COVID-19 hemodynamic and thrombotic effect on the eye microcirculation after hospitalization: A quantitative case-control study
Abstract
BACKGROUND & OBJECTIVE:
To quantify the hemodynamic and thrombotic effect of COVID-19 on the eye microcirculation of patients with thromboprophylaxis, shortly after hospital discharge.
METHODS:
This case-control study included 17 COVID-19 survivors (named “COVID-19 Group”) and 17 healthy volunteers (named “Control Group”). Axial blood velocity (Vax) and percentage of occluded vessels (POV) were quantified by Conjunctival Video Capillaroscopy (CVC). Microvessels were identified and classified as “capillaries” (CAP), “postcapillary venules of size 1” (PC1), and “postcapillary venules of size 2” (PC2).
RESULTS:
The COVID-19 Group did not differ significantly in basic demographics from the Control Group. In the COVID-19 Group, there was a statistically significant (p < 0.001) reduction of Vax (39%, 49% and 47%, for CAP, PC1, and PC2, respectively) in comparison to the Control Group and a sizeable (p < 0.001) increase of POV (600%) in comparison to the Control Group.
CONCLUSIONS:
COVID-19 not only reduces significantly axial blood velocity in the capillaries and postcapillary venules of the eye but has also a devastating effect on microthrombosis (POV) despite thromboprophylaxis treatment. This gives a possible explanation for long COVID and a hint about the existence of a possibly unknown coagulation factor.
1Introduction
We are now in the third year of the COVID-19 pandemic and over 6.3 million deaths have been reported globally [1]. Apart from the high mortality of the COVID-19 disease, numerous symptoms have been observed in patients surviving COVID-19 and the term “long COVID syndrome” has been proposed [2, 3].
COVID-19 is not only a respiratory disease with diffuse alveolar damage [4] but also a multisystem thrombotic syndrome with many extrapulmonary manifestations in the visual, neuromuscular, cardiovascular, gastrointestinal, urinary and integumentary system [5, 6].
From the first year of the pandemic (2020) it became apparent that, in addition to the macrothrombotic events, the COVID-19 mediated endothelial injury would trigger coagulation mechanisms leading to widespread thrombotic microangiopathy [6–10]. Various mechanisms have been proposed for the cause of this microangiopathy, such as endothelial glycocalyx damage [11–13], endothelial cell derived microvesicles (MV) [14] or extracellular vesicles (EV) [15], endothelial exocytosis [16], immunothrombosis with neutrophil extracellular traps (NETs) [17] and degradation of endothelial junctional proteins [18]. Regardless of the underlying endothelial mechanism, the extensive microthrombosis [9] guided the clinical doctors to the proposition of tailored antithrombotic strategies [19].
In addition, from the classical interpretation of the Virchow’s triad, it is evident that microvascular blood flow plays a role in the thrombotic process and further investigation of hemodynamic aspects in COVID-19 was recently proposed [20]. Furthermore, the benefit on the microcirculation of using antithrombotics is still a matter of debate [21]. In order to assess such potential pharmacological benefits in the treatment of COVID-19 more quantitative data are needed from the human microcirculation in vivo.
In this context, the purpose of this work was to use Conjunctival Video Capillaroscopy (CVC) to quantify microvascular hemodynamics and thrombosis, for the first time, in the eyes of COVID-19 survivors receiving thromboprophylaxis.
CVC is currently an available noninvasive clinical imaging tool for the quantification of blood velocity in the eye microvessels with diameters less than 25μm and can be implemented in various imaging set-ups based on high magnification and high speed optical (slit lamp) biomicroscopy [22]. The measurement procedure is performed without tissue contact, the diameter can easily be measured and the venular side can be discriminated from the arteriolar side. Another noninvasive imaging tool, for measuring blood velocity in the smallest eye microvessels, is adaptive optics scanning laser ophthalmoscopy (AOSLO). Warner et al. measured temporal and spatial variations of blood velocity in the healthy human retina using dual beam AOSLO, without reporting capillary diameters [23] which correlate with velocity [24].
CVC hemodynamic metrics have been shown to correlate with hypertension [25], cardiovascular risk [26], diabetic microvasculopathy [27], sickle cell retinopathy [28], acute myocardial infarction [29], oral contraceptives [30] and the administration of medical supplements [31].
2Methods
2.1Study design
This observational, fully quantitative, case-control study was based on measurements from images acquired at the University Hospital of Larissa from April 2021 to February 2022. The study was approved from the University Hospital of Larissa Scientific Committee (11155/2021), and the research was performed according to the Declaration of Helsinki. Informed consent was obtained from all participants in the study.
2.2Subjects
Microvascular images of the temporal side of the bulbar conjunctiva were taken from the right eye of 17 normal volunteers (named “Control Group”) and 17 COVID-19 survivors shortly after exiting the hospital (named “COVID-19 Group”).
All normal volunteers of the Control Group had no systemic or ocular disease, no alcohol or smoking habit, were not under any medication and had never been tested positive for SARS-CoV-2.
Inclusion criteria for the COVID-19 Group were hospitalization for COVID-19, oxygen therapy, Acute Respiratory Distress Syndrome (ARDS) with HI (Horowitz Index) less than 300 mmHg and a 28-day maximum time interval between hospital exit and image recording (duration from discharge). Exclusion criterion for the COVID-19 Group was rheumatic disease.
Clinical data for both groups, including diastolic and systolic arterial pressure (DP and SP), body height and body weight and cardiac beats per minute (BPM) were measured on site before and after image recording. Subjects with diastolic blood pressure greater than 95 mmHg were excluded from the study (both groups) as hypertensive. Body mass index (BMI) and body surface area (BSA, Du Bois formula) were calculated from the body height and weight and mean arterial pressure (MP) was calculated from DP and SP (MP = DP + (SP-DP)/3). Image recordings were performed in a temperature controlled environment (21 to 23°C) after waiting 30 minutes for adaptation.
