First Report of Tocilizumab Use in a Cohort of Latin American Patients Hospitalized for Severe COVID-19 Pneumonia
- 1Department of Rheumatology, Clínica Alemana, Santiago, Chile
- 2Facultad de Medicina Clínica Alemana, Universidad del Desarrollo, Santiago, Chile
- 3Department of Pediatrics, Clínica Alemana, Santiago, Chile
- 4Immunology and Rheumatology Unit, Roberto del Río Hospital, Santiago, Chile
- 5Department of Immunology, Clínica Alemana, Santiago, Chile
- 6Department of Infectology, Clínica Alemana, Santiago, Chile
- 7Clinical Laboratory, Clínica Alemana, Santiago, Chile
- 8Department of Respiratory Diseases, Clínica Alemana, Santiago, Chile
- 9Department of Hematology, Clínica Alemana, Santiago, Chile
- 10Critical Patient Unit, Clínica Alemana, Santiago, Chile
Introduction/objectives: An interleukin-6 inhibition strategy could be effective in selected COVID-19 patients. The objective is to present our experience of tocilizumab use in patients with severe COVID-19.
Methods: Observational retrospective cohort study. Hospitalized patients were evaluated by our multidisciplinary team for eventual use of tocilizumab. Patients with progressive ventilatory impairment and evidence of a hyperinflammatory state despite usual treatment received tocilizumab 8 mg/kg intravenous (maximum dose 800 mg), in addition to standard treatment. The use and time of use of mechanical ventilation (MV), the change of the Alveolar-arterial (A-a) gradient, of the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) and of inflammation laboratory parameters after 72 h of tocilizumab use was evaluated.
Results: 29 patients received tocilizumab. 93.1% were men, 37.9% were obese, and 34.5% had hypertension. Of the 20 patients who were not on MV when receiving tocilizumab, 11 required non-invasive MV, for an average of 5 days, and one of them required intubation. A-a gradient, PaO2/FiO2, and inflammation parameters improved significantly. A better lymphocyte count, which improved significantly after tocilizumab use, was significantly associated with less use of MV. Five patients presented positive culture samples after tocilizumab, three being of clinical significance. A lower lymphocyte count was associated with having a positive culture. No other significant adverse events were seen.
Conclusion: Our study suggests the utility and shows the safety of tocilizumab use in COVID-19 patients who have respiratory failure and evidence of hyperinflammation. Lymphocyte improvement was a predictor of good response.
Keypoints
- The use of tocilizumab in patients with severe COVID-19 was safe.
- Most of the patients presented a good response in terms of ventilatory and inflammatory parameters.
- Lymphocyte improvement after using tocilizumab was the main predictor of a good outcome.
Introduction
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), can lead to life-threatening respiratory symptoms (1).
The pathogenesis of the disease is complex and not yet fully understood (2, 3), however, as a consequence of lytic viral infection macrophage activation, and immune cell recruitment to alveoli, a significant increase in the secretion of proinflammatory cytokines occurs, referred to as “cytokine release syndrome” (CRS) (4, 5).
Different phases have been proposed in the course of this disease, stage I (mild) of early infection and active viral replication, stage II (moderate) of lung involvement without (IIa) or with hypoxia (IIb), where viral replication and local inflammation can coexist, and stage III (severe), with systemic hyperinflammation (6). Several immunomodulatory strategies have been proposed to treat hyperinflammation in phases II and III and are currently being studied (7–11).
Interleukin-6 (IL-6) seems to have a pivotal role in the production of diffuse alveolar damage and acute respiratory distress syndrome (12, 13). Thus, it is possible to conceive an IL-6 inhibition strategy to control CRS in patients with progressive hyperinflammation. Tocilizumab is a humanized anti IL-6 receptor antibody approved for the treatment of rheumatoid arthritis, juvenile idiopathic arthritis, giant cell arteritis, and life-threatening cytokine release induced by chimeric-antigen receptor T-cell therapy (14).
Faced with the possibility of not having the health resources to confront the pandemic, and in the absence of treatments with demonstrated efficacy, we constituted a multidisciplinary, composed of physicians from various specialties including rheumatology, immunology, hematology, infectious diseases, intensive care medicine and pulmonologists, with the aim of developing a guide to consider the use of tocilizumab, with a strict protocol to evaluate the patient and suggest its use for specific patients.
Our purpose is to present our experience of tocilizumab use in a cohort of patients with severe COVID-19, with emphasis on ventilatory results, inflammatory parameters, and adverse events.
Methods
Study Design
Observational retrospective cohort study.
Setting
Patients hospitalized in Clínica Alemana, Santiago, Chile, with a diagnosis of COVID-19, for whom evaluation was requested by our multidisciplinary team for eventual tocilizumab use. The results of the patients evaluated between April 13 and June 16, 2020 who received tocilizumab and who were discharged or died before July 18, 2020 are reported. All data was collected by this multidisciplinary team from the patient's medical record by the multidisciplinary team.
Participants and Intervention
The multidisciplinary team evaluated each case by reviewing the patient's medical file and discussing with the treating physician. Criteria were previously established to consider the use of tocilizumab:
Inclusion Criteria
1. Age ≥ 18 years.
2. SARS-CoV-2 confirmed infection according to institutional criteria [polymerase chain reaction (PCR), antibodies, and/or compatible images].
