
Extracorporeal membrane oxygenation in patients with COVID-19: 1-year experience
Introduction
The rapid spread of coronavirus disease 2019 (COVID-19) dramatically affected international healthcare delivery with a high proportion of patients requiring respiratory support and intensive care unit (ICU) admission (1,2). Several studies reported high ICU mortality rates ranging from 31% to 42% (3). Acute respiratory distress syndrome (ARDS) is a common complication which contributed to the high mortality rates reported in previous studies (4). In some patients with severe ARDS or acute respiratory failure, conventional therapies (e.g., MV) are not successful and extracorporeal membrane oxygenation (ECMO) therapy is indicated (5,6). Even though ECMO is a complex therapy provided only by specialist centers with sufficient resources (7), the Extracorporeal Life Support Organization (ELSO) guidelines recommended its use in carefully selected patients (8). This recommendation was supported by an early analysis including 1,035 ECMO-supported patients with COVID-19 that showed reasonable results with a 90-day mortality of 37.4% (9). Furthermore, a meta-analysis including nearly 1900 patients reported a mortality rate of 37.1% (10). These findings were comparable to mortality rates in non-COVID-19 related ARDS patients (11).
A second wave of critically ill patients with COVID-19 arose in Germany after September 2020; however, literature on ECMO outcomes since the second pandemic wave is limited. Meanwhile, the updated guidelines published by the ELSO stated that overall mortality of patients with COVID-19 receiving ECMO may be increasing (12). Thus, there is a need for new analysis of ECMO therapy data, including admissions of patients with COVID-19 during the second epidemic wave.
Hence, we conducted a retrospective, single-center study to evaluate the characteristics, physiologic parameters, and outcomes of patients with COVID-19 who received ECMO therapy. The aim of this study was to: (I) describe our experience of ECMO therapy in patients with COVID-19 after 1 year of practice; and (II) compare the baseline characteristics and outcomes between patients of the first and second epidemic waves.
We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/jtd-21-971).
Methods
Patients and diagnosis of COVID-19
This single-center, retrospective study included all patients aged ≥18 years admitted to the hospital between March 1, 2020 and February 28, 2021, who were diagnosed with COVID-19 according to the World Health Organization interim guidance (6) and developed severe COVID-19 disease with ARDS requiring support through ECMO. ARDS was determined according to the 2011 Berlin Definition of the European Society of Intensive Care Medicine (13). Regarding ECMO types, we included patients receiving veno-venous (VV) and veno-arterial (VA) configurations.
Data collection
We collected data including demographics, medical history, time course of laboratory and MV parameters, and ECMO settings throughout the entire duration of hospital stay. We also gathered data regarding the amount of packed red blood cells (PRBC) and albumin units utilized during ECMO therapy. Of note, PRBC units contained 200–300 mL. Data were collected at two time points (September 1, 2020 and March 21, 2021). Patients admitted to the hospital after September 1, 2020 were assigned to the second epidemic wave.
Screening for the occurrence of complications was conducted daily according to our standard clinical protocol. Laboratory analyses were routinely performed daily; blood gas analyses were performed at intervals of 1–2 h. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics board of RWTH Uniklinik Aachen (No. 20-085) and individual consent for this retrospective analysis was waived.
ECMO settings
Critically ill patients with COVID-19 were considered for ECMO treatment based on the following criteria: (I) Presence of indications for ECMO as suggested by the ELSO guidelines (14); and (II) Failure of all other treatments options (i.e., lung protective invasive MV, prone positioning, neuro-muscular blockade, and inhaled nitric oxide [iNO] rescue therapy). The decision on initiation of ECMO treatment was determined by consensus of our (mobile) ECMO-team consisting of internal medicine intensivists, cardiothoracic surgeons, and pneumologists.
