognosis-and-epidemiology (accessed April 5, 2020).
13. Marik P. EVMS Critical Care COVID-19 Management Protocol. Norfolk, VA: Eastern Virginia Medical School (2020). Available online at: https://www.evms.edu/media/evms_public/departments/internal_medicine/EVMS_Critical_Care_COVID-19_Protocol.pdf
14. Hsieh YH, Lee JY, Chang HL. SARS Epidemiology modeling. Emerg Infect Dis. (2004) 10:1165–7. doi: 10.3201/eid1006.031023
15. Massachusetts General Hospital. Massachusetts General Hospital COVID-19 Treatment Guidance. (2020). Available online at: https://www.massgeneral.org/assets/MGH/pdf/news/coronavirus/mass-general-COVID-19-treatment-guidance.pdf
16. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-Co-V-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
17. Clinical Management of Severe Acute Respiratory Infection When COVID-19 is Suspected (2020). Available online at: https://www.who.int./publications/i/item/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected (accessed April 6, 2020).
18. Hsueh PR, Huang LM, Chen PJ, Kao CL, Yang PC. Chronological evolution of IgM, IgA, IgG and nantibodies after infection with SARS-associated coronavirus. Clin Microbiol Infect. (2004) 10:1062–6. doi: 10.1111/j.1469-0691.2004.01009.x
19. Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today's diseases? Lancet Infect Dis. (2003) 3:722–7. doi: 10.1016/S1473-3099(03)00806-5
20. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. (2000) 342:1334–49. doi: 10.1056/NEJM200005043421806
21. Huang C, Wang Y, Li X, Sun W, Wang D, Fu B, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
22. People Who Are at Higher Risk for Severe Illness. (2020). Available online at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/groups-at-higher-risk.html (accessed April 13, 2020).
23. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
24. The ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. (2012) 307:2526–33. doi: 10.1001/jama.2012.5669
25. Riviello ED, Kiviri W, Twagirumugabe T, Mueller A, Banner-Goodspeed VM, Officer L, et al. Hospital incidence and outcomes of the acute respiratory distress syndrome using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med. (2016) 193:52–9. doi: 10.1164/rccm.201503-0584OC
26. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
27. Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. NEJM. (2020) 382:2327–36. doi: 10.1056/NEJMoa2007016
28. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. NEJM. (2020) 382:1787–99. doi: 10.1056/NEJMoa2001282
29. Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VCC, et al. Infectious Diseases Society of America guidelines on the treatment and management of patients with COVID-19. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa478. [Epub ahead of print].
30. Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. (2017) 93:449–63. doi: 10.2183/pjab.93.027
31. Nagata T, Lefor AK, Hasegawa M, Ishii M. Favipiravir: a new medication for the Ebola virus disease pandemic. Disaster Med Public Health Prepared. (2015) 9:79–81. doi: 10.1017/dmp.2014.151
32. Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, et al. Experimental treatment with Favipiravir for COVID-19: an open-label control study. Engineering. (2020). doi: 10.1016/j.eng.2020.03.007. [Epub ahead of print].
33. Kadam RU, Wilson IA. Structural basis of influenza virus fusion inhibition by the antiviral drug Arbidol. Proc Natl Acad Sci USA. (2017) 114:206–14. doi: 10.1073/pnas.1617020114
34. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. (2020) 71:769–77. doi: 10.1093/cid/ciaa272
35. Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. (2020) 97:396–403. doi: 10.1016/j.ijid.2020.06.099
36. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized trial. Int J Antimicrob Agents.(2020) 56:105949. doi: 10.1016/j.ijantimicag.2020.105949
37. Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. (2020) 80:e1–e6. doi: 10.1016/j.jinf.2020.03.004
38. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheum. (2020) 16:155–66. doi: 10.1038/s41584-020-0372-x
39. Sahraei Z, Shabani M, Shokouhi S, Saffaei A. Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine. Int J Antimicrob Agents. (2020) 55:105945. doi: 10.1016/j.ijantimicag.2020.105945
40. Bethel M, Yang FM, Li S, Nahman NS, Oliver AM, Machua W, et al. Hydroxychloroquine in patients with systemic lupus erythematosus with end-stage renal disease. J Investig Med. (2016) 64:908–10. doi: 10.1136/jim-2016-000065
41. April J, Ung C, Young LH, Melles RB, Choi HK. Hydroxychloroquine retinopathy—implications of research advances for rheumatology care. Nat Rev Rheumatol. (2018) 14:693–703. doi: 10.1038/s41584-018-0111-8
42. van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood monomuclear cells. J Rheumatol. (1997) 24:55–60.