3. ≥ 7 days from the onset of symptoms.
4. Evidence of severe COVID-19:
a. At least two of the following:
i. Respiratory rate > 24x.
ii. Multiple pulmonary infiltrates on chest radiography or computed axial tomography and/or increase in infiltrates.
iii. O2 saturation <93% with a fraction of inspired oxygen (FiO2) of 21% or O2 requirement to maintain saturation > 92%.
iv. Fever > 38°C.
b. And at least two of the following:
i. C-reactive protein (CRP) > 5 mg/dL (or relative increase >50% in <48 h).
ii. Alveolar-Arterial (A-a) O2 gradient with deterioration > 50%.
iii. Increase ≥25% of ferritin with respect to the previous value within the last 48 h or isolated value ≥ 2,000 ng/mL.
iv. Neutrophil-lymphocyte ratio (NLR) > 3.12 or lymphopenia <700/mm3.
v. Fibrinogen increase ≥25% or ≥800 mg/dL.
Exclusion Criteria
1. Not controlled infection by bacteria, fungi or non-COVID viruses.
2. Active tuberculosis.
3. Any concurrent immunomodulatory medication or intervention that the treating physician believes would put the patient at increased risk.
4. History of diverticulitis or intestinal perforation.
5. Absolute neutrophil count <1,000/mm3, platelets <50,000/mm3.
6. Known allergy to tocilizumab, or to any other anti-IL-6 agent.
7. Any condition that, in the opinion of the treating physician or the multidisciplinary group, would increase the patient's risk when receiving tocilizumab.
If the patient met the criteria, the indication for tocilizumab was discussed within the multidisciplinary group. If compassionate tocilizumab use was recommended, this suggestion was communicated to the treating physician and to the patient or his/her family, explaining the risks and benefits, specifying that the use was off-label. If its use was accepted, tocilizumab was administered as a single 60 min intravenous drip infusion. The dose used was 8 mg/kg, with a maximum of 800 mg.
Variables
The baseline characteristics of the patients were recorded. Data from clinical chart was recorded, specifying the level of care, the type of ventilatory support [nasal cannula, non-invasive mechanical ventilation (NIMV), invasive mechanical ventilation (IMV)], the use of prone position, hydroxychloroquine (HCQ), convalescent plasma, corticosteroids (CS), and tocilizumab. Several laboratory parameters were considered including lymphocyte and neutrophil counts, erythrocyte sedimentation rate (ESR), CRP, ferritin, D-dimer, fibrinogen, Lactate dehydrogenase (LDH), transaminases, and IL-6. The A-a gradient and the ratio of arterial oxygen partial pressure to fractional inspired oxygen (PaO2/FiO2) were calculated.
The change, between the time of tocilizumab use and 72 h after, in laboratory and ventilatory parameters was analyzed.
The use and time of use of mechanical ventilation (MV), and the percentage improvement of the A-a gradient and of PaO2/FiO2 after 72 h of tocilizumab use were defined as outcomes.
Deaths, non-covid-19 infections and events not attributable to the usual course of the disease were recorded and analyzed.
Data Sources/Measurement
Data was obtained from the patient's clinical record in the institutional electronic clinical file. The use of MV was evaluated as a qualitative and quantitative variable (days of use), and improvements in the A-a gradient and PaO2/FiO2 were evaluated quantitatively. Non-COVID-19 infections and adverse events not attributable to the usual course of the disease were evaluated as qualitative variables.
Statistical Methods
The data was analyzed using IBM SPSS Statistics for Mac OS, Version 24.0. Armonk, NY. Categorical variables were described as frequencies and/or percentages, and quantitative variables were described as mean and standard deviation (SD) for those normally distributed or median and interquartile range (IQR) for those not normally distributed. Changes in parameters were analyzed using T-test for paired variables or Wilcoxon signed-rank sum test, as appropriate. The associations between variables and outcomes were evaluated using linear or logistic regressions for univariate and multivariate analysis. The covariates included in the multivariate analysis model were age, sex, body mass index, diabetes, hypertension, asthma, obstructive sleep apnea, smoking status, days with symptoms. Statistical significance was defined as P < 0.05. In case of missing data, the analysis was performed with the available data.
Institutional Review Board
The Scientific Ethics Committee of the Clínica Alemana-Universidad del Desarrollo medicine faculty approved the study prior to data collection.
Role of the Funding Source
This study did not receive any type of financing.
Results
Participants
Twenty-nine of 68 evaluated patients (42.6%) received Tocilizumab.