The ECMO devices used in our ICU were iLA ACTIVVE XLUNG kits (XENIOS, Heilbronn, Germany) and Cardiohelp HLS systems Version 7.0 (Maquet Cardiopulmonary GmbH, Rastatt, Germany). Data on ECMO settings, utilization (i.e., VV or VA), cannulation sites, utilization switch (i.e., from VV to VA or veno-venous arterial) were recorded. Our standard approach for the treatment of isolated respiratory failure was VV ECMO.
Anticoagulation regime
The following hemostatic parameters were measured daily: activated partial thromboplastin time (aPTT), international normalized ratio (INR), platelet count, fibrinogen, antithrombin, D-dimer, and activated clotting time (ACT). The aPTT and ACT were measured thrice and four times daily, respectively, as control for adequate coagulation and not as target values. In addition, factor XIII was measured thrice weekly. The following protocol was used in all patients of the study. In the absence of other relevant indications for a higher anticoagulation target (e.g., atrial fibrillation or mechanical heart valve prosthesis), we primarily administered 400 IE/h of unfractionated heparin. In the absence of bleeding complications at the beginning, anticoagulation was tapered stepwise to achieve an aPTT of 40–60 seconds and we tolerated an ACT ≤180 s. If necessary, the dose of unfractionated heparin was reduced or its administration paused. Other target values were: platelet count >50 G/L; fibrinogen >150 mg/dL; and INR <1.5. In case of bleeding, we adjusted the target values using fresh frozen plasma or PRBC. We aimed for an ACT <160 s, normalized the INR, and raised the platelet count to ≥80 G/L and fibrinogen to >200 mg/dL.
Bleeding complications
All bleeding events that led to the use of two or more units of whole blood or red cells and/or a fall in hemoglobin by >1.24 mmol/L were identified as major according to the definition established by the International Society on Thrombosis and Haemostasis (ISTH) (15). We selected the ISTH classification because of its applicability to patients treated with anticoagulants and ECMO being a non-surgical treatment.
Transfusion of PRBC was very frequent, aiming to maintain a hemoglobin value of 9 g/dL in VV-ECMO. However, as our main ECMO target aside from ultraprotective ventilation was sufficient oxygen delivery, we calculated the ratio of oxygen delivery (Do2) to oxygen consumption (Vo2) several times daily and aimed for a ratio of ≥3:1; and ideally of ≥4:1 with ECMO blood flow as low as possible. Thus, in some patients, a transfusion of PRBC was required due to low Do2:Vo2 ratios, although the level of hemoglobin was approximately 10 g/dL.
Statistical analysis
Categorical variables are presented as absolute numbers and percentages. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test and presented as the median and interquartile range (IQR). For comparison between patients from the first and second waves, univariate analyses were performed using the Mann–Whitney U test for continuous variables and Fisher’s exact test for categorical variables. Analyses of laboratory parameters at three-time points were conducted using Friedman’s nonparametric test with Dunn’s correction for repeated measurement.
All statistical comparisons were two-sided. P-values <0.05 denoted statistically significant differences. Statistical analysis was performed using the SPSS Version 26.0 (IBM Corp., Armonk, NY, USA) software. Time courses of laboratory parameters were created using the GraphPad Prism Version 8.0 (GraphPad Software, San Diego, CA, USA) software. Kaplan-Meier survival estimates, including visualization, were obtained using the open-source Jamovi Version 1.2.22.0 software.
Results
Baseline characteristics
During the study period, a total of 39 patients were treated with ECMO at our university hospital. The proportion of patients transferred from other referring hospitals was 85%, and mobile ECMO support (from our retrieval team) was provided to 23% of the patients (Table 1). VA ECMO was indicated in one patient due to severe right ventricular decompensation after pulmonary embolism in both lungs; all other patients received VV ECMO. We reported an overall median age of 56 (IQR: 50–60) years, body mass index (BMI) of 29.7 (IQR: 26.3–35.2) kg/m2, and (pre-ECMO) hospitalization time of 12 (IQR: 6–19) days. The most frequent comorbidities were arterial hypertension and diabetes mellitus type 2 (62% and 39%, respectively). Several treatments were performed before and/or during implantation of ECMO: prone positioning (100%), iNO (74%), corticosteroids (62%), and neuromuscular blocking agents (NMB) (56%). The most frequent complications prior to ECMO were pneumomediastinum, hypercapnia, pulmonary hypertension, and secondary hepatopathy in 23%, 18%, 15%, and 13% of the patients, respectively. We reported a median Respiratory ECMO Survival Prediction (RESP) of 0 (IQR: −1–2) and Sequential Organ Failure Assessment (SOFA) of 10 (IQR: 8–11). The median (pre-ECMO) MV time was 6 (IQR: 3–15) days, and 49% of the patients received MV for >7 days.
