Abstract
Prone position (PP) is known to improve oxygenation and reduce mortality in COVID-19 patients. This systematic review and meta-analysis aimed to determine the effects of PP on respiratory parameters and outcomes. PubMed, EMBASE, ProQuest, SCOPUS, Web of Sciences, Cochrane library, and Google Scholar were searched up to 1st January 2021. Twenty-eight studies were included. The Cochran's Q-test and I2 statistic were assessed heterogeneity, the random-effects model was estimated the pooled mean difference (PMD), and a meta-regression method has utilized the factors affecting heterogeneity between studies. PMD with 95% confidence interval (CI) of PaO2/FIO2 Ratio in before–after design, quasi-experimental design and in overall was 55.74, 56.38, and 56.20 mmHg. These values for Spo2 (Sao2) were 3.38, 17.03, and 7.58. PP in COVID-19 patients lead to significantly decrease of the Paco2 (PMD: − 8.69; 95% CI − 14.69 to − 2.69 mmHg) but significantly increase the PaO2 (PMD: 37.74; 95% CI 7.16–68.33 mmHg). PP has no significant effect on the respiratory rate. Based on meta-regression, the study design has a significant effect on the heterogeneity of Spo2 (Sao2) (Coefficient: 12.80; p < 0.001). No significant associations were observed for other respiratory parameters with sample size and study design. The pooled estimate for death rate and intubation rates were 19.03 (8.19–32.61) and 30.68 (21.39–40.75). The prone positioning was associated with improved oxygenation parameters and reduced mortality and intubation rate in COVID-19 related respiratory failure.
Similar content being viewed by others
Introduction
Recently a new virus called coronavirus 2019 (COVID-19) is spreading all around the world1,2 and caused a global pandemic with increasing incidence, mortality, and medical resource consumption which impose enormous socio-economic burdens3,4. COVID-19 disease ranges from mild respiratory tract illness to severe progressive pneumonia, primarily manifesting as acute respiratory distress syndrome (ARDS) requiring admission to the intensive care unit (ICU)4. ARDS occurs in 20–41% of patients5. The mortality rate among ARDS patients is high and has been reported to be between 30 and 40%6,7. Higher mortality of COVID-19 patients may be related to higher incidences of barotrauma and ventilator-induced lung injury (VILI)8. The COVID-19 pandemic presented a unique challenge for the health care systems. The shortage of resources is one of these problems that pandemic imposed, include human resources, ICU beds, and mechanical ventilators9. In the absence of effective therapies for COVID-19, the implementation of supportive care is essential10. Prone positioning is one of these interventions for patients with severe ARDS, which could improve oxygenation and has a survival benefit11 and also could improve outcomes in COVID-19 patients. It has been suggested as the standard of care in international guidelines12. Proning can reduce ventral-dorsal trans-pulmonary pressure differences and lung compression by the heart and diaphragm, resulting in lung perfusion improvement. Proning has been demonstrated to improve lung compliance and lung recruitability and reduce VILI incidence8. Studies of prone ventilation in COVID-19 ARDS patients have shown improvement in the ratio of the partial pressure of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2) (PaO2/FiO2)8 by 35 mmHg13. Prone positioning may play a role in reducing systemic inflammation by increasing alveolar fluid drainage. Inflammatory responses related to ARDS or secondary to VILI may be attributed with pulmonary and extra-pulmonary organ dysfunction and strategies to reduce inflammation may lead to increased survival8. Prone positioning also increases chest wall elastance and amplifies active expiration during coughing14. Studies report that prone positioning reduced 28-day and 90-day mortality rates15,16 and accelerated the time for extubation15. The World Health Organization (WHO) recommends its use for periods of 12–16 h/day17,18,19.
Correct selection of patients and applying the accurate treatment protocol for prone positioning are crucial to its efficacy6. Special precautions are required for placing and monitoring a patient in the prone position20. Intubated patients in prone positioning are at risk, such as accidental removal of the tracheal tube, pressure ulcer, facial edema, gastroesophageal reflux, and other problems. Overall, it seems that correct patient selection, timely initiation, and duration of patient’s placement in this position can all affect the effectiveness of this intervention6. Considering that COVID-19 is a novel disease that caused many difficulties and due to lack of sufficient evidence, the need to assess the effects of prone positioning as a supportive care in hypoxemic patients is necessary, so we conducted this systematic review and meta-analysis to determine the effects of prone position on respiratory parameters and outcomes of COVID-19 patients.