2.3Imaging setup
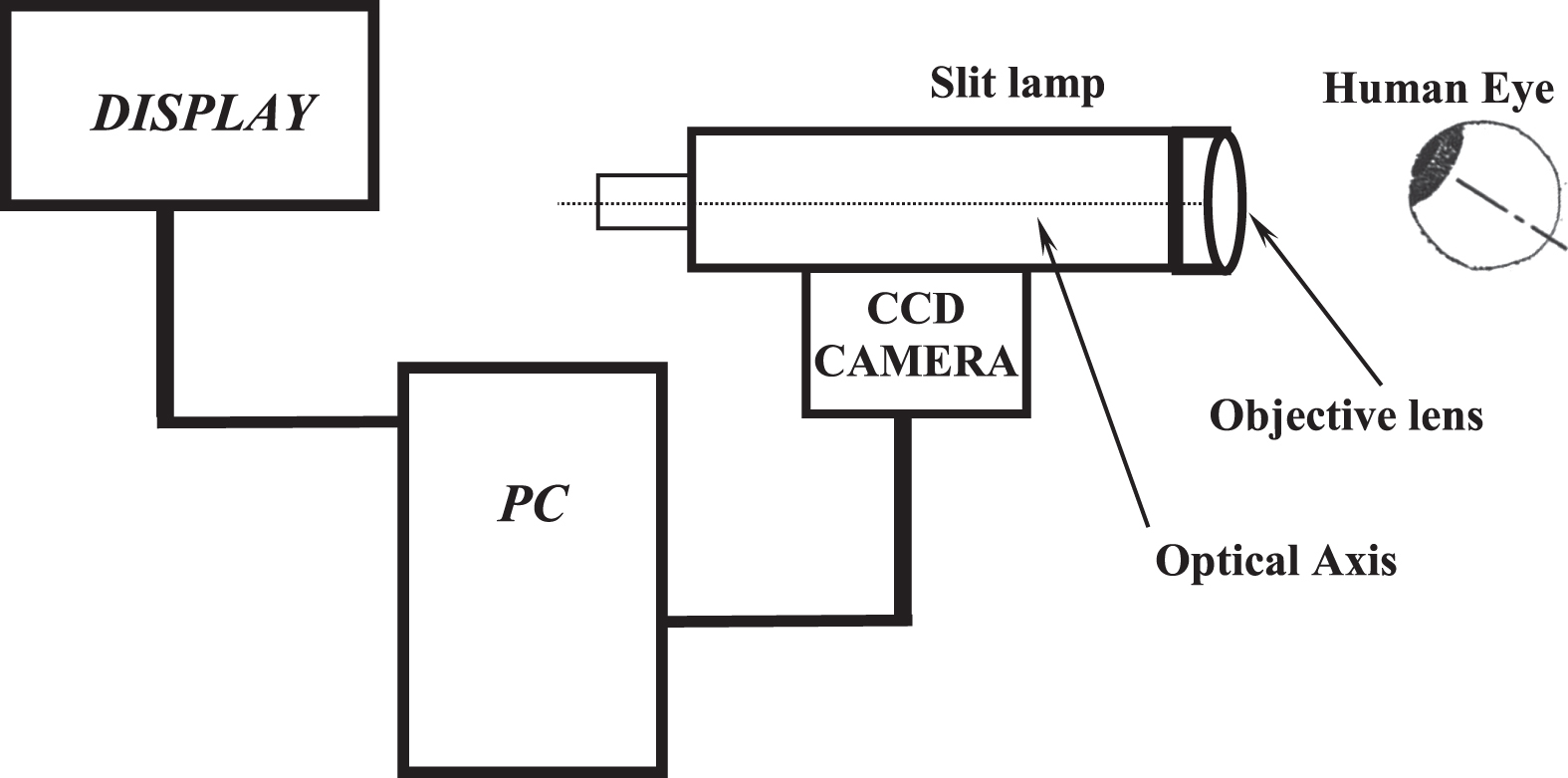
The imaging setup (Fig. 1) consisted of a special slit lamp (Nikon FS-3V), a high-speed CCD camera (12 bit, PCO Computer Optics GmbH, Germany) and a personal computer (Pentium 4, 3 GHz). The imaging system [32] with an enhanced maximum magnification of 242× and a digital resolution of 1.257±0.004μm/pixel produced digital images of 320×240 pixels at a high frame rate of 96 frames per second.
Fig. 1
The imaging set up.
2.4Hemodynamics
The internal diameter (D) of each microvessel was estimated from the recorded image using the Pythagoras’s theorem on the coordinates of the intersection points between a vertical line to the vessel axis and the outer limits of the erythrocyte column [32]. Axial erythrocyte velocity (Vax) of each microvessel was measured using the axial distance DC travelled by a red blood cell or a plasma gap, over a fixed time interval Δt which was known from the frame rate of the camera as equal to 10.04 ms.
2.5Percentage of occluded vessels (POV)
The percentage of occluded vessels (POV) was introduced in this work as the ratio of the number of occluded vessels (No) over the total number of vessels (NT), in percent (%), at a given microvascular field of view:
POV was defined in microvascular fields comprising at least 4 microvessels in total. A microvessel was considered as occluded when the flow inside was completely stopped or when blood velocity Vax was less than 0.1 mm/s [22]. The POV is a microvascular parameter complementary to the proportion of perfused vessels (PPV) [33] and it is an easily measurable index of thrombotic microangiopathy proportional to the microvascular damage sustained by a tissue.
2.6Statistical analysis
The Microsoft Office EXCEL 2016 (professional edition) and the SOFA (version 1.4, Paton-Simpson & Associates Ltd) software were used for statistics.
In order to take into account the effect of microvessel diameter on microvascular blood flow velocity [24] the diameters (D) of 9 and 14μm, were selected as size discriminators, for classifying microvessels of D < 9μm as “capillaries” (CAP), of 9≤D < 14μm as “postcapillary venules of size 1” (PC1) and of 14≤D≤24μm as “postcapillary venules of size 2” (PC2). Measurements were taken only from the venular side of the microvascular bed to avoid the effects of the pre-capillary arteriolar pulse [34].
To cover all variable distributions, normal or not, the differences between groups were examined using the Mann-Whitney U test. The significance level was set at 0.05.
3Results
More than 230 thousand digital images of the conjunctival microvasculature were recorded and then processed manually to take measurements from 683 capillaries and postcapillary venules.
3.1Baseline characteristics
In Table 1 there is a summary of the demographic and clinical data for the 2 groups. There was no statistically significant difference in age, body mass index (BMI), body surface area (BSA), cardiac beats per minute (BPM) and arterial mean pressure (MP) between the 2 groups. In each group, the changes in BPM and MP before and after the recording procedure were not significant.
Table 1
Demographic and clinical data of the Control Group and COVID-19 Group
| Variable | Control group | COVID-19 group | p value |
| N | 17 | 17 | – |
| Age (years) | 53±9 | 57±7 | >0.06 |
| BMI (kgr/m2) | 27±3 | 28±5 | >0.25 |
| BSA (m2) | 1.9±0.2 | 2.0±0.2 | >0.06 |
| BPM | 68±6 | 65±11 | >0.44 |
| MP (mmHg) | 91±8 | 94±9 | >0.59 |
N = number of subjects, BMI = Body Mass Index, BSA = Body Surface Area (Du Bois), BPM = cardiac Beats Per Minute, MP = arterial Mean Pressure. Data are expressed as Mean±Standard deviation. There was no significant difference in age, BMI, BSA, BPM and MP between groups.