Descriptive Data
Baseline Characteristics
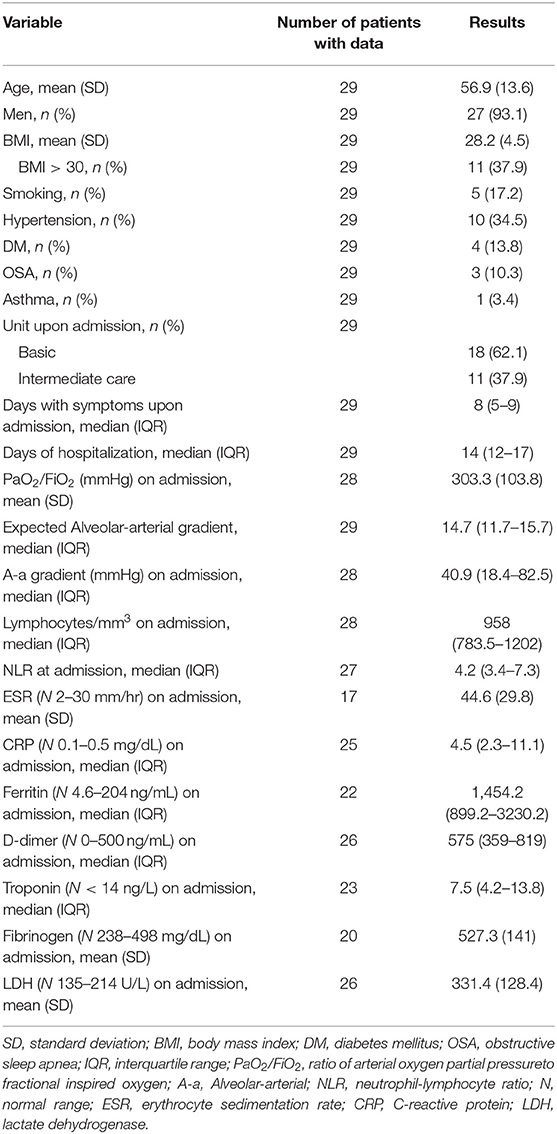
The mean age of the patients was 56.9 years, and only two were women. 37.9% were obese and the most frequent comorbidity was hypertension. All patients presented lymphopenia and elevated inflammatory markers (Table 1).
Tocilizumab
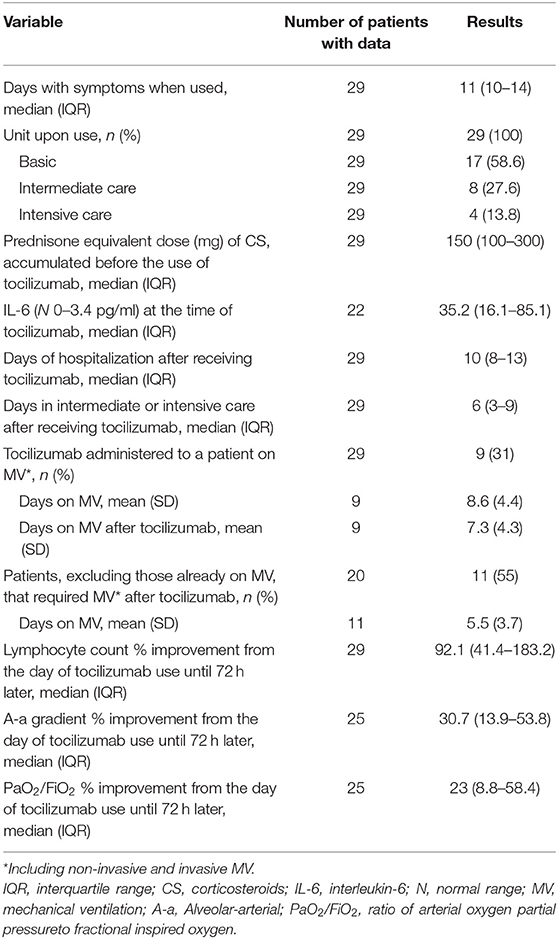
Tocilizumab was used at a median of 4 days of hospitalization and 11 with symptoms. Of the 17 patients (58.6%) who received tocilizumab while in the basic hospitalization unit, 11 (64.7%) were subsequently transferred to intermediate care, and of those 11 patients, three (27.3%) required admission to intensive care. Of the eight patients who received the medication while at the intermediate care unit, two (25%) had to be admitted to intensive care afterwards. The other four patients received the medication while at the intensive care unit. The median cumulative dose of CS (equivalent to prednisone) at the time of tocilizumab use was 150 mg. IL-6 was elevated before tocilizumab use, and afterwards it was measured again in five patients (median 104; IQR 89.8–446) (Table 2).
Other Interventions
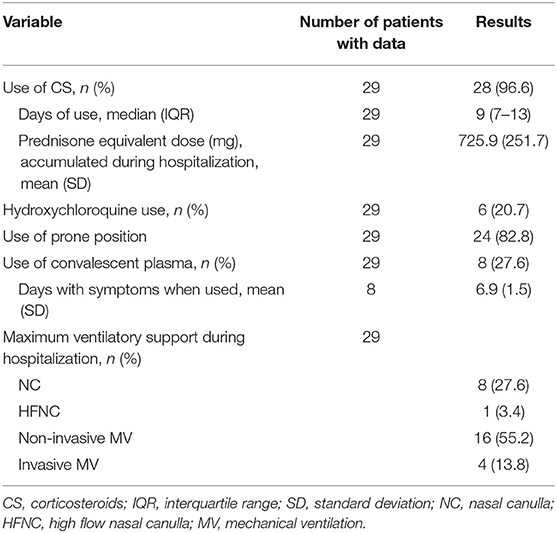
Six patients received HCQ (400 mg every 12 h the first day and then 200 mg every 12 h for 4, 5 days in total). None of the patients used antiviral therapy and all received prophylactic anticoagulation. All patients used CS, but the type and dose were not standard and could change during the hospitalization of the same patient, so the analysis was performed by calculating the cumulative dose equivalent to prednisone (Table 3).