Materials and methods
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for designing and implementing systematic review studies, the following steps were taken: a systematic literature search, organization of documents for the review, abstracting and quality assessment of each study, synthesizing data, and writing the report21. The protocol of the study was registered in the International Prospective Register Of Systematic Reviews (PROSPERO) at the National Institute For Health Research. Registration number in PROSPERO is CRD42021257619.
Search strategy
According to the PICO framework, the systematic literature search was conducted on PubMed, EMBASE, ProQuest, SCOPUS, Web of Sciences, Cochrane library, and Google Scholar databases. MeSH Keywords were connected with AND, OR and NOT prone position and respiratory parameters, and their suggested entry terms were the main keywords in the search strategy.
-
1.
'Coronavirus Disease 2019 [Title/Abstract], OR 'COVID-19' [Title/Abstract], OR 'Coronavirus' [Title/Abstract], OR 'SARS-cov-2' [Title/Abstract], OR 'Sever acute respiratory syndrome coronavirus-2' [Title/Abstract], '2019-nCov' [Title/Abstract], OR 'SARS-Cov' [Title/Abstract]
-
2.
'Prone' [Title/Abstract], OR 'Prone position' [Title/Abstract]
-
3.
'Oxygenation' [Title/Abstract], OR 'Cell Respiration' [Title/Abstract], OR 'Cell Respirations' [Title/Abstract]
-
4.
'Respiratory Distress Syndrome' [Title/Abstract], OR 'Acute respiratory distress syndrome' [Title/Abstract] OR 'Hypoxemic' [Title/Abstract], OR 'Respiratory Insufficiency' [Title/Abstract], OR 'Dyspnea' [Title/Abstract]
-
5.
1 AND 2
-
6.
1 AND 2 AND 3
-
7.
2 AND 3AND 4
-
8.
1 AND 2 AND 3 AND 4
Population, Intervention, Comparators, Outcomes (PICO) criteria for this study includes (P): patients with COVID-19. (I): prone position. (C): no intervention, (O): respiratory parameters and outcome.
Inclusion and exclusion criteria
Type of studies
Studies including quasi-experimental and before–after designs were included if the effects of prone position on respiratory parameters were reported as an outcome. Also, studies met the inclusion criteria if they were published until 1st January 2021. There was no language filtering. The case report, case series, reviews, and studies with incomplete data were excluded.
Type of participants
The studies were selected if participants were patients with Reverse transcription polymerase chain reaction (RT-PCR) confirmed test or if imaging findings showed evidences of COVID-19, patients with COVID-19 need oxygenation (face mask, nasal cannula, invasive mechanical ventilation, non-invasive mechanical ventilation). Pregnant women, patients who have prone positioning contraindication such as skeletal fractures were excluded.
Type of intervention
Patients were instructed to stay in the prone position based on the proning protocol of each study for at least 30–60 min and then return to the supine position. Standard prone position was considered for 16 h/day (some studies considered the duration of prone position ≥ 3–4 h, or until the patient is uncomfortable). The average time of prolonged sessions was considered up to 36 h. However, in one study, a 5-min protocol was used. Respiratory parameters were measured three times in most studies (before positioning, during prone position, and after prone position).
Type of outcomes measure
The primary outcome was the respiratory parameters and respiratory status. The secondary outcomes were death rate and intubation rates (Supplementary 1).
Study selection
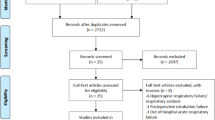
Two authors independently evaluated the eligibility of these articles, and any disagreements were resolved by consensus. Several articles were excluded due to being irrelevant or duplicated. Finally, 28 full-text articles were included in the systematic review and 26 articles in the meta-analysis (Fig. 1).