Table 1
| Characteristic | Total (n=39) | Wave 1 (n=19) | Wave 2 (n=20) | P value |
|---|---|---|---|---|
| Age (y) | 56 [50–60] | 57 [50–62] | 53 [50–59] | 0.461 |
| Female gender | 11 [28] | 6 [32] | 5 [25] | 0.731 |
| Weight (kg) | 90 [82–110] | 90 [80–100] | 93 [85–110] | 0.113 |
| Height (cm) | 175 [167–180] | 176 [170–185] | 175 [166–180] | 0.247 |
| BMI (kg/m2) | 29.7 [26.3–35.2] | 28.2 [24.7–31.1] | 31.1 [27.8–39.2] | 0.022* |
| Coronary artery disease | 1 [3] | 1 [5] | 0 [0] | 0.487 |
| Prior myocardial infarction | 3 [8] | 1 [5] | 2 [10] | 1.000 |
| Arterial hypertension | 24 [62] | 14 [74] | 10 [50] | 0.191 |
| COPD | 4 [10] | 3 [16] | 1 [5] | 0.342 |
| Diabetes mellitus type 2 | 15 [39] | 7 [37] | 8 [40] | 1.000 |
| Chronic kidney disease† | 2 [5] | 2 [11] | 0 [0] | 0.231 |
| Immunosuppressive medication | 1 [3] | 1 [5] | 0 [0] | 0.487 |
| CNS dysfunction | 0 [0] | 0 [0] | 0 [0] | 1.000 |
| History of malignancy | 2 [5] | 1 [5] | 1 [5] | 1.000 |
| Liver cirrhosis | 2 [5] | 0 [0] | 2 [10] | 0.487 |
| Hospitalization and treatment | ||||
| Transferred from another hospital | 33 [85] | 14 [74] | 19 [95] | 0.091 |
| Mobil ECMO | 9 [23] | 4 [21] | 5 [25] | 1.000 |
| VA ECMO indication | 1 [3] | 1 [5] | 0 [0] | 0.487 |
| Pre-ECMO LOS in-hospital (d)†† | 12 [6–19] | 8 [5–15] | 14 [8–20] | 0.141 |
| Pre-ECMO LOS in-ICU (d)†† | 8 [3–14] | 5 [1–12] | 10 [6–15.5] | 0.101 |
| Prone positioning | 39 [100] | 19 [100] | 20 [100] | 1.000 |
| Tracheostomy | 20 [51] | 11 [58] | 9 [45] | 0.527 |
| iNO inhalation | 29 [74] | 14 [74] | 15 [75] | 1.000 |
| Neuromuscular blocking agents | 22 [56] | 11 [58] | 11 [55] | 1.000 |
| Cytokine absorption§ | 13 [33] | 9 [47] | 4 [20] | 0.096 |
| Antibiotics§§ | 20 [51] | 9 [47] | 11 [55] | 0.752 |
| Antifungal medication§§ | 6 [15] | 3 [16] | 3 [15] | 1.000 |
| Antiviral medication | 7 [18] | 4 [21] | 3 [15] | 0.695 |
| Corticosteroids | 24 [62] | 9 [47] | 15 [75] | 0.105 |
| Sildenafil | 14 [36] | 6 [32] | 8 [40] | 0.741 |
| Complications and risk scores | ||||
| Secondary hepatopathy | 5 [13] | 4 [21] | 1 [5] | 0.182 |
| Pulmonary hypertension | 6 [15] | 6 [32] | 0 [0] | 0.008* |
| Hypercapnia at ECMO-initiation | 7 [18] | 5 [26] | 2 [10] | 0.235 |
| Pneumomediastinum on CT/X-ray | 9 [23] | 3 [16] | 6 [30] | 0.451 |
| Prior SOFA score | 10 [8–11] | 11 [7–13] | 10 [8–11] | 0.588 |
| Prior RESP Score | 0 [−1 to 2] | 0 [−3 to 2] | 0 [−1 to 2] | 0.214 |
| Mechanical ventilation | ||||
| Prior MV time (d)†† | 6 [3–15] | 4 [3–15] | 9 [3–15] | 0.708 |
| Prior MV longer than 7 days†† | 19 [49] | 8 [42] | 11 [55] | 0.527 |
| FiO2 (percentage) | 60 [50–80] | 60 [50–80] | 60 [50–80] | 0.647 |