Outcome Data
Laboratory Parameters
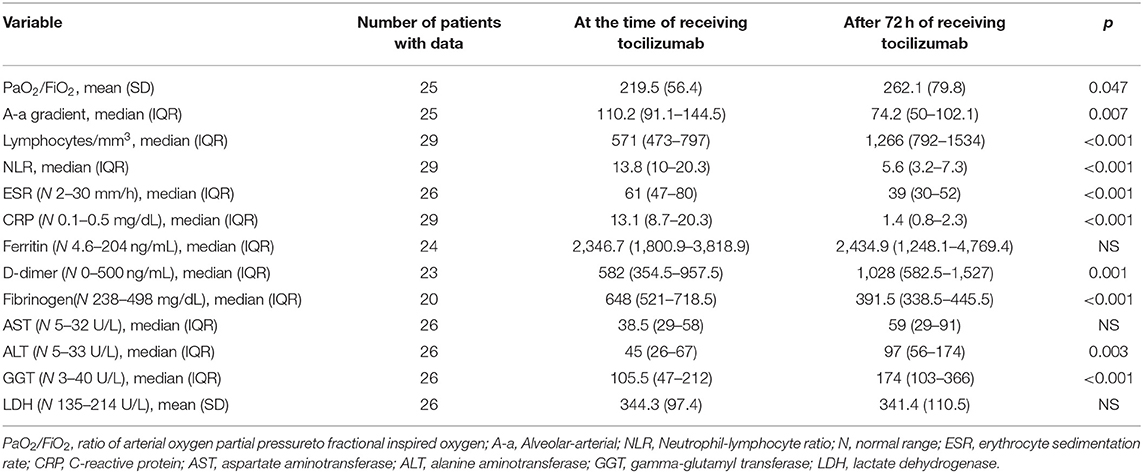
From admission to the day of tocilizumab use, lymphocytes decreased by a mean of 30.7% (SD 36). After 72 h of using tocilizumab there was a significant improvement in the lymphocyte count, NLR, ESR, CPR, and fibrinogen. A mild and transient, but statistically significant, rise in alanine aminotransferase, gamma-glutamyl transferase and D-dimer was observed (Table 4).
Ventilatory Parameters
The improvement in PaO2/FiO2 (23%) and A-a gradient (30.7%) after 72 h of tocilizumab use was statistically significant. Nine patients received tocilizumab while on MV. Of the remaining 20 patients, 11 (55%) required MV after using tocilizumab. Of these 11 only one required IMV (the other three patients who used IMV required it before tocilizumab use) (Tables 2, 4).
Infections
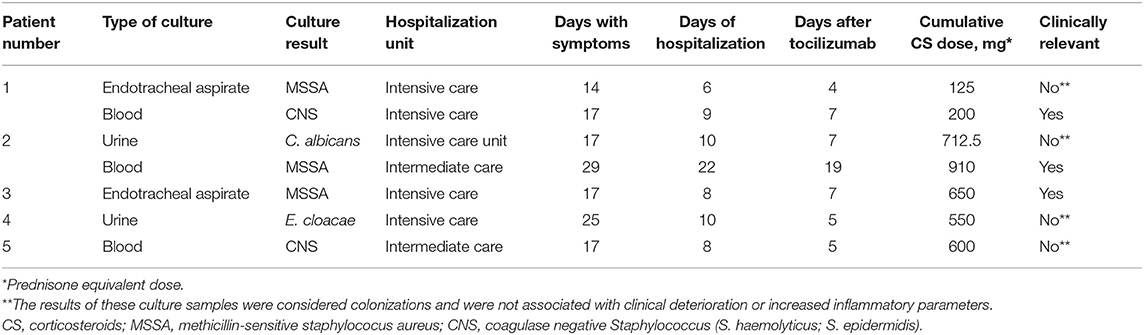
Seven culture samples were positive after tocilizumab use, in five patients (17.2%). Only three of them presented a proven clinical infection (Table 5).
The first patient received tocilizumab on the second day of hospitalization while on NIMV [with a procalcitonin (PCT) of 0.28 ng/mL], was intubated on the third day, and on the sixth day a methicillin-sensitive Staphylococcus aureus was identified in the endotracheal aspirate culture, without clinical impact. On the ninth day of hospitalization a methicillin-resistant Staphylococcus epidermidis was identified in two blood and one catheter tip samples. This was compatible with a central line associated bacteremia and the central line was removed. The fever disappeared without additional antibiotic treatment. The patient was discharged after 29 days of hospitalization.
The second patient had a urine culture positive for candida albicans (>100,000 colony forming units) with non-inflammatory urine (PCT of 0.45 ng/mL at the time of tocilizumab use). This was an asymptomatic candiduria. Twelve days later the patient presented two positive blood cultures for methicillin-sensitive Staphylococcus aureus. The patient had an elbow bursitis and a retroperitoneal hematoma at the same time. The patient was successfully treated with intravenous cefazolin and was discharged after 44 days of hospitalization.