Risk of bias and quality assessment
The methodological quality of the included studies in this review was conducted by the Mixed Methods Appraisal Tool (MMAT). The quality assessment was conducted independently by two authors. The MMAT was developed to appraise different empirical studies categorized into five categories: qualitative, randomized controlled trial, nonrandomized, quantitative descriptive, and mixed methods studies22. This tool consists of five items for each category, each of which could be marked as Yes, No, or cannot tell. Based on the scoring system, score one is assigned to Yes and score 0 to all other answers. In other words, the total score would be the percentage of affirmative responses. To evaluate the final scores qualitatively, the scores above half (more than 50%) were considered high quality.
Data extraction
Data were collected as follows: reference, location, type of study, sample size, age, duration of the prone position, proning protocol, timing of measurement, and respiratory parameters.
Unification of units
All respiratory parameters converted to mmHg. For conversion of respiratory parameters to get from SI units (KPa) to mmHg was multiplied by 7.501.
Statistical analysis
All analyses were conducted with Stata software version 14.0 (College Station, Texas). For each study, the mean and standard deviation (SD) of respiratory parameters in the prone position and supine position was extracted and if Median and IQR was reported; we changed it to mean with [(min + max + 2*Median)/4] or [(med + q1 + q3)/3] and SD with [IQR/1.35]. Then mean difference (MD) of respiratory parameters for each study was calculated by mean1 minus to mean 2. Due to different studies design (Before–After or Quasi-Experimental design), in the before–after design, we calculated the change score MD (mean after prone position minus mean before prone position), and in Quasi-Experimental design, we calculated MD (mean in supine position minus mean in prone position). Then Standard deviation in Before–After design and Quasi-Experimental design was calculated based on formulas (1) and (2):
where SDbefore, SDafter, and Corr is the standard deviation in before prone position, standard deviation after prone position, and correlation coefficient between before and after
where SDprone position, SDsupine position, n1, and n2 is the standard deviation in prone position group, the standard deviation in supine position group, the sample size in the prone position and supine position groups. Then pooled MD (PMD) was calculated by the “Metan” command23,24. Heterogeneity was determined using Cochran’s Q test of heterogeneity, and the I2 index was used to quantify heterogeneity. In accordance with the Higgins classification approach, I2 values above 0.7 were considered as high heterogeneity. To estimate the PMD for respiratory parameters and subgroup analysis (study design and ventilation), the fixed-effect model was used, and when the heterogeneity was greater than 0.7, the random-effects model was used. The meta-regression analysis was used to examine the effect of study design, sample size, BMI, age and prone position (PP) duration as factors affecting heterogeneity among studies. The “Meta bias” command was used to check for publication bias, and if there was any publication bias, the PMD was adjusted with the “Metatrim” command using the trim-and-fill method. In all analyses, a significance level of 0.05 was considered.
Result
Overall, 1970 studies were found through databases. After excluding redundant papers, 855 studies remained. After reading abstracts, 775 studies were excluded from the list. Then, the full text of the remaining 80 studies was reviewed, and 52 studies were excluded. Finally, 28 studies included in qualitative analysis and 26 studies with a total sample size of 1272 participants were included in the quantitative analysis. The flowchart of this selection process is shown in Fig. 1. Studies were published during 2020–2021, most studies were done in the UK, China, and Spain with three studies and range participants age were 17–83 years old (Tables 1 and 2). Supplementary 2 shows risk of bias assessment for included studies. All studies were high quality (more than 50% scores).
Pooled mean difference of respiratory parameters in total and based on subgroups
Figure 2 showed the forest plot for MD of PaO2/FIO2 Ratio in included studies. The minimum and maximum reported MD of PaO2/FIO2 reported by Abou-Arab et al. (MD: 0.00; 95% CI 7.21–7.21 mmHg) in France25 and by Mittermaier et al. (MD: 187.90; 95% CI 156.14–199.66 mmHg) in Germany30. Based on Fig. 2 using the random-effects model approach; the PMD in the study with before–after design, quasi-experimental design and in total was 55.74 (95% CI 28.13–83.35) mmHg, 56.38 (95% CI 8.47–104.29) mmHg, and 56.20 (95% CI 33.16–79.24) mmHg; respectively. This means that in general, the prone position in COVID-19 patients leads to significant improvement of PaO2/FIO2 Ratio, so that in before–after design, quasi-experimental design, and in total, the mean of PaO2/FIO2 Ratio significantly increased 55.74, 56.38, and 56.20 mmHg; respectively.