All patients had pneumonia (different lung extension and distribution) upon admission (severe COVID-19 disease). During hospitalization and after 48 hours, all COVID-19 patients needed oxygen therapy with High Flow Nasal Cannula (HFNC) with settings adjusted on the patient’s requirements. Concerning pharmacological agents during hospitalization, 15 (88%) patients received remdesivir (an inhibitor of the viral RNA-dependent, RNA polymerase) since these patients were in the early disease phase (2–4 days with symptoms) and required low-flow oxygen supply upon admission. 15 patients (88%) received tocilizumab (a humanized monoclonal antibody against the interleukin-6 receptor) because of rapid clinical deterioration and high levels of C-reactive protein and ferritin. It is worth mentioning that the other 2 patients refused to receive tolicizumab although there was a clear indication. In addition, 15 (88%) patients received Low Molecular Weight Heparin (LMWH) in thromboembolism prophylaxis dosage, while 3 of them (18%) received a full dose of enoxaparin since they developed atrial fibrillation and a high CHADS2 (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Stroke [double weight]) score during hospitalization, and 2 (11%) of them were also on antiplatelet therapy with acetylsalicylic acid (ASA) because of coronary artery disease.
After hospital discharge, all patients had extensive radiological findings due to previous ARDS, with deteriorated pulmonary function. Nine patients (50%) needed oxygen therapy at home for at least one month and all patients were on antithrombotic prophylaxis until they were fully mobilized (LMWH is recommended up to 40 days in severe and critically ill patients after discharge), while 2 (11%) of them continued their chronic medication with acetylsalicylic acid (ASA). Those 3 patients with atrial fibrillation who were receiving a therapeutic dose of LMWH continued their antithrombotic therapy after discharge with a full dose of rivaroxaban. In Table 2 there is a summary of the COVID-19 Group clinical and pharmacological data during and after hospitalization.
Table 2
Clinical and pharmacological data of the COVID-19 Group (17 subjects) during hospitalization and after hospitalization
| Variable | COVID-19 group | COVID-19 group |
| During Hospitalization | After Hospitalization | |
| HI (mmHg) | 139±55 | – |
| Oxygen therapy, n (%) | 17 (100%) | 9 (50%) |
| ICU, n (%) | 2 (12%) | – |
| Antiviral therapy (remdesivir), n (%) | 15 (88%) | – |
| Anti-IL6R (tocilizumab), n (%) | 15 (88%) | – |
| AP, n (%) | 15 * (88%) | 17 * (100%) |
| ASA, n (%) | 2 (11%) | 2 (11%) |
| Duration from discharge (days) | – | 9±8 |
Data are expressed as Mean±Standard deviation or as number of subjects (n) and the corresponding percentage (%). Five subjects in the COVID-19 Group were diabetic (29%) and 3 subjects were smokers (18%). HI = Horowitz Index, ICU = Intensive Care Unit, IL6R = InterLeukine-6 Receptor, AP = Antithrombotic Prophylaxis (* 3 of those patients developed atrial fibrillation and received full therapeutic dose of enoxaparin during hospitalization and rivaroxaban after hospitalization), ASA = AcetylSalicylic Acid.
3.2Effect of COVID-19 on axial blood velocity
Microvessel diameters (D) ranged from 4.5 to 24.4μm in the Control Group and from 4 to 24μm in the COVID-19 Group. A view of the velocity measurements in the capillaries (CAP, diameters less than 9μm) of both groups is shown in Fig. 2.
The diameter averages and 95% confidence intervals (95CI) for all groups and microvessel sizes are shown in Fig. 3(α). For all microvessel sizes (CAP, PC1 and PC2) there was no statistical difference in diameter between groups.
Fig. 2
A view of the velocity measurements in the capillaries (CAP) of both groups. Blood axial velocity (Vax) measurements are shown in black dots and X marks for the Control Group and COVID-19 Group, respectively. The difference was statistically significant (p < 0.001).
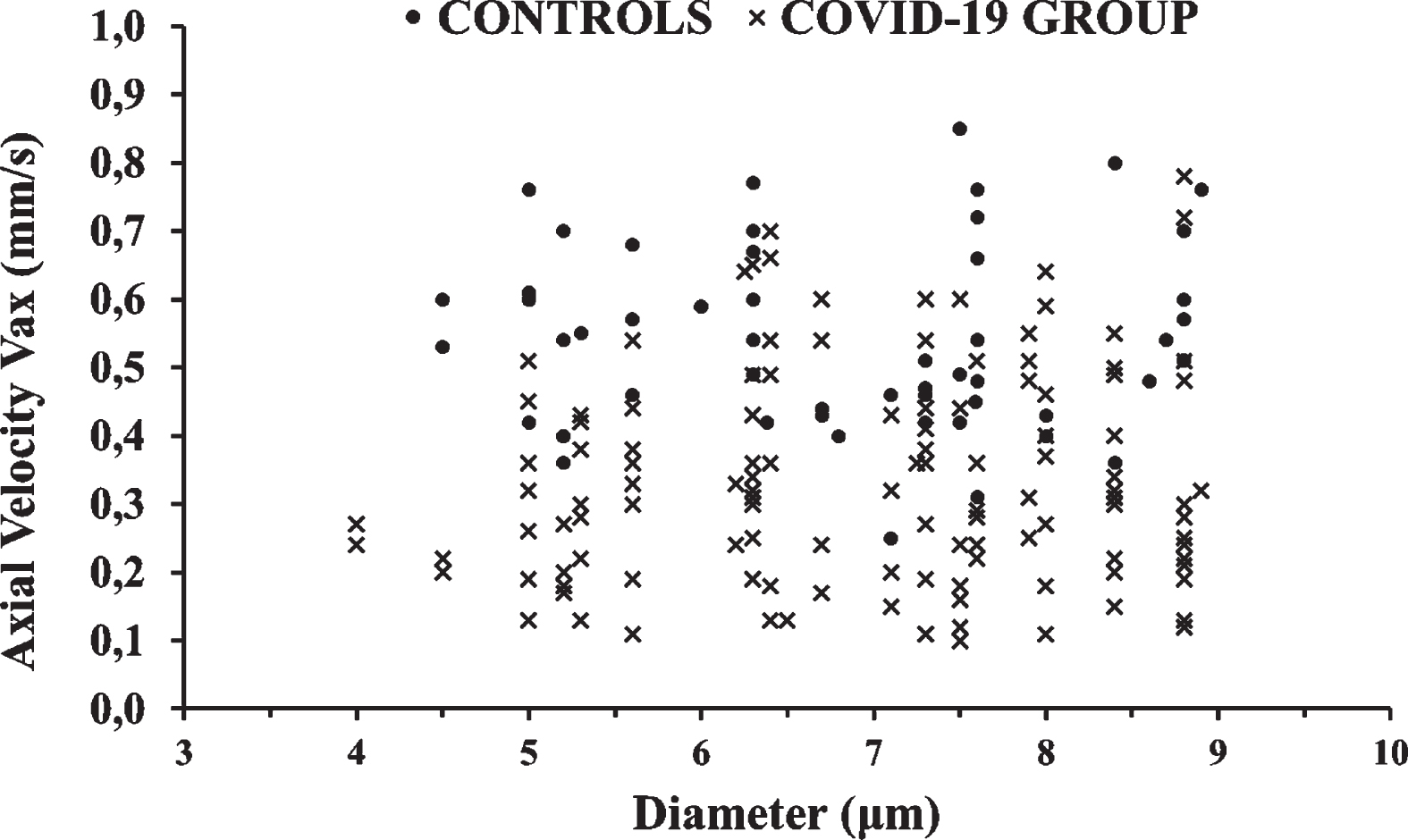
Fig. 3
The Control Group and COVID-19 Group results are shown in white and gray columns, respectively. Column heights are mean values and black bars represent the 95% confidence interval of the mean. (a) There was no statistical difference in diameter D between groups, for capillaries (CAP), postcapillary venules of size 1 (PC1) and postcapillary venules of size 2 (PC2). (b) The average COVID-19 Group axial velocity (Vax) was 39%, 47% and 49% lower than the average Control Group Vax, in the capillaries (CAP), postcapillary venules of size 1 (PC1) and postcapillary venules of size 2 (PC2), respectively (*P < 0.001).