The third patient presented a positive culture of the endotracheal aspirate for a methicillin-sensitive Staphylococcus aureus. The bronchoalveolar lavage culture of the same day was negative. The patient was successfully treated with intravenous antibiotics.
In all patients the PCT measured on the day of tocilizumab use had a median value of 0.17 ng/mL (IQR 0.1–0.38; <0.5 ng/mL = low risk of sepsis). In the five patients who presented a positive culture, PCT had a median value of 0.26 (IQR 0.17–0.28), at the time of tocilizumab use. There was no statistically significant difference in the PCT between these two groups.
Thus, three patients presented a clinically relevant infection, and none of these had severe course.
Adverse Events
Events out of the expected course for COVID-19, after receiving tocilizumab, were described in six male patients (20.7%).
One patient presented leukopenia of 2,800/mm3 with neutropenia of 1,425/mm3, which normalized 10 days after discharge. Two patients had mild elevation of transaminases. The study of known hepatotropic agents was negative, thus a direct effect of SARS-CoV-2 infection or drugs was considered as possible causes. One of these patients had already had his post-discharge evaluation and transaminases had normalized.
On the 27th day of hospitalization, a patient (patient number 1 in Table 5) who required intubation presented a small left para-aortic pleural collection, possibly with blood content, on computed axial tomography. This did not mean any change for the course of his hospitalization, and he was discharged after 29 days of hospitalization.
Two patients presented pulmonary thromboembolism. The first case occurred in a patient on his eighth day of hospitalization and on his seventeenth day of symptoms, 7 days after receiving tocilizumab and after 3 days connected to IMV, being on prophylactic anticoagulation, without D-dimer level of that day. Pulmonary thromboembolism was bilateral and difficult to anticoagulate, and the hospitalization lasted 26 days, but the patient was discharged in good condition. The second presented a left basal subsegmental pulmonary thromboembolism, on day 17 of hospitalization and on the 25th day of symptoms, 14 days after receiving tocilizumab and after 15 days on MV (nine of them on IMV), being on prophylactic anticoagulation, with a D-dimer of 1.751 ng/mL (11 days before it was 5.858 ng/mL). After 7 days of anticoagulation, the patient presented an extensive retroperitoneal hematoma. The patient was discharged after 36 days of hospitalization in good condition.
The only variable that was significantly associated with presenting pulmonary thromboembolism was the number of days of hospitalization at the intermediate or intensive care unit [Exp (B) 1.332; 95% C.I. 1.004–1.765; p = 0.46].
Deaths
Two patients died (6.9%). The first patient required IMV after 1 day of hospitalization and received tocilizumab in this condition on the fifth day of hospitalization and twentieth of symptoms. The patient managed to transition to NIMV temporarily. The only positive culture was a urine culture with Enterobacter cloacae (patient number 4 in Table 5). The patient followed a progressive course with respiratory and hemodynamic deterioration, with elevated levels of troponin and N-terminal pro brain natriuretic peptide, and presented a sharp drop in his hematocrit (from 35 to 23%, being anticoagulated with dalteparin) before dying in the eleventh day of hospitalization.
The second patient, with history of obstructive sleep apnea and possible interstitial lung disease, received tocilizumab on the third day of hospitalization and eighth of symptoms while on NIMV. The patient did not present notable infections or events, but had progressive respiratory deterioration, and after 7 days in NIMV it was decided to limit therapeutic efforts and the patient was not intubated. The patient died on the twelfth day of hospitalization.
No significant associations were found between the different variables and death.