Figure 3 and Table 3 showed the PMD of other respiratory parameters in included studies. The PMD of SPO2 (Sao2) in the study with before–after design, quasi-experimental design, and in total was 3.38 (95% CI 1.68–5.09), 17.03 (95% CI 12.19–21.88), and 7.58 (95% CI 4.93–10.23); respectively. This means that the prone position in COVID-19 patients leads to significant improvement corresponding to Spo2 (Sao2). Also the PMD of Paco2 in COVID-19 patients was significantly decreased in quasi-experimental design (PMD: − 18.49; 95% CI − 34.50 to − 2.47 mmHg) and in total (PMD: − 8.69; 95% CI − 14.69 to − 2.69 mmHg). No significant change was observed for PMD of PaCo2 in the before–after design. The PMD of other respiratory parameters showed in Table 3 and Fig. 3. It should be noted that prone position leads to improvement of PaO2 but does not have any effects on the respiratory rate in general, especially in the quasi-experimental design. The pooled estimate and 95% CI for death rate and intubation rate were 19.03 (8.19–32.61) and 30.68 (21.39–40.75); respectively (Fig. 4).
Figure 5 showed PMD of respiratory parameters based on ventilation status. PMD of Spo2 (Sao2) in Intubation and Non-intubation subgroup was 10.56 (95% CI − 18.15 to 39.26) and 8.57 (95% CI 3.47–13.67); respectively. This means that the prone position in COVID-19 patients with non-intubation leads to significant improvement corresponding to Spo2 (Sao2) but Intubation have no effects on Spo2 (Sao2) improvement. Also PMD of PaO2/FIO2in Intubation and non-intubation subgroup was 65.03 (95% CI 6.06–123.99) and 49.56 (95% CI 26.56–72.56); respectively. This means that the prone position in COVID-19 patients leads to significant improvement of PaO2/FIO2 Ratio, but this value for Intubated patients was higher than non-intubated groups. Situation of other parameter was showed in Fig. 5.
Publication bias
Based on Egger's test results, significant publication bias was observed for PaO2/FIO2 Ratio (Coefficient: 5.63; 95% CI 0.91–10.35; p: 0.024). Therefore, the fill- and trim-adjusted PaO2/FIO2 Ratio (PMD: 57.41, 95% CI 32.19–81.01 mmHg) was generated, which was not significantly different from the original PaO2/FIO2 Ratio (PMD: 56.20; 95% CI 33.16–79.24 mmHg). It means that the result of the meta-analysis was robust. No significant publication bias was observed for other respiratory parameters.
Heterogeneity and meta-regression results
According to Cochran’s Q test of heterogeneity, there was significant heterogeneity among studies (p < 0.001). Except for PaCo2 in the before–after design, the heterogeneity amount was more than 85% based on the I2 index, which indicates high heterogeneity. Table 4 presents the results of the univariate meta-regression; there are significant associations between study, results with study design corresponding to SPO2 (Sao2) percent (Coefficient: 12.80; p < 0.001). No significant associations were observed for other respiratory parameters with sample size, study design, BMI, age and PP duration (Table 4).
Discussion
This systematic review analyzed the effects of prone position on respiratory parameters, intubation, and death rate. We found that prone position initiation leads to improved oxygenation parameters (PaO2/FiO2 ratio, SpO2, PaO2, and PaCO2) in patients with mild to severe respiratory failure due to confirmed COVID-19. However, the prone position did not change the respiratory rate in patients with hypoxemic respiratory failure suffering from COVID-19.