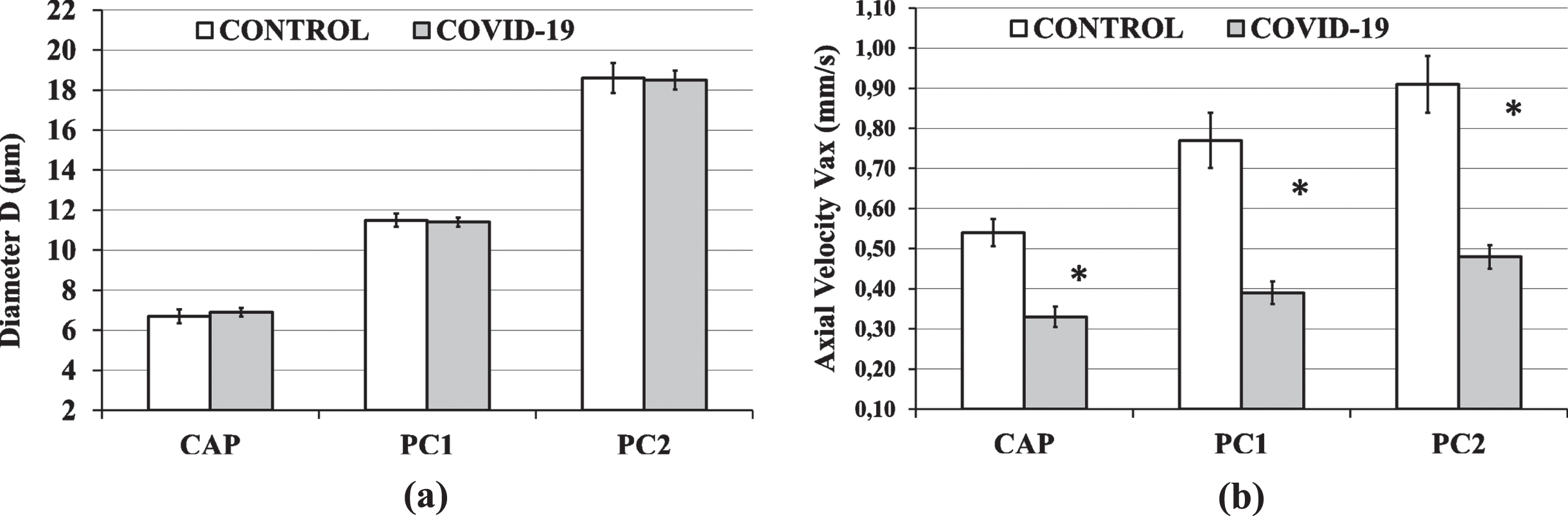
Microvessel axial blood velocities (Vax) ranged from 0.25 to 1.68 mm/s in the Control Group and from 0.10 to 0.99 mm/s in the COVID-19 Group. Descriptive statistics of Vax in the capillaries (CAP), postcapillary venules of size 1 (PC1) and postcapillary venules of size 2 (PC2) for both groups are shown in Table 3. For all microvessel sizes, the Vax in the COVID-19 Group was significantly lower (p < 0.001) than the Vax in the Control Group. In more detail, the average COVID-19 Group axial velocity (Vax) was 39%, 47% and 49% lower than the average Control Group Vax, in the capillaries (CAP), postcapillary venules of size 1 (PC1) and postcapillary venules of size 2 (PC2), respectively. The CAP, PC1 and PC2 velocity averages and 95% confidence intervals (95CI) for both groups are shown in Fig. 3(b).
Table 3
Statistics of axial blood velocity (Vax) in the capillaries (CAP), postcapillary venules of size 1 (PC1) and postcapillary venules of size 2 (PC2) of healthy subjects (Control Group) and COVID-19 patients after discharge with thromboprophylaxis (COVID-19 Group)
| Statistical | Vax (mm/s) | |||||
| parameter | CAP | PC1 | PC2 | |||
| Control group | COVID-19 group | Control group | COVID-19 group | Control group | COVID-19 group | |
| NM | 57 | 132 | 69 | 160 | 69 | 196 |
| Mean±SD | 0.54±0.13 | 0.33±0.15 | 0.77±0.29 | 0.39±0.18 | 0.91±0.30 | 0.48±0.21 |
| Median | 0.54 | 0.31 | 0.73 | 0.36 | 0.89 | 0.48 |
NM is the number of measurements for each group with each measurement corresponding to a different microvessel. SD is standard deviation. Vax in the COVID-19 Group was significantly lower (p < 0.001) than Vax in the Control Group at all microvessel classes (CAP, PC1 and PC2).