Results of the Univariate and Multivariate Analysis
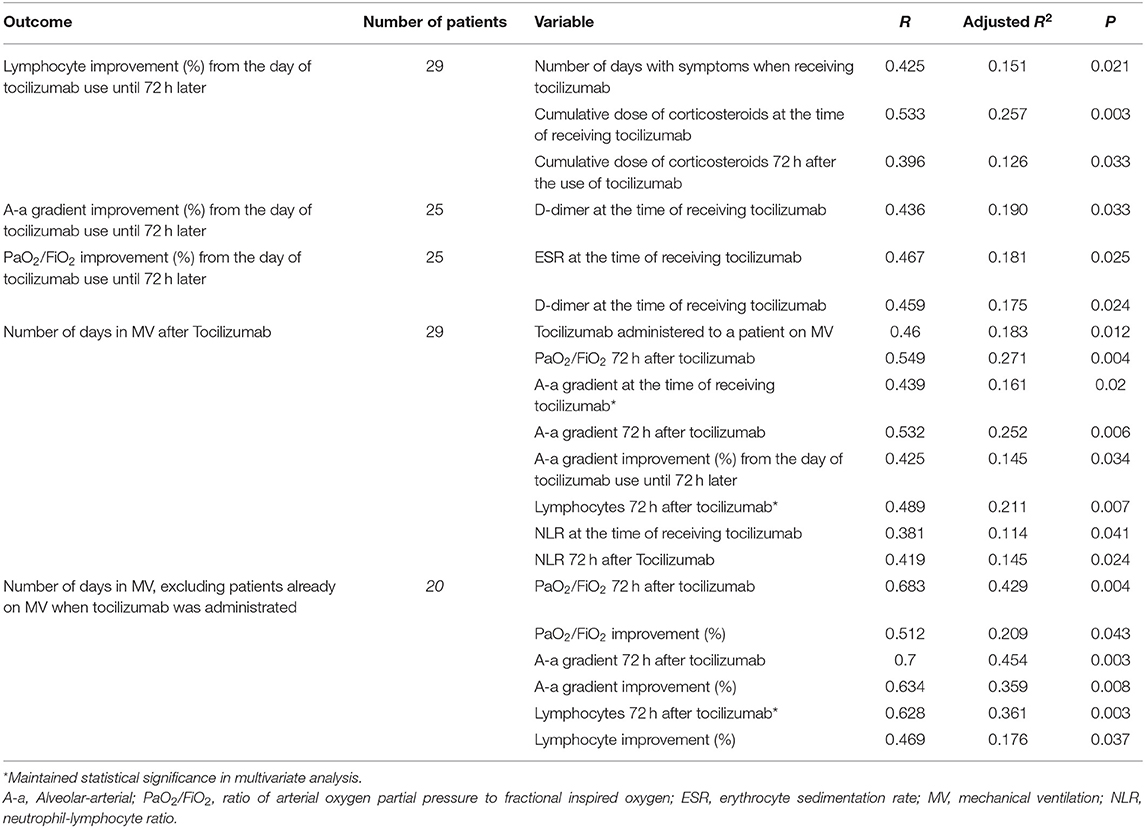
Lymphocyte Count
A greater percentage improvement in the lymphocyte count between the day of tocilizumab use and 72 h afterwards was significantly associated with a higher number of days with symptoms when tocilizumab was used, and a higher cumulative dose of corticosteroids. These associations did not maintain statistical significance in the multivariate analysis (Table 6).
Ventilatory Parameters
A greater percentage improvement in the A-a gradient was significantly associated with a lower level of D-dimer when receiving tocilizumab, and a greater improvement in PaO2/FiO2 was associated with a higher level of ESR and lower D-dimer at the time of receiving tocilizumab. These associations were weak, and none remained significant after multivariate analysis (Table 6).
Mechanical Ventilation
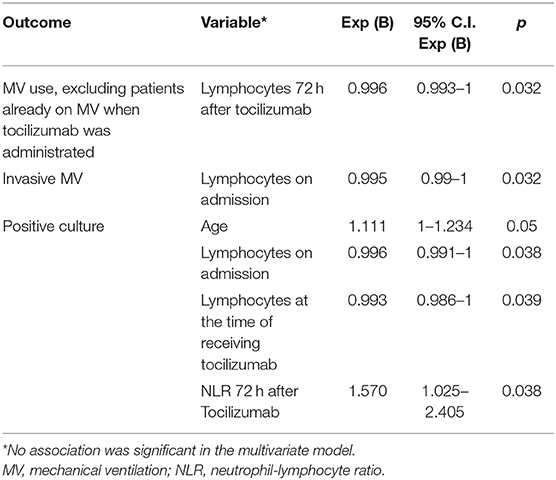
A lower lymphocyte count 72 h after tocilizumab use was associated with requiring MV in patients who were not on MV at the time of receiving tocilizumab (Table 7). In the 20 patients who received tocilizumab without using MV, there were no significant differences between the 11 patients who required MV later and the nine who did not, except in the lymphocyte count 72 h after receiving tocilizumab (p = 0.004) and their percentage of improvement (p = 0.016). At the time of receiving tocilizumab, the median number of lymphocytes in those who subsequently required MV was 682 (IQR 484–767) and rose to 807 (IQR 666–1335) 72 h after tocilizumab, a median improvement of 51.7% (IQR 30.3–79.3). In those who did not require MV after tocilizumab, the lymphocytes rose from 737 (IQR 571–806) to 1,602 (IQR 1402–2087), a median improvement of 157.8% (IQR 99.7–180.6).A greater number of days in MV after tocilizumab was significantly associated with worse PaO2/FiO2 and A-a gradient, and with lower lymphocyte count, but only the A-a gradient and the lymphocyte count maintained statistical significance in the multivariate analysis (Table 6). Furthermore, a lower number of lymphocytes on admission was associated with requiring IMV (Table 7).
Infections
Older age, low lymphocyte count and a high NLR were associated with a greater possibility of presenting a positive culture (Table 7).
Deaths
No associations were found.
Discussion
This is the first Latin American report to evaluate the use of tocilizumab in patients hospitalized for severe COVID-19.
Our cohort corresponds to relatively young Latin American patients, mainly men, with a significant prevalence of comorbidities known to be strong risk factors for severe COVID-19 (15). The patients presented a severe disease and 69% required MV. All patients presented biomarkers compatible with hyperinflammation, impaired gas exchange, and rapid worsening of these despite receiving usual treatment. The use of hydroxychloroquine was infrequent since its prescription decreased when reports were published that warned of the ineffectiveness and risk of adverse events of its use (16).