| pO2:FiO2 (ratio) | 1.04 [0.82–1.29] | 1.06 [0.71–1.36] | 1.02 [0.83–1.22] | 0.923 |
| Pinsp (mbar) | 27 [24–30] | 28 [25–30] | 25.5 [23–28] | 0.204 |
| PEEP (cmH2O) | 12 [10–14] | 14 [10–15] | 12 [10–13.5] | 0.044* |
Continuous variables are presented as median and interquartile range [IQR] or n (%). *, P values under 0.05 are considered as significant and tagged with an asterisk; †, Including all patients with a MDRD-GFR <60 mL/min; ††, Including time in the previous hospital; §, Started before ECMO or incorporated in the ECMO circuit; §§, Medication due to superinfection. BMI, body mass index kg/m2; CNS, Central nervous system; COPD, chronic obstructive pulmonary disease; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit; iNO, inhaled nitric oxide; LOS, length of stay; MV, Mechanical ventilation; PEEP, Positive end-expiratory pressure; Pinsp, Peak inspiratory pressure; RESP, The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; SOFA, Sequential Organ Failure Assessment Score; VA, veno-arterial.
We performed a comparison of the baseline characteristics of patients between the first (n=19) and second (n=20) waves. Compared with patients of the first wave, those of the second wave had significantly higher median BMI [28.2 (IQR: 24.7–31.1) vs. 31.1 (IQR: 27.8–39.2) kg/m2, respectively, P=0.022], significantly lower median positive end-expiratory pressure (PEEP) [14 (IQR: 10–15) vs. 12 (IQR 10–13.5) cmH2O, respectively, P=0.044], and a significantly lower rate of (pre-ECMO) pulmonary hypertension (32% vs. 0%, respectively, P=0.008). The median age of patients was 57 (IQR: 50–62) and 53 (IQR 50–59) years, respectively (P=0.461). The median (pre-ECMO) duration of hospitalization was 8 (IQR: 5–15) and 14 (IQR: 8–20) days, respectively (P=0.141). Regarding treatments before and/or during initiation of ECMO, patients of the second wave received less CytoSorb (cytokine absorption) therapy (47% vs. 20%, respectively, P=0.096) and more corticosteroids (47% vs. 75%, respectively, P=0.105). Patients of the second wave showed a higher median (pre-ECMO) MV time [4 (IQR: 3–15) vs. 9 (IQR: 3–15) days, respectively, P=0.708]. The median RESP scores were similar in both groups [0 (IQR: −3–2) vs. 0 (IQR: −1–2), respectively, P=0.214]. Further details are presented in Table 1.
Laboratory parameters
The 23 different laboratory measurements are presented in Table 2. In the total study sample (n=39), we reported a median partial pressure of oxygen (pO2) of 68 (IQR: 54–76) mmHg, partial pressure of carbon dioxide (pCO2) of 65 (IQR: 51–77) mmHg, C-reactive protein of 211 (IQR: 100–287) mg/L, D-dimer of 4,547 (IQR: 1,876–11,006) µg/dL, and fibrinogen of 466 (IQR: 409–717) mg/dL prior to implantation of ECMO.