3.3Effect of COVID-19 on microvessel occlusion
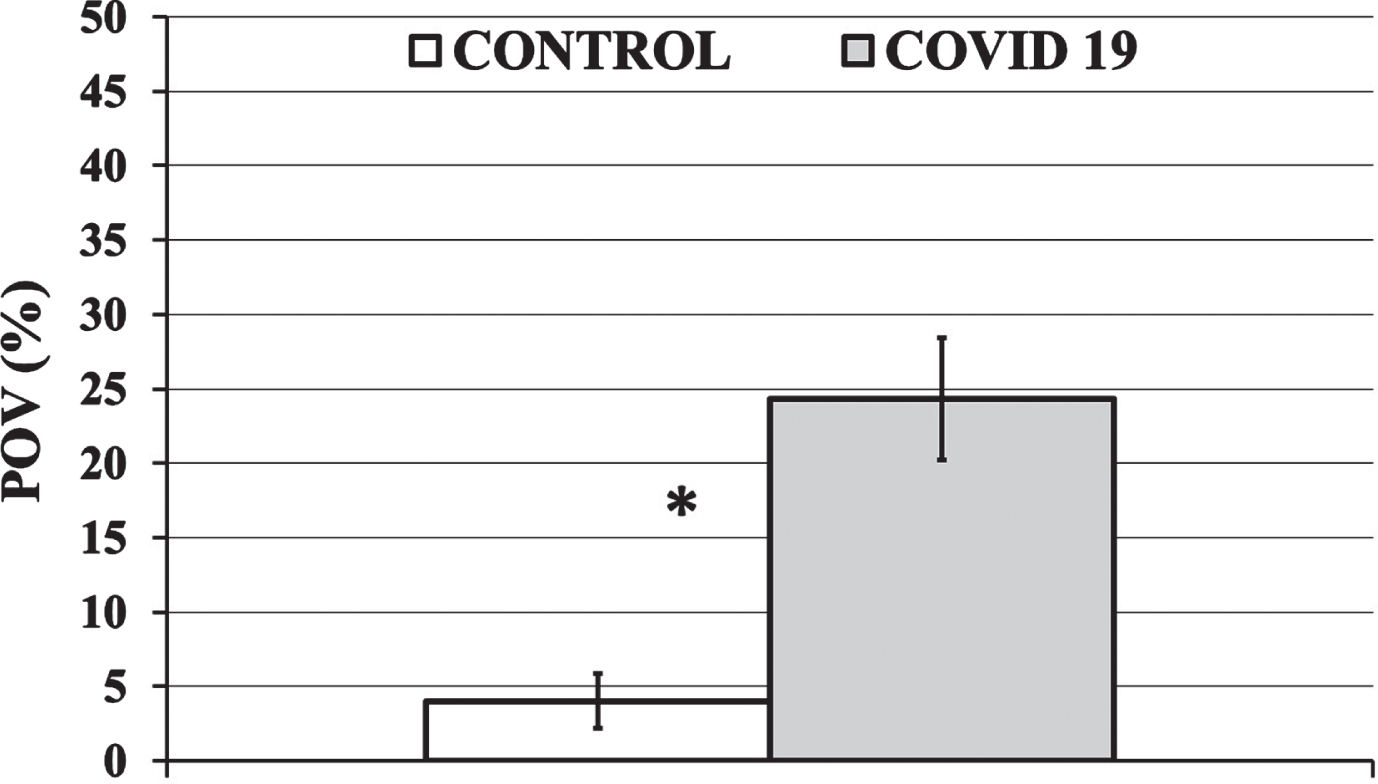
An example of an occluded microvessel is shown in Fig. 4. The percentage of occluded vessels (POV) ranged from 0 to 43% in the Control Group and from 0 to 100% in the COVID-19 Group. The POV in the Control Group was significantly lower (p < 0.001) than the POV in the COVID-19 Group (Fig. 5). In more detail, the average COVID-19 Group POV (24±25% (Average±SD)) was 600% higher than the average Control Group POV (4±8%). The median POV value was 18% for the COVID-19 Group and 0% for the Control Group.
Fig. 4
An example of an occluded microvessel (black arrow). Two images of the same microvessel were taken at different instances. It is clear that, even though image (a) was taken 146 ms earlier than image (b), the red blood cell column has not advanced and that blood flow has stopped.
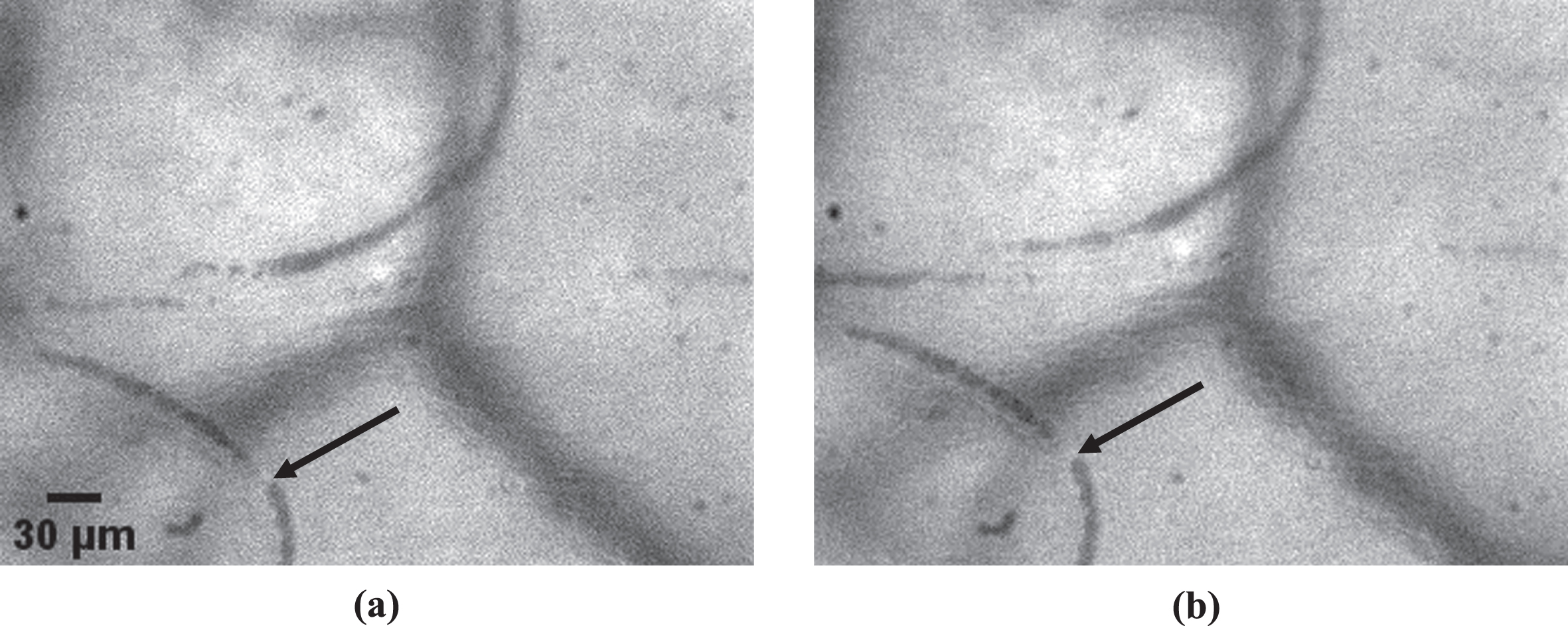
Fig. 5
The Percentage of Occluded Vessels (POV) for the Control and COVID-19 Group is shown in a white and gray column, respectively. Average values are presented as column heights and black bars represent the 95% Confidence Interval (95CI) of the average value. The average POV of the COVID-19 Group was 6 times (600%) higher than that of the Control Group (*P < 0.001).