Rapid clinical deterioration in most of our patients, in a phase of hyperinflammatory disease, led to the indication of immunomodulatory therapy, at first with CS. In practice, we used a sequential strategy in which the use of tocilizumab was decided only once despite the use of corticosteroids a worsening of the patient condition was observed. CS usefulness in COVID-19 remains controversial (17), however, in a recently published trial, the use of dexamethasone (6 mg every day for a median 6 days, approximately a cumulative dose equivalent to 225 mg of prednisone) resulted in lower 28-day mortality (18). In our cohort the cumulative dose of CS, equivalent to prednisone, was higher, with a median of 725.9 mg. A recently published retrospective analysis also showed good results with a high dose of CS and subsequent use of tocilizumab (19). Undoubtedly, the routine CS use with a high cumulative dose results in an important confounding factor when interpreting efficacy and safety data.
In our patients, 72 h after tocilizumab use, a dramatic improvement was observed in ventilatory parameters and in most CRS biomarkers. D-dimer was highly variable from day to day in our patients. Although its values increased 72 h after tocilizumab and seemed to be associated with improvements in A-a gradient and PaO2/Fio2, the changes in its levels over the course of the hospitalization make it difficult to draw conclusions about its associations and predictive role.
A higher lymphocyte count was associated with less use of MV. Lymphopenia is a common finding in severe COVID-19, with almost all subsets affected, and it has been suggested as a poor prognostic factor and reliable indicator of severity and mortality risk (20–22). It is not clear if SARS-CoV-2 directly affects T cells or if the triggered cytokine release drives depletion and exhaustion of T cells (23) and impairs the cytotoxic activity of CD8 T cells and NK cells, which are crucial for antiviral immunity (24). Strategies to improve T cell counts and function may be critical to reduce disease severity. It is expected that the use of CS would be associated with greater lymphopenia and worse NLR, but what we observed was that after the use of tocilizumab these parameters improved significantly. It can be hypothesized that blocking IL-6 could help in COVID-19 not only by inhibiting directly the exaggerated inflammatory response, but also by restoring cellular immunity which is crucial for virus clearance.
After multivariate analysis, the decrease in the A-a gradient, but not the increase of PaO2/FiO2, correlated with a lower number of days of MV use after tocilizumab infusion. This is because the gradient is a more sensitive index to evaluate disturbances in gas exchanges (25).
Assessing the safety of tocilizumab use in a COVID-19 patient is of the utmost importance. Despite the increase in transaminase values after tocilizumab use in our patients, this was transitory and not associated with liver failure. Two patients (6.9%) suffered from pulmonary thromboembolism, which seems to be within the reported rates for severe COVID-19 (26).
In our experience, infections are the most important factor influencing fear of tocilizumab use in COVID-19 patients. Five patients of our cohort had a positive blood or urine culture, all of them hospitalized more than 7 days and in intermediate or intensive care units when presenting the positive culture. Only three patients presented a clinically relevant infection, and none of these presented a severe course. Lower lymphocyte count and worse NLR were associated with a greater probability of having a positive culture. More than an immunosuppressive agent, tocilizumab acts as an immunomodulator, and our patients significantly raised their lymphocytes after its use, which was associated with a lower risk of infection.
Two of our patients died (6.9%). The mortality rate in our center, as of July 16, 2020, counting 1,076 hospitalized patients, is 5.3%. The rate of our cohort is slightly higher, but it should be considered that it consists of patients selected because of their severity.
Our study has several limitations. It is an observational study without control group. It represents a small number of patients from a single center. In the analysis of the results, it was not possible to have some data in all the patients. This report may not capture adverse events that may occur in the long term.
Our study is relevant because it represents the experience of a Latin American country, with a population poorly represented in other series. In addition, we provide more information to assist in choosing the best “window of opportunity” for tocilizumab use, specially the work of a multidisciplinary team that evaluated each patient in detail. Our team analyzed the pathophysiological rationality and the accumulated evidence and established criteria to consider the use of tocilizumab that considered the presence of an uncontrolled inflammatory response. Even when the patients met the established criteria, there was a group discussion weighing the risks and possible benefits before suggesting the use of the drug. Furthermore, we describe the use of tocilizumab together with high doses of CS, we detail the infections, adverse events, and deaths, and we analyze the variables associated with a better hospital stay. The provided data is useful to improve patient selection, trying to minimize risks and optimize efficacy.
Our study suggests the utility and shows the safety of tocilizumab use in COVID-19 patients who have respiratory failure and evidence of CRS. Lymphocyte improvement after using tocilizumab was the best predictor of good response to tocilizumab.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Scientific Ethics Committee of the Clínica Alemana-Universidad del Desarrollo medicine faculty prior to data collection. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SI perfomed the data analysis. All authors contributed equally with literature search, study design, data collection, data interpretation, and writing.
Conflict of Interest
OV reports personal fees from Bristol, personal fees from Abbvie, personal fees from Novartis, personal fees from Janssen, outside the submitted work. SI reports personal fees from Bristol, personal fees from Abbvie, personal fees from Novartis, non-financial support from Janssen, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank and acknowledge all of the staff of the COVID-19 unit at Clínica Alemana for their work and support in carrying out this project. MP wishes to thank FONDECYT 11181222 for its support.