Most of the studies (18/28 studies) demonstrated significant improvement in PaO2/FiO2 ratio after prone positioning. Moreover, the improvement of SpO2 (SaO2) and PaO2 has been shown in 15 and 7 studies, respectively. Although the effect of prone position after resupination has declined in five studies1,5,8,16,29, early prone positioning should be considered as first-line therapy in ARDS patients43. Initiation of prone position in ARDS patients by reducing shunt, and V/Q mismatch, brings about an increase in the recruitment of non-aerated areas of the lungs, secretion clearance, improvement work of breathing (WOB) and oxygenation, and reduction of mortality compared with the supine position44,45,46. Prone position by enhancement in PaO2/FiO2 ratio not only leads to a decrease in the classification of respiratory failure but also prevents further complications due to ARDS, such as multi-organ failure (MOF), which is the most common cause of mortality in this devastating condition47.
The efficacy of prone positioning may be affected by various protocols, such as different settings (ICU or emergency department), the timing of initiation (early or late), duration (prolonged or short sessions), positioning (prone position with or without lateral position), respiratory support in intubated or non-intubated patients (mechanical ventilation, NIV, nasal cannula, helmet, face mask) and the severity of ARDS48. Even though in this study PaO2/FiO2 ratio was significantly higher in the prone-positioning group with mild to severe ARDS, a further meta-analysis need to assess the impact of prone position in a different classification of ARDS with mild (PaO2/FiO2 = 201–300 mmHg), moderate (PaO2/FiO2 = 101–200 mmHg), and severe (PaO2/FiO2 < 100 mmHg) condition. In this systematic review, the prone position time varied from less than 1 to 16 h in a day. In eight studies, the prone positioning has been implemented for about 16 h a day. The prolonged prone positioning (no less than 10–12 h and ideally for 16–20 h) leads to improved oxygenation and a significant reduction in mortality in patients with severe ARDS. On the other hand, reducing the number of turning in patients with critical conditions can decrease the risk of more complications48. Although PaCO2 did not demonstrate a difference in five studies5,16,25,29,40, the PMD of PaCO2 in COVID-19 patients significantly decreased totally. The prone position by increasing the dorsal recruitment, PaCO2 clearance, and decreasing the dead space can also lead to better ventilation. Moreover, a higher PaCO2 clearance due to the prone position is related to a significant decrease in 28-day mortality54. In terms of respiratory rate, in few studies, the respiratory rate reduction was significant, but we found that respiratory rate did not change during the prone positioning in the overall analysis.
Our systematic review and meta-analysis demonstrated that prone positioning leads to a lower mortality rate in confirmed COVID-19 patients. Although in this systematic review and meta-analysis, many studies have assessed the impact of prone position on the short term (28 days) mortality, where they benefit from prone positioning protocols, the effect of prone positioning in the long-term (3 months or more) mortality is unclear. Therefore, further studies will be needed to demonstrate the relationship between prone positioning in COVID-19 patients and long-term mortality. Furthermore, this study confirmed that the improvement of oxygenation parameters due to the prone position might be associated with a lower intubation rate in COVID-19 patients.
Conclusions
In our systematic review of 28 studies, prone positioning has been compared with supine positioning in hypoxic adult patients with COVID-19. We found prone position by optimizing lung recruitment, and the V/Q mismatch can improve oxygenation parameters such as PaO2/FIO2 Ratio, Spo2 (Sao2), PaO2, PaCO2. Nevertheless, the prone position did not change their respiratory rate. Moreover, the initiation of prone position might be associated with a lower mortality and intubation rate. Since most patients demonstrated improved oxygenation and lower mortality and intubation rate, we recommend the prone position in patients COVID-19.Similar to other studies, our research had some limitations. (1) Some studies did not report values of the respiratory parameters in different groups and just reported significantly parameter (like that p-value); which we have to exclude this studies from the quantitative analysis that this limitation was not be resolved even by data requesting from corresponding authors. We would like to perform the gender-specific estimation, but it was not possible due to insufficient data in the primary studies; (2) also we tend to estimate the pooled MD in different geographical regions or country-specific estimation based on available methods50, since the infrequent studies number, this estimation will not be robust.