43. Pharmacists Advancing Healthcare. Assessment of Evidence for COVID-19-Related Treatments. Available online at: https://www.ashp.org/-/media/assets/pharmacy-practice/resource-centers/Coronavirus/docs/ASHP-COVID-19-Evidence-Table.ashx
44. Cally L, Druce JD, Catton MG. The FDA-approved drug Ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. (2020) 178:104787. doi: 10.1016/j.antiviral.2020.104787
45. Roberts SS, Miller RK, Jones JK, Lindsay KL, Greene MF, Maddrey WC, et al. The Ribavirin pregnancy registry: findings after 5 years of enrollment, 2003-2009. Birth Defects Res A Clin Mol Teratol. (2010) 88:551–9. doi: 10.1002/bdra.20682
46. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. (2020) 23:1582–9. doi: 10.1001/jama.2020.4783
47. Ohe M, Shida H, Jodo S, Kisunoki Y, Seki M, Furuya K, et al. Macrolide treatment for COVID-19: will this be the way forward? Biosci Trends. (2020) 14:159–60. doi: 10.5582/bst.2020.03058
48. Amsden GW. Anti-inflammatory effects of macrolides—an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother. (2005) 55:10–21. doi: 10.1093/jac/dkh519
49. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Sevestre J, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med Infect Dis. (2020) 34:101663. doi: 10.1016/j.tmaid.2020.101663
50. Simpson TF, Kovacs RJ, Stecker EC. Ventricular arrhythmia risk due to hydroxychloroquine-azithromycin treatment for COVID-19. Cardiol Mag. (2020). Available online at: https://www.acc.org/latest-in-cardiology/articles/2020/03/27/14/00/ventricular-arrhythmia-risk-due-to-hydroxychloroquine-azithromycin-treatment-for-covid-19
51. Sodhi M, Etminan M. Therapeutic potential for tetracyclines in the treatment of COVID-19. Pharmacotherapy. (2020) 40:487–8. doi: 10.1002/phar.2395
52. Puckett Y, Gabbar A, Bokhari AA. Prednisone. StatPearls (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK534809/
53. Lee N, Chan KCA, Hui DS, Ng EKO, Wu A, Chiu RWK, et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. (2004) 31:304–9. doi: 10.1016/j.jcv.2004.07.006
54. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemostasis. (2020) 18:1094–9. doi: 10.1111/jth.14817
55. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with Coronavirus Disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. (2020) 180:1–11. doi: 10.1001/jamainternmed.2020.0994
56. Walker KH, Pearson JC. Therapeutics and Clinical Trials. Brigham and Women's Hospital: COVID-19 Clinical Guidelines. (2020). Available online at: https://covidprotocols.org/protocols/04-therapeutics-and-clinical-trials
57. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. (2020) 46:846–8. doi: 10.1007/s00134-020-05991-x
58. Xu X, Han M, Li T, Sun W, Wang D, Fu B, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. (2020) 117:10970–5. doi: 10.1073/pnas.2005615117
59. Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. (2020) 187:104791. doi: 10.1016/j.antiviral.2020.104791
60. Konig MF, Powell M, Staedtke V, Bai RY, Thomas DL, Fischer N, et al. Targeting the catecholamine-cytokine axis to prevent SARS-CoV-2 cytokine storm syndrome. medRxiv [Preprint]. (2020). doi: 10.1101/2020.04.02.20051565
61. Eli Lilly. Lilly Begins Clinical Testing of Therapies for COVID-19. (2020). Available online at: https://investor.lilly.com/news-releases/news-release-details/lilly-begins-clinical-testing-therapies-covid-19
62. Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO-19 randomized clinical trial. JAMA Netw Open. (2020) 3:e2013136. doi: 10.1001/jamanetworkopen.2020.13136
63. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. (2020) 117:9490–6. doi: 10.1073/pnas.2004168117
64. Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, et al. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest. (2006) 129:1441–52. doi: 10.1378/chest.129.6.1441
65. Lescure FX, Bouadma L, Nguyen D, Parisey M, Wicky PH, Behillil S, et al. Clinical and virological data of the first cases of COVID-19 in Europe: a case series. Lancet Infect Dis. (2020) 20:697–706. doi: 10.1016/S1473-3099(20)30200-0
66. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
Keywords: COVID 19, infectious disease, disease management, directed treatment, cover-19 testing, clinical course
Citation: Aguilar RB, Hardigan P, Mayi B, Sider D, Piotrkowski J, Mehta JP, Dev J, Seijo Y, Camargo AL, Andux L, Hagen K and Hernandez MB (2020) Current Understanding of COVID-19 Clinical Course and Investigational Treatments. Front. Med. 7:555301. doi: 10.3389/fmed.2020.555301
Received: 24 April 2020; Accepted: 03 September 2020;
Published: 21 October 2020.
Edited by:
Abdallah Samy, Ain Shams University, EgyptReviewed by:
Yongwen Chen, Third Military Medical University, ChinaMahmoud Mohamed Shehata, National Research Centre, Egypt
Copyright © 2020 Aguilar, Hardigan, Mayi, Sider, Piotrkowski, Mehta, Dev, Seijo, Camargo, Andux, Hagen and Hernandez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Patrick Hardigan, patrick@nova.edu
 Richard B. Aguilar1
Richard B. Aguilar1  Patrick Hardigan
Patrick Hardigan Bindu Mayi
Bindu Mayi Jared Piotrkowski
Jared Piotrkowski Jinesh P. Mehta
Jinesh P. Mehta Yelenis Seijo
Yelenis Seijo Marlow B. Hernandez
Marlow B. Hernandez