4Discussion
The outbreak of the coronavirus disease (COVID-19) at the end of 2019 and the subsequent pandemic is a historic landmark for challenging the public health system worldwide. The cause of the disease is an RNA beta coronavirus (SARS-CoV-2) which infects endothelial cells after binding to the angiotensin converting enzyme 2 (ACE2) receptor and entering the cell interior with the help of the transmembrane protease serine type 2 (TMPRSS2). Then a cascade of events may follow, depending on the subject, including endothelial cell activation and dysfunction [13, 14, 35], diffuse alveolar damage, increased blood viscosity [36, 37], hypercoagulation, thrombotic microangiopathy, acute respiratory distress syndrome (ARDS), local and systemic hypoxia, and death.
It is evident that the endothelium has a pivotal role in the development of the COVID-19 and this correlates to its essential part for normal coagulation function after triggering the intrinsic and/or the extrinsic pathway [35] depending on the conditions. COVID-19 causes imbalance in various coagulation related blood substances [38–41]. The injured endothelium in an interplay with the other two components of the Virchow’s triad namely, abnormal hemodynamics and hypercoagulability [42], induces extensive microthrombosis [9].
However, clinical trials have not shown whether antithrombotics reduce microthrombosis [21]. Lodigiani et al. [43] reported a higher rate of venous thromboembolic events in COVID-19 patients than in non-COVID patients despite anticoagulant prophylaxis for both groups. Kelliher et al. reported a high incidence of thrombosis despite thromboprophylaxis in COVID-19 patients during hospitalization [44]. In a recent clinical trial [45] therapeutic doses of LMWH reduced major thromboembolism and death compared with standard heparin thromboprophylaxis among inpatients with COVID-19. In another clinical trial [46] with COVID-19 patients after hospitalization, thromboprophylaxis with rivaroxaban improved clinical outcome. In general, there is great variability in the anticoagulation strategy in COVID-19 [47]. Currently high-quality data regarding microthrombotic status and microvascular hemodynamics of COVID-19 patients in vivo after hospital discharge are rare.
In our case-control study, we quantified microvascular hemodynamics and microvascular thrombosis, in the conjunctival microcirculation in vivo, in COVID-19 patients shortly after hospitalization (Table 2) and compared the results to those of a Control Group.
Regarding microvascular hemodynamics, the axial blood velocity (Vax) of the COVID-19 Group was remarkably low with an average Vax of 0.33 mm/s in the capillaries and 0.39 mm/s in the postcapillary venules of size 1 (Table 3). The COVID-19 lower Vax of this work supports a recently published sophisticated simulation [48] according to which, when the initial microvascular blood flow velocity is lower than 0.30 mm/s the white blood cells (WBCs) attach to the thrombus. These simulation results [48] indicated that WBCs could directly participate in the microthrombus growth and, provided a mechanistic rationale for microthrombosis in COVID-19 patients.
Regarding microvascular thrombosis in the COVID-19 Group, POV was impressively higher (600%) than that in the Control Group (Fig. 5), despite thromboprophylaxis. This result sets serious questions regarding the efficiency of thromboprophylactic doses for COVID-19 patients at the microvascular level. Low Molecular Weight Heparin (LMWH), binds to and potentiates antithrombin (a circulating anticoagulant) to form a complex that irreversibly inactivates clotting factor Xa in the common pathway of coagulation. The high degree of microthrombosis in our work, despite thromboprophylaxis, indicates that perhaps there is an unknown coagulation factor XC (C from COVID) that circumvents the action of common pathway anticoagulants and promotes coagulation. This unknown factor XC could be related to one of the mechanisms that were proposed as the cause of the microvascular damage such as degradation of endothelial junctional proteins [18] and glycocalyx [11–13], endothelial cell derived microvesicles (MV) [14], endothelial exocytosis [16] and, immunothrombosis with NETs [17].
It is now well known that some people cannot fully recover after COVID-19 disease, presenting long-term symptoms which are usually named “long-COVID-19”, “long COVID syndrome”, “post-COVID-19 condition”, or “post-acute COVID-19 syndrome” (PACS) [2, 3, 49]. Huang et al. reported that 76% of 1733 COVID-19 patients discharged from hospital had at least one symptom 6 months after the acute infection, with most common symptoms being fatigue, muscle weakness, sleep difficulties and anxiety or depression [50]. There were similar reports for milder cases of COVID-19 without hospitalization [2], where 61% of patients older than 46 years had persistent symptoms at a 6-month follow-up. When the NICE guideline was published on long COVID it was clear that gaps in our knowledge regarding long COVID-19 symptom etiology remained considerable [3]. The results of our work contribute to this “knowledge gap” and, with the reported reduced blood flow and therefore oxygen supply, give a possible explanation for the long COVID-19 symptoms. The underline assumption for such an explanation would be that similar microvascular abnormalities exist in other tissues and systems, a plausible premise considering the numerous extrapulmonary manifestations of microvascular damage due to COVID-19 [6, 7, 8].
A limitation of this observational study was the small sample size. However, the differences between the groups were so high that the results are statistically significant at a high level with 80% statistical power.
5Conclusion
It was found that COVID-19 reduces significantly axial blood velocity in all conjunctival capillaries and postcapillary venules with a diameter of less than 24μm and increases substantially microvessel occlusion percentage, even after hospital discharge and thromboprophylaxis. The scientific explanation of this effect remains unknown but the results give a possible explanation for long COVID and a hint about the existence of a possibly unknown coagulation factor. Our results contribute to the accumulation of quantitative knowledge regarding microvascular status in COVID-19 patients with thromboprophylaxis, in vivo, after hospitalization.
Declaration of competing interest
The authors have declared no conflicts of interest.
Funding
No specific funding was received from any funding bodies in the public, commercial or not for-profit sectors to carry out the work described in this manuscript.