Abbreviations
- PMD:
-
Pooled mean difference
- CI:
-
Confidence interval
- ARDS:
-
Acute Respiratory Distress Syndrome
- VILI:
-
Ventilator-induced lung injury
- PaO2 :
-
Pressure of arterial oxygen
- FIO2 :
-
Fraction of inspired oxygen
- WHO:
-
World Health Organization
- PP:
-
Prone position
- NIV:
-
Non invasive ventilation
- IMV:
-
Intermittent mandatory ventilation
- HFNO:
-
High flow nasal oxygen
- PEEP:
-
Positive end expiratory pressure
- CPAP:
-
Continuous positive airway pressure
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
References
Carsetti, A., Paciarini, A.D., Marini, B., Pantanetti, S., Adrario, E., Donati, A. Prolonged prone position ventilation for SARS-CoV-2 patients is feasible and effective. Crit. Care. 24(1), 1–3 (2020).
Shaterian, N. & Abdi, F. Is cesarean section a safe delivery method to prevent mother to child transmission of SARS-CoV-2?. Tehran Univ. Med. J. TUMS Publ. 78(5), 337–338 (2020).
Roozbeh, N., Amirian, A. & Abdi, F. Coronavirus and male infertility: Letter to the editor. Tehran Univ. Med. J. 78(9), 630–631 (2020).
Ni, Z. et al. Efficacy of early prone or lateral positioning in patients with severe COVID-19: a single-center prospective cohort. Precision Clinical Medicine, 1–12 (2020).
Coppo, A. et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 8, 765–774 (2020).
Ghelichkhani, P. & Esmaeili, M. Prone position in management of COVID-19 patients; a commentary. Arch. Acad. Emerg. Med. 8(1), e48, 1–3 (2020).
Abdi, F. & Amirian, A. Diagnosis of SARS-CoV-2 vertical transmission using the amniotic fluid test. J. Mil. Med. 22(6), 670–671 (2020).
Khullar, R., Shah, S., Singh, G., Bae, J., Gattu, R., Jain, S., et al. Effects of prone ventilation on oxygenation, inflammation, and lung infiltrates in COVID-19 related acute respiratory distress syndrome: A retrospective cohort study. J. Clin. Med. 9(12), 4129 (2020).
Padrao, E.H., Valente, F.S., Besen, B.A.M.P., Rahhal, H., Mesquita, P.S., de Alencar, J.C.G., et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: Exploratory findings in a single-center retrospective cohort study. Acad. Emerg. Med. 0(0), 1–11 (2020).
Koeckerling, D. et al. Awake prone positioning in COVID-19. Thorax 75, 833–834 (2020).
Shelhamer, M., Wesson, P. D., Solari, I. L., Jensen, D. L., Steele, W. A., Dimitrov, V. G., et al. Prone positioning in moderate to severe acute respiratory distress syndrome due to COVID-19: a cohort study and analysis of physiology. J. Intensive Care Med. 36(2), 241–252 (2021).
Berrill, M. Evaluation of oxygenation in 129 proning sessions in 34 mechanically ventilated COVID-19 patients. J. Intensive Care Med. [Original Res.] 36(2), 229–232 (2021).
Sryma, P. B. et al. Reinventing the wheel in ARDS: Awake proning in COVID-19. Arch Bronconeumol. 56(11), 747–763 (2020).
Karpov, A., Mitra, A.R., Crowe, S., Haljan, G. Prone position after liberation from prolonged mechanical ventilation in COVID-19 respiratory failure. Crit. Care Res. Pract. 688120, 1–7 (2020).
Rahmani, F., Salmasi, S. & Rezaeifar, P. Prone position effects in the treatment of COVID-19 patients. Caspian J. Intern. Med. 11(1), S580–S582 (2020).
Weiss, T.T., Cerda, F., Scott, J.B., Kaur, R., Sungurlu, S., Mirza, S.H., et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: A retrospective observational cohort study. Br. J. Anaesthesia, 126(1), 48–55. (2020).
Taboada, M., Rodríguez, N., Riveiro, V., Abelleira, R., Ricoy, J., Lama, A., et al. Short-term outcomes of 50 patients with acute respiratory distress by COVID-19 where prone positioning was used outside the ICU. J. Clin. Anesthesia. 67, 110028 (2020).
Qadri, S. K. et al. Critically ill patients with COVID-19: A narrative review on prone position. Pulm Ther. 6, 233–246 (2020).