Table 2
| Parameter | Total (n=39) | Wave 1 (n=19) | Wave 2 (n=20) | P value |
|---|---|---|---|---|
| pO2 (mmHg) | 68 [54–76] | 68 [54–72] | 68 [53.25–77.5] | 0.771 |
| pCO2 (mmHg) | 65 [51–77] | 66 [48–78] | 62 [52–73] | 0.771 |
| pH | 7.33 [7.26–7.43] | 7.29 [7.2–7.39] | 7.42 [7.3–7.45] | 0.005* |
| Bicarbonate (mmol/L) | 31 [26.4–34.4] | 27 [24.7–34.4] | 31.8 [29.6–34.8] | 0.033* |
| Lactate (mmol/L) | 1.9 [1.3–2.9] | 1.9 [1.3–3] | 1.85 [1.4–2.8] | 0.945 |
| Hb (g/dL) | 9.8 [9–11.4] | 9.8 [8.7–10.5] | 9.8 [9.2–11.6] | 0.531 |
| pfHb (mg/L) | 46 [28–68] | 36 [22–50] | 58 [44–73] | 0.013* |
| Leucocytes (/nL) | 13 [10–19] | 14 [10–22] | 12 [10–16] | 0.214 |
| Platelet (G/L) | 240 [170–340] | 222 [154–389] | 246 [176–340] | 0.728 |
| PCT (%) | 2.2 [0.6–8.3] | 4.5 [0.6–6.49] | 1.5 [0.6–9.1] | 0.813 |
| aPTT (s) | 33 [29–39] | 34 [29–39] | 32 [30–39] | 0.989 |
| INR (ratio) | 1.2 [1.1–1.3] | 1.3 [1.2–1.3] | 1.2 [1.1–1.3] | 0.204 |
| ATIII (%) | 66 [55–77] | 65 [54–76] | 67 [56–88] | 0.328 |
| D-dimer (µg/dL) | 4,547 [1,876–11,006] | 4,345 [1,745–12,046] | 4,869 [2,117–10,698] | 0.967 |
| CRP (mg/L) | 211 [100–287] | 211 [120–280] | 208 [77–346] | 0.835 |
| Fibrinogen (mg/dL) | 466 [409–717] | 656 [432–717] | 455 [379–853] | 0.607 |
| PCT (%) | 2.2 [0.6–8.3] | 4.5 [0.6–6.5] | 1.5 [0.6–9.1] | 0.813 |
| LDH (U/L) | 459 [333–661] | 403 [311–639] | 504 [411–687] | 0.07 |
| ALT (U/L) | 44 [32–75] | 33 [27–45] | 52 [42–92] | 0.018* |
| AST (U/L) | 61 [38–93] | 66 [37–126] | 58 [44–82] | 0.989 |
| BUN (mg/dL) | 68 [46–111] | 66 [43–116] | 68 [59–110] | 1.000 |
| Creatinine (mg/dL) | 1.1 [0.7–1.8] | 1.2 [0.8–1.8] | 0.9 [0.6–2.1] | 0.204 |
| Bilirubin (mg/dL) | 0.6 [0.4–1.6] | 0.6 [0.4–2.2] | 0.6 [0.4–1.1] | 0.901 |
Continuous variables are presented as median and interquartile range [IQR] or n (%). *, P values under 0.05 are considered as significant and tagged with an asterisk. All parameters were measured 1–6 hours before ECMO-initiation. ALT, alanine transaminase; AST, aspartate transaminase; ATIII, Antithrombin III, aPTT, partial thromboplastin time; BUN, blood urea nitrogen; CRP, C-reactive protein; ECMO, extracorporeal membrane oxygenation; Hb, Hemoglobin; LDH, lactate dehydrogenase; PCT, procalcitonin; pfHb, plasma-free hemoglobin; WBC, white blood cells.