References
[1] | WHO (World Health Organization). COVID-19 Weekly Epidemiological Update. Edition 97, published 22 June 2022. |
[2] | Blomberg B , Mohn KG , Brokstad KA , Zhou F , Linchausen DW , Hansen BA , et al., Long COVID in a prospective cohort of home-isolated patients, Nat Med (2021) ;27: (9):1607–1613. doi: 10.1038/s41591-021-01433-3. |
[3] | Venkatesan P NICE guideline on long COVID, Lancet Respir Med (2021) ;9: (2):129. doi: 10.1016/S2213-2600(21)00031-X. |
[4] | Satturwar S , Fowkes M , Farver C , Wil son AM , Eccher A , Girolami I , et al., Postmortem findings associated with SARS-CoV- Systematicreview and meta-analysis, Am J Surg Pathol (2021) ;45: (5):587–603. doi: 10.1097/PAS.0000000000001650. |
[5] | Haberecker M , Schwarz EI , Steiger P , Frontzek K , Scholkmann F , Zeng X , et al., Autopsy-based pulmonary and vascular pathology: Pulmonary endotheliitis and multi-organ involvement in COVID-19 associated deaths, Respiration (2022) ;101: (2):155–165. doi: 10.1159/000518914. |
[6] | Gupta A , Madhavan MV , Sehgal K , Nair N , Mahajan S , Sehrawat TS , et al., Extrapulmonary manifestations of COVID-19, Nat Med.- (2020) ;26: (7):1017–1032. doi: 10.1038/s41591-020-0968-3. |
[7] | Ackermann M , Verleden SE , Kuehnel M , Haverich A , Welte T , Laenger F , Vanstapel A , et al., Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19, N Engl J Med (2020) ;383: (2):120–128. doi: 10.1056/NEJMoa2015432. |
[8] | Masi P , Hékimian G , Lejeune M , Chommeloux J , Desnos C , PinetonDe Chambrun M , et al., Systemic inflammatory response syndrome is amajor contributor to COVID-19-associated coagulopathy: Insights froma prospective, single-center cohort study, Circulation (2020) ;142: (6):611–614. doi: 10.1161/CIRCULATIONAHA.120.048925. |
[9] | Jung EM , Stroszczynski C , Jung F , Contrast enhanced ultrasonography (CEUS) to detect abdominal microcirculatory disorders in severe cases of COVID-19 infection: First experience, Clin Hemorheol Microcirc (2020) ;74: (4):353–361. doi: 10.3233/CH-209003. |
[10] | Martini R , The compelling arguments for the need of microvascular investigation in COVID-19 critical patients, Clin Hemorheol Microcirc (2020) ;75: (1):27–34. doi: 10.3233/CH-200895. |
[11] | Yamaoka-Tojo M , Vascular endothelial glycocalyx damage in COVID-19, Int J Mol Sci (2020) ;21: (24):9712. doi: 10.3390/ijms21249712. |
[12] | Rovas A , Osiaevi I , Buscher K , Sackarnd J , Tepasse PR , Fobker M , et al., Microvascular dysfunction in COVID- The MYSTIC study, Angiogenesis (2021) ;24: (1):145–157. doi: 10.1007/s10456-020-09753-7. |
[13] | Astapenko D , Tomasova A , Ticha A , Hyspler R , Chua HS , Manzoor M , et al., Endothelial glycocalyx damage in patients with severe COVID-19 on mechanical ventilation - A prospective observational pilot study, Clin Hemorheol Microcirc (2022) ;81: (3):205–219. doi: 10.3233/CH-221401. |
[14] | Jung F , Krüger-Genge A , Franke RP , Hufert F , Küpper JH , COVID-19 and the endothelium, Clin Hemorheol Microcirc (2020) ;75: (1):7–11. doi: 10.3233/CH-209007. |
[15] | Inal J , Complement-mediated Extracellular Vesicle release as a measure of endothelial dysfunction and prognostic marker for COVID-19 in peripheral blood - Letter to the Editor, Clin Hemorheol Microcirc (2020) ;75: (4):383–386. doi: 10.3233/CH-200958. |
[16] | Lowenstein CJ , Solomon SD , Severe COVID-19 is a microvascular disease, Circulation (2020) ;142: (17):1609–1611. doi: 10.1161/CIRCULATIONAHA.120.050354. |
[17] | Bonaventura A , Vecchié A , Dagna L , Martinod K , Dixon DL , VanTassell BW , et al., Endothelial dysfunction and immunothrombosis askey pathogenic mechanisms in COVID-19, Nat Rev Immunol (2021) ;21: (5):319–329. doi: 10.1038/s41577-021-00536-9. |
[18] | Raghavan S , Kenchappa DB , Leo MD . SARS-CoV-2 spike protein induces degradation of junctional proteins that maintain endothelial barrier integrity, Front Cardiovasc Med (2021) ;8: :687783. doi: 10.3389/fcvm.2021.687783. |
[19] | Pellegrini D , Kawakami R , Guagliumi G , Sakamoto A , Kawai K , Gianatti A , et al., Microthrombi as a major cause of cardiac injury in COVID- A pathologic study, Circulation.- (2021) ;143: (10):1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. |
[20] | Sastry S , Cuomo F , Muthusamy J , COVID-19 and thrombosis: The role ofhemodynamics, Thromb Res.51-57 (2022) ;212: –10.1016/j.thromres.2022.02.016. |
[21] | Spyropoulos AC , Bonaca MP , Studying the coagulopathy of COVID-19, Lancet (2022) ):399: (10320)::118–119. doi:10.1016/S0140-6736(21)01906-1. |
[22] | Koutsiaris AG , Meta-analysis of conjunctival microvascular hemorheology metrics, Microvasc Res (2022) ;142: :691043. doi: 10.1016/j.mvr.2022.104369. |
[23] | Warner RL , Gast TJ , Sapoznik KA , Carmichael-Martins A , Burns SA , Measuring temporal and spatial variability of red blood cell velocity in human retinal vessels, Invest Ophthalmol Vis Sci (2021) ;62: (14):29. doi: 10.1167/iovs.62.14.29. |
[24] | Koutsiaris AG , Correlation of axial blood velocity to venular and arteriolar diameter in the human eye in vivo, Clin Hemorheol Microcirc (2015) ;61: (3):429–38. doi: 10.3233/CH-141888. |
[25] | Jung F , Pindur G , Ohlmann P , Spitzer G , Sternitzky R , Franke RP , et al., Microcirculation in hypertensive patients, Biorheology (2013) ;50: (5-6):241–255. doi: 10.3233/BIR-130645. |
[26] | Karanam VC , Tamariz L , Batawi H , Wang J , Galor A , Functional slit lamp biomicroscopy metrics correlate with cardiovascular risk, The Ocular Surface (2019) ;17: :64–69. doi:10.1016/j.jtos.2018.09.002. |
[27] | Khansari MM , Wanek J , Tan M , Joslin CE , Kresovich JK , Camardo N , Assessment of conjunctival microvascular hemodynamics in stages of diabetic microvasculopathy, Sci Rep (2022) ;7: :6. doi: 10.1038/srep45916. |