References
1. He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol. (2020) 92:719–25. doi: 10.1002/jmv.25766
2. Cao X. COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol. (2020) 20:269–70. doi: 10.1038/s41577-020-0308-3
3. Sinha P, Matthay MA, Calfee CS. Is a “Cytokine Storm” relevant to COVID-19? JAMA Intern Med. (2020) 180:1152–4. doi: 10.1001/jamainternmed.2020.3313
4. Moore BJB, June CH. Cytokine release syndrome in severe COVID-19. Science. (2020) 368:473–4. doi: 10.1126/science.abb8925
5. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20:363–74. doi: 10.1038/s41577-020-0311-8
6. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. (2020) 39:405–7. doi: 10.1016/j.healun.2020.03.012
7. Zhang J, Xie B, Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav Immun. (2020) 87:59–73. doi: 10.1016/j.bbi.2020.04.046
8. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 323:1824–36. doi: 10.1001/jama.2020.6019
9. Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. (2020) 19:102567. doi: 10.1016/j.autrev.2020.102567
10. Sarzi-Puttini P, Giorgi V, Sirotti S, Marotto D, Ardizzone S, Rizzardini G, et al. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin Exp Rheumatol. (2020) 38:337–42.
11. Wu R, Wang L, Kuo HCD, Shannar A, Peter R, Chou PJ, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. (2020) 6:56–70. doi: 10.1007/s40495-020-00216-7
12. Antwi-Amoabeng D, Kanji Z, Ford B, Beutler BD, Riddle MS, Siddiqui F. Clinical outcomes in COVID-19 patients treated with tocilizumab: an individual patient data systematic review. J Med Virol. (2020) 92:2516–22. doi: 10.1002/jmv.26038
13. Coomes EA, Haghbayan H. Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev Med Virol. (2020) e2141. doi: 10.1101/2020.03.30.20048058
14. Le RQ, Li L, Yuan W, Shord SS, Nie L, Habtemariam BA, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist. (2018) 23:943–7. doi: 10.1634/theoncologist.2018-0028
15. Tian W, Jiang W, Yao J, Nicholson CJ, Li RH, Sigurslid HH, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. (2020) 92:1875–83. doi: 10.1002/jmv.26050
16. Ibáñez S, Martínez O, Valenzuela F, Silva F, Valenzuela O. Hydroxychloroquine and chloroquine in COVID-19: should they be used as standard therapy? Clin Rheumatol. (2020) 39:2461–5. doi: 10.1007/s10067-020-05202-4
17. Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. (2020) 34:1503–11. doi: 10.1038/s41375-020-0848-3
18. Group TRC. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. N Engl J Med. (2020). doi: 10.1056/nejmoa2021436. [Epub ahead of print].
19. Ramiro S, Mostard RLM, Magro-Checa C, Van Dongen CM, Dormans T, Buijs J, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. (2020) 79:1143–51. doi: 10.1136/annrheumdis-2020-218479
20. Huang I, Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. (2020) 8:36. doi: 10.1186/s40560-020-00453-4
21. Conrozier T, Lohse A, Balblanc JC, Dussert P, Royer PY, Bossert M, et al. Biomarker variation in patients successfully treated with tocilizumab for severe coronavirus disease 2019 (COVID-19): results of a multidisciplinary collaboration. Clin Exp Rheumatol. (2020) 38:742–7.
22. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
23. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, et al. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. (2020) 11:827. doi: 10.3389/fimmu.2020.00827
24. Mazzoni A, Salvati L, Maggi L, Capone M, Vanni A, Spinicci M, et al. Impaired immune cell cytotoxicity in severe COVID-19 is IL-6 dependent. J Clin Invest. (2020) 130:4694–703. doi: 10.1172/JCI138554
25. Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care Lond Engl. (2020) 24:154. doi: 10.1186/s13054-020-02880-z
Keywords: tocilizumab, COVID-19, SARS-CoV 2, lymphocyte, mechanical ventilalion
Citation: Valenzuela O, Ibáñez S, Poli MC, Roessler P, Aylwin M, Roizen G, Iruretagoyena M, Agar V, Donoso J, Fierro M and Montes J (2020) First Report of Tocilizumab Use in a Cohort of Latin American Patients Hospitalized for Severe COVID-19 Pneumonia. Front. Med. 7:596916. doi: 10.3389/fmed.2020.596916
Received: 20 August 2020; Accepted: 16 October 2020;
Published: 16 November 2020.
Edited by:
Monica Catarina Botelho, Instituto Nacional de Saúde Doutor Ricardo Jorge (INSA), PortugalReviewed by:
Annalisa Ruggeri, Bambino Gesù Ospedale Pediatrico, ItalyPeter Papadakos, University of Rochester, United States
Copyright © 2020 Valenzuela, Ibáñez, Poli, Roessler, Aylwin, Roizen, Iruretagoyena, Agar, Donoso, Fierro and Montes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastián Ibáñez, sibanez@alemana.cl
 Omar Valenzuela1,2
Omar Valenzuela1,2  Sebastián Ibáñez
Sebastián Ibáñez Mirentxu Iruretagoyena
Mirentxu Iruretagoyena