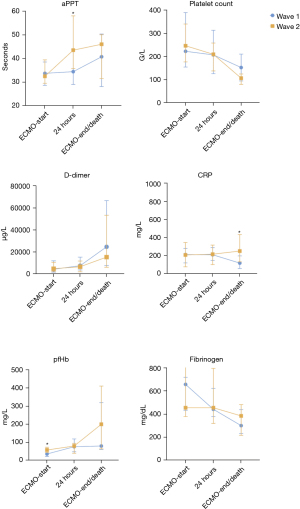
We compared the laboratory parameters of patients between the first (n=19) and second (n=20) waves at three different time points and found multiple statistically significant differences (Table 2, Figure 1, Table S1). Before implantation of ECMO: patients of the second wave had significantly higher pH (median: 7.29 vs. 7.42, respectively, P=0.005), bicarbonate (median: 27 vs. 31.8 mmol/L, respectively, P=0.033), plasma free hemoglobin (pfHb) (median: 36 vs. 58 mg/L, respectively, P=0.013), and alanine aminotransferase (ALT) (median: 33 vs. 52 U/L, respectively, P=0.018) levels. After implantation of ECMO (24 h): patients of the second wave showed significantly higher aPTT (median: 34 vs. 44 s, respectively, P=0.038) and antithrombin III (median: 48% vs. 71%, respectively, P=0.008) levels, and significantly lower creatinine (median: 1.5 vs. 1 mg/dL, respectively, P=0.033) levels. Before explantation of ECMO: patients of the second wave showed significantly higher C-reactive protein (median: 118 vs. 251 mg/L, respectively, P=0.006) and significantly lower blood urea nitrogen (median: 98 vs. 70 s, respectively, P=0.004) levels.
Outcomes
Outcomes, complications, and administration of blood products are presented in Table 3. Of the 39 patients, 41% were successfully weaned from ECMO therapy and survived until discharge. A total of 23 patients expired, and there were no patients who remained hospitalized. The median duration of ECMO was 19 (IQR: 11–29) days. In the one patient who received VA ECMO, we reported recovery of right ventricular function and successful weaning from ECMO after 27 days. Thromboembolic events (TEE) occurred in 36% of the patients, and pulmonary artery embolism was the most frequent (21%). Major bleeding events (MBE) occurred in 62% of the patients; the most frequent locations were endobronchial and mucosal bleedings in the upper respiratory tract (23% each). The incidence of acute kidney failure was 72%; renal replacement therapy was applied to all cases. The median amount of PRBC used during ECMO therapy was 1.5 (IQR: 0.8–2.0) units per day.
Table 3
| Outcome | Total (n=39) | Wave 1 (n=19) | Wave 2 (n=20) | P value |
|---|---|---|---|---|
| Survived until discharge, n (%)† | 16 [41] | 10 [53] | 6 [30] | 0.200 |
| ECMO duration (d) | 19 [11–29] | 16 [11–24] | 24.5 [15.3–33] | 0.074 |
| Thromboembolic events, n (%)†† | 14 [36] | 8 [42] | 6 [30] | 0.514 |
| Pulmonary artery embolism | 8 [21] | 5 [26] | 3 [15] | 0.451 |
| Peripheral venous thrombosis | 5 [13] | 3 [16] | 2 [10] | 0.661 |
| Peripheral arterial thrombosis | 2 [5] | 1 [5] | 1 [5] | 1.000 |
| ECMO-circuit thrombus | 3 [8] | 0 [0] | 3 [15] | 0.231 |
| Major bleeding events, n (%)†† | 24 [62] | 8 [42] | 16 [80] | 0.022* |
| Endobronchial | 9 [23] | 2 [11] | 7 [35] | 0.127 |
| Mucosal | 9 [23] | 3 [16] | 6 [30] | 0.451 |
| Cannulation side | 6 [15] | 2 [11] | 4 [20] | 0.661 |
| Gastrointestinal | 3 [8] | 1 [5] | 2 [10] | 1.000 |
| Cerebral | 3 [8] | 0 [0] | 3 [15] | 0.231 |
| Hemothorax | 1 [3] | 1 [5] | 0 [0] | 0.487 |
| Pericardial tamponade | 3 [8] | 2 [11] | 1 [5] | 0.605 |
| Other | 3 [8] | 0 [0] | 3 [15] | 0.231 |
| Acute kidney failure§ | 28 [72] | 13 [68] | 15 [75] | 0.447 |
| Blood products†† | ||||
| PRBC (units) | 26 [14–46] | 26 [14–38] | 23 [12.8–59] | 0.731 |
| PRBC (units/d) | 1.5 [0.8–2] | 1.5 [1.1–2.7] | 1 [0.7–1.8] | 0.285 |
| Total Albumin (g) | 120 [0–300] | 200 [0–320] | 0 [0–255] | 0.161 |
| FFP (units) | 0 [0–8] | 0 [0–8] | 4 [0–8] | 0.41 |
| PC (units) | 2 [0–10] | 0 [0–10] | 2 [0–10.5] | 0.35 |