[28] | Kord Valeshabad A , Wanek J , Zelkha R , Lim JI , Camardo N , Gaynes B , et al., Conjunctival microvascular haemodynamics in sickle cell retinopathy, Acta Ophthalmol (2015) ;93: (4):e275–80. doi: 10.1111/aos.12593. |
[29] | Brennan PF , McNeil AJ , Jing M , Awuah A , Moore JS , Mailey J , et al., Assessment of the conjunctival microcirculation for patients presenting with acute myocardial infarction compared to healthy controls, Sci Rep (2021) ;11: (1):7660. doi: 10.1038/s41598-021-87315-7. |
[30] | Arend O , Wolf S , Harris A , Jung F , Reim M , Effects of oral contraceptives on conjunctival microcirculation, Clin Hemorheol Microcirc (1993) ;13: (4):435–445. doi: 10.3233/CH-1993-13402. |
[31] | Liu Z , Jiang H , Townsend JH , Wang J , Improved conjunctival microcirculation in diabetic retinopathy patients with MTHFR polymorphisms after Ocufolintrademark Administration, Microvasc Res (2020) ;132: :10406610.1016/j.mvr.2020.104066. |
[32] | Koutsiaris AG , Tachmitzi SV , Batis N , Kotoula MG , Karabatsas CH , Tsironi E , et al. Volume flow and wall shear stress quantification in the human conjunctival capillaries and post-capillary venules in vivo , Biorheology (2007) ;44: (5-6):375–86. |
[33] | Massey MJ , Shapiro NI , A guide to human in vivo microcirculatory flow image analysis, Crit Care (2016) ;20: :35. doi: 10.1186/s13054-016-1213-9. |
[34] | Koutsiaris AG , Tachmitzi SV , Papavasileiou P , Batis N , Kotoula MG , Giannoukas AD , et al., Blood velocity pulse quantification in the human conjunctival pre-capillary arterioles, Microvasc Res (2010) ;80: :202–208. doi:10.1016/j.mvr.2010.05.001. |
[35] | Chen AT , Wang CY , Zhu WL , Chen W , Coagulation disorders and thrombosis in COVID-19 patients and a possible mechanism involving endothelial cells: A review, Aging Dis (2022) ;13: (1):144–156. doi: 10.14336/AD.2021.0704. |
[36] | Nader E , Nougier C , Boisson C , Poutrel S , Catella J , Martin F , et al., Increased blood viscosity and red blood cell aggregation in patients with COVID-19, Am J Hematol (2022) ;97: (3):283–292. doi: 10.1002/ajh.26440. |
[37] | Shaik A , Chen Q , Mar P , Kim H , Mejia P , Pacheco H , et al., Blood hyperviscosity in acute and recent COVID-19 infection. Clin Hemorheol Microcirc. 2022. doi: 10.3233/CH-221429. |
[38] | Alzoughool F , Alanagreh L , Abumweis S , Atoum M , Cerebrovascular comorbidity, high blood levels of C-reactive protein and D-dimer are associated with disease outcomes in COVID-19 patients, Clin Hemorheol Mi crocirc (2021) ;77: (3):311–322. doi: 10.3233/CH-201002. |
[39] | Xing Y , Yang W , Jin Y , Wang C , Guan X ,. D-dimer daily continuous tendency predicts the short-term prognosis for COVID-19 independently: A retrospective study from Northeast China, Clin Hemorheol Microcirc (2021) ;79: (2):269–277. doi: 10.3233/CH-201071. |
[40] | Watson O , Pillai S , Howard M , Cezar-Zaldua J , Whitley J , Burgess B , et al., Impaired fibrinolysis in severe Covid-19 infection is detectable in early stages of the disease. Clin Hemorheol Microcirc. 2022. doi: 10.3233/CH-221491. |
[41] | George A , Deepika C , Mohan G , Rajendran V , Shastry S , Blarakrishnan JM , Rao S , ADAMTS13 factor deficiency in severe COVID-19 may not be immune mediated-report from a pilot study. Clin Hemorheol Microcirc. In press 2022. doi: 10.3233/CH-221514. |
[42] | Farber PL , Can erythrocytes behavior in microcirculation help the understanding the physiopathology and improve prevention and treatment for covid-19? Clin Hemorheol Microcirc (2021) ;78: (1):41–47. doi: 10.3233/CH-201082. |
[43] | Lodigiani C , Iapichino G , Carenzo L , Cecconi M , Ferrazzi P , Sebastian T , et al., Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy, Thromb Res (2020) ;191: :9–14. doi: 10.1016/j.thromres.2020.04.024. |
[44] | Kelliher S , Weiss L , Cullivan S , O’Rourke E , Murphy CA , Toolan S , et al., Non-severe COVID-19 is associated with endothelial damage and hypercoagulability despite pharmacological thromboprophylaxis, J Thromb Haemost (2022) ;20: (4):1008–1014. doi: 10.1111/jth.15660. |
[45] | Spyropoulos AC , Goldin M , Giannis D , Diab W , Wang J , Khanijo S , et al., Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID- The HEP-COVID randomized clinical trial, JAMA Intern Med.- (2021) ;181: (12):1612–1620. doi: 10.1001/jamainternmed.2021.6203. |
[46] | Ramacciotti E , Barile Agati L , Calderaro D , Aguiar VCR , Spyropoulos AC , de Oliveira CCC , et al., Rivaroxaban versus no anticoagulation for post-discharge thromboprophylaxis after hospitalisation for COVID-19 (MICHELLE): An open-label, multicentre, randomised, controlled trial, Lancet (2022) ;399: (10319):50–59. doi: 10.1016/S0140-6736(21)02392-8. |
[47] | Ngu S , Smith JK , Goldin M , Use of anticoagulants in COVID- A review, Am J Ther (2022) ;19: . doi: 10.1097/MJT.0000000000001515. |
[48] | Li H , Deng Y , Li Z , Dorken Gallastegi A , Mantzoros CS , Frydman GH , et al., Multiphysics and multiscale modeling of microthrombosis in COVID-19, PLoS Comput Biol. (2022) ;18: (3):e1009892. doi: 10.1371/journal.pcbi.1009892. |
[49] | Izquierdo-Pujol J , Moron-Lopez S , Dalmau J , Gonzalez-Aumatell A , Carreras-Abad C , Mendez M , et al., Post COVID-19 condition inchildren and adolescents: An emerging problem, Front Pediatr (2022) ;10: :894204. doi: 10.3389/fped.2022.894204. |
[50] | Huang C , Huang L , Wang Y , Li X , Ren L , Gu X , et al., 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study, Lancet (2021) ):397: (10270):220–232. doi:10.1016/S0140-6736(20)32656-8. |