Dong, W., Gong, Y., Feng, J., Bai, L., Qing, H., Zhou, P., et al. Early awake prone and lateral position in non-intubated severe and critical patients with COVID-19 in Wuhan: A respective cohort study. https://doi.org/10.1101/2020.05.09.20091454 (2020).
Makic, M. B. F. Prone position of patients with COVID-19 and acute respiratory distress syndrome. J. Perianesth. Nurs. 35, 437–438 (2020).
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. P. preferred reporting items for systematic reviews and mata-analysis: The PRISMA statement. PLoS Med. 6(7), e1000097 (2009).
Hong, Q. N. et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ. Inf. 34(4), 285–291 (2018).
Soltani, S., Tabibzadeh, A., Zakeri, A., Zakeri, A.M., Latifi, T., Shabani, M., et al. COVID-19 associated central nervous system manifestations, mental and neurological symptoms: a systematic review and meta-analysis. Rev. Neurosci. 32(3), 351–361 (2021).
Hashemi, H. et al. Global and regional prevalence of strabismus: A comprehensive systematic review and meta-analysis. Strabismus. 27(2), 54–65 (2019).
Abou-Arab, O., Haye, G., Beyls, C., Huette, P., Roger, P.-A., Guilbart, M., et al. Hypoxemia and prone position in mechanically ventilated COVID-19 patients: A prospective cohort study. Can. J. Anesth. 68, 262–263 (2020).
Ferrando, C., Mellado-Artigas, R., Gea, A., Arruti, E., Aldecoa, C., Adalia, R., et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: A multicenter, adjusted cohort study. Crit. Care. 24(597), 1–11 (2020).
Caputo, N. D., Strayer, R. J. & Levitan, R. Early self-proning in awake, non-intubated patients in the emergency department: A single ED’s experience during the COVID-19 pandemic. Acad. Emerg. Med. 27, 375–378 (2020).
Elharrar, X. et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 323, 2336–2338 (2020).
Retucci, M., Aliberti, S., Ceruti, C., Santambrogio, M., Tammaro, S., Cuccarini, F., et al. Prone and lateral positioning in spontaneously breathing patients with COVID-19 pneumonia undergoing noninvasive helmet CPAP treatment. CHEST. 158(6), 2431–2435 (2020).
Mittermaiera, M., Pickerodt, P., Kurth, F., Jarcy, L.B.D., Uhrig, A., Garcia, C., et al. Evaluation of PEEP and prone positioning in early COVID-19 ARDS. EClinicalMedicine. 28, 100579, 1–9 (2020).
Taboada, M., Rodríguez, N., Riveiro, V., Baluja, A. & Atanassoff, P. G. Prone positioning in awake non-ICU patients with ARDS caused by COVID-19. Anaesth. Crit. Care Pain Med. [Letter to the Editor]. 39, 581–583 (2020).
Sartini, C. et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 323(22), 2338–2340 (2020).
Thompson, A. E., Ranard, B. L., Wei, Y. & Jelic, S. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Internal Med. 180(11), 1537–1539 (2020).
Tu, G.-W., Liao, Y.-X., Li, Q.-Y., Dong, H., Yang, L.-Y., Zhang, X.-Y., et al. Prone positioning in high-flow nasal cannula for COVID-19 patients with severe hypoxemia: a pilot study. Ann. Transl. Med. 8(9), 598 (2020).
Winearls, S., Swingwood, E.L., Hardaker, C.L., Smith, A.M., Easton, F.M., Millington, K.J., et al. Early conscious prone positioning in patients with COVID-19 receiving continuous positive airway pressure: A retrospective analysis. BMJ Open Resp. Res. 7(e0007), 1–4 (2020).
Sharp, T., Al-Faham, Z., Brown, M., Martin-Lazaro, J., Morales, J.C. Prone position in COVID-19: Can we tackle rising dead space? J. Intensive Care Soc. 1–4 (2020).
Wendt, C., Mobus, K., Wiener, D., Eskin, B., Allegra, J.R. Prone positioning on non-intubated COVID 19 patients in hypoxic respiratory distress: Single site retrospective health records review. J. Emerg. Nursing. 47(2), 279–287 (2021).