Continuous variables are presented as median and interquartile range [IQR] or n (%). *, P values under 0.05 are considered as significant and tagged with an asterisk; †, discharge to a rehabilitation center, general ward of other hospital or home. We reported death in all other patients; ††, during ECMO-therapy; §, all patients with acute kidney failure in stages 2 or 3 by KDIGO guidelines. ECMO, extracorporeal membrane oxygenation; FFP, fresh frozen plasma; LOS, length of stay, PC, platelet cells; PRBC, packed red blood cells.
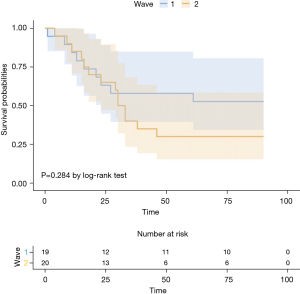
We also compared the outcomes of patients of the first (n=19) and second (n=20) waves. Compared with patients of the first wave, those of the second wave had a significantly higher rate of MBE (42% vs. 80%, respectively, P=0.022), lower survival until discharge (53% vs. 30%, respectively, P=0.200) and longer duration of ECMO [16 (IQR: 11–24) vs. 24.5 (IQR: 15.3–33) days, respectively, P=0.074]. The hazard ratio for death within 90 days after initiation of ECMO in the second wave, compared with the first wave, was 1.57 (95% CI: 0.68–3.65, P=0.284) (Figure 2). Patients of the second wave exhibited a lower incidence of thromboembolic events (42% vs. 30%, P=0.514). Acute kidney failure occurred in 68% and 75% of the patients, respectively, (P=0.447). Despite the higher rate of bleeding events, the median amount of PRBC administered was lower in the second wave [1.5 (IQR: 0.8–2.0) vs. 1 (IQR: 0.7–1.8) units per day, respectively, P=0.285]. Further details are presented in Table 3.
Discussion
Since the beginning of 2020, a large number of patients with COVID-19 received support with ECMO at our center; the majority were transferred from other hospitals. Most of our patients received NMB agents, antibiotics, steroids, and iNO prior to initiation of ECMO. A high proportion of our patients remained for >1 week in the ICU and 49% received MV for >7 days. The rate of survival to discharge was 41% in total. However, the number of ECMO non survivors were higher during the second wave, in line with the higher mortality reported during the second wave globally. Similar trends were observed in other centers using ECMO during the second wave, irrespective of the burden of the pandemic. Survival to discharge was 53% and 30% in the first and second wave, respectively, (P=0.200); even though the median RESP in both groups was 33–57%. The difference in survival was statistically non-significant in our study, but the impact on clinical practice is highly relevant since this negative trend has been reported by the ELSO registry (16). Broman and colleagues reported that successful weaning was accomplished in 58% (841 of 1,442) of patients in the first wave, compared with 47% (718 of 1,723) in the second wave (P<0.0001) (16). Patients from our center were also submitted to the ELSO registry, and we noticed similar baseline characteristics, such as age, BMI, gender, comorbidity, and superinfection before ECMO-initiation (17). A second observation by Broman et al. was that the number of patients on long-term ECMO (>28 days) increased (16). We also reported longer ECMO runs in patients from the second wave (16 vs. 24.5 days, P=0.074). In another registry led by the Japan ECMOnet for COVID-19 group, survival rates were approximately 10% less in patients of the second wave (18). Regarding ECMO duration, the Japanese ECMOnet registry showed increased mortality in patients who underwent ECMO for 16–20 days, and a 65% mortality risk in patients under ECMO for more than 16 days (18).