Burton-Papp, H.C., Jackson, A.I.R., Beecham, R., Ferrari, M., Nasim-Mohi, M., Grocott, M.P.W., et al. Conscious prone positioning during non-invasive ventilation in COVID-19 patients: Experience from a single centre [version 1; peer review: 2 approved]: F1000Research2020 9(859), (2020).
Jagan, N., Morrow, L.E., Walters, R.W., Klein, L.P., Wallen, T.J., Chung, J., et al. The POSITIONED study: Prone positioning in nonventilated coronavirus disease 2019 patients—A retrospective analysis. Crit. Care Explorations. 2(e0229) (2020).
Şan, İ., Yıldırım, Ç., Bekgoz, B., Gemcioğlu, E. Transport of awake hypoxemic probable COVID 19 patients in the prone position. Am. J. Emerg. Med. 1–8 (2020).
Solverson, K., Weatherald, J., Parhar, K.K.S. Tolerability and safety of awake prone positioning COVID-19 patients with severe hypoxemic respiratory failure. Can. J. Anesth. [Rep. Original Investigat.] 68, 64–70 (2020).
Zang X. et al. Efficacy of early prone position for COVID‑19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med 46, 1927–1929 (2020).
Guerin, C., Baboi, L. & Richard, J. Mechanisms of the effects of prone positioning in acute respiratory distress syndrome. Intensive Care Med. 40(11), 1634–1642 (2014).
Kallet, R. H. A comprehensive review of prone position in ARDS. Respir. Care 60(11), 1660–1687 (2015).
Guerin, C. et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 368(23), 2159–2168 (2013).
Scholten, E. L., Beitler, J. R., Prisk, G. K. & Malhotra, A. Treatment of ARDS with prone positioning. Chest 151(1), 215–224 (2017).
Wright, A. D. & Flynn, M. Using the prone position for ventilated patients with respiratory failure: A review. Nurs. Crit. Care 16(1),19–27 (2011).
Malhotra, A., & Kacmarek, R. M. Prone ventilation for adult patients with acute respiratory distress syndrome. https://www.uptodate.com/contents/prone-ventilation-for-adult-patients-with-acute-respiratorydistress-syndrome (2020).
Gattinoni, L. et al. (eds) Prone Positioning in Acute Respiratory Distress Syndrome. Seminars in Respiratory and Critical Care Medicine (Thieme Medical Publishers, 2019).
Hashemi, H. et al. Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 34(8), 1357–1370 (2020).
Yap, C. Y. & Aw, T. Arterial blood gases. Proc. Singapore Healthcare 20(3), 227–235 (2011).
Singh, V., Khatana, S., & Gupta, P. Blood gas analysis for bedside diagnosis. Natl. J. Maxillofacial Surg. 4(2), 136–141 (2013).
Berry, W., Barreiro, G., Dziekan, G., Enright, A., Evans, P., & Funk, L. Pulse oximetry training manual. (World Health Organization) (2011).
Auliawati, D., Suparyatha, I. B. G., Wati, D. K., Hartawan, I. N. B. & Subanada, I. B. SpO2/ FiO2 ratio as an oxygenation parameter in pediatric acute respiratory distress syndrome. Bali Med. J. 5(2), 358–361 (2016).
Nicolo, A., Massaroni, C., Schena, E., & Sacchetti, M. The importance of respiratory rate monitoring: From healthcare to sport and exercise. Sensors. 20(6396) (2020).
Acknowledgements
We appreciate the reviewers comment.
Funding
This study has no funding.
Author information
Authors and Affiliations
Contributions
F.B. and F.A. conceived the study, interpreted the data, drafted the manuscript and approved the final version of the paper. R.P. critically analyzed the data. M.G.H., F.Z., N.M. interpreted the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Behesht Aeen, F., Pakzad, R., Goudarzi Rad, M. et al. Effect of prone position on respiratory parameters, intubation and death rate in COVID-19 patients: systematic review and meta-analysis. Sci Rep 11, 14407 (2021). https://doi.org/10.1038/s41598-021-93739-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-93739-y
This article is cited by
-
Managing Severe Hypoxic Respiratory Failure in COVID-19
Current Emergency and Hospital Medicine Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.