Evidence for a direct cause of high mortality rates in the second wave cannot be provided, however, there are multiple factors that need to be discussed. Patients from the second wave remained for long periods in (ICUs of) referring hospitals and already received steroids and other adjuvants (Table 1). Concurrently, the median time on MV prior ECMO was 9 days in patients of the second wave. MV for <7 days is recommended for ECLS in patients with COVID-19 (14); of note, 55% of patients from the second wave exceeded this limit (Table 1). This was due to late requests for ECMO in our hospital, which have been associated with worse outcomes (19). Specifically, for COVID-19 patients, the Japanese registry and a German analysis reported lower mortality in patients with early onset of ECMO therapy after initiation of MV (18,20). Due to long ICU periods and severe illness, more patients develop acute kidney failure and receive dialysis before the initiation of ECMO. Karagiannidis et al. showed that ECMO-centers in Germany selected more patients with need for dialysis than other countries (20). The prevalence of acute kidney failure was also high in our study, especially during the second wave (Table 3). Looking at the overall COVID-19 population, we found different a different trend between the first and second wave. In Germany, the mortality of mechanically ventilated patients with COVID-19 in ICUs during the first wave was reported to be 53% (21). Unlike ECMO outcomes, an analysis with patients from the second wave showed that the prognosis of ICU patients, those requiring mechanical ventilation and those not, remained the same (21). An important difference is that compared with in the first wave, 50% less of all hospitalised patients were admitted to the ICU during the second wave (21). Possible reasons for this difference are clearly defined algorithms for non-invasive treatment strategies, and the early administration of pharmacological treatments, such as dexamethasone. Regarding ECMO patients, a selection bias with patients who did not respond to adjuvant therapies and underwent long hospitalization periods could have affected the outcomes (16). Another important factor is that health care providers experienced higher work load during the second wave, because the absolute number of ICU admissions steadily increased and almost doubled compared with that of the first wave (21). Having said this, we did not experience shortcomings of (ECMO) resources in our center.
A relevant finding of our study were significantly more bleeding events in the second wave (Table 3). A study conducted by Aubron and colleagues investigated bleeding complications in ECMO patients and reported that bleeding was independently associated with worse survival (22). In this study, definition of bleeding was similar to the ISTH classification that we used, and furthermore, bleeding rate was 60% which was comparably high in our cohort (22). More bleeding complications in the second wave could be explained by longer ECMO runs and more severe sepsis (Table 3). Laboratory measurements (24 h after initiation of ECMO) revealed significantly higher PTT values in patients of the second wave (Table S1). Our approach aimed at a PTT under 60 s, and the median PTT of the patients was 32 (IQR: 30–39) s and 44 (IQR: 36–58) s before and 24 h after the initiation of ECMO, respectively. As ELSO guidelines recommend PTT values 1.4- or 1.5-fold higher than normal (23), we assume that these values are reasonable. Platelet count was another important laboratory measurement. Compared with patients of the first wave, those of the second wave had lower platelet levels before explantation of ECMO [152 (IQR: 94–210) vs. 106 (IQR: 79–124) days, respectively, P=0.07]. Low platelet levels or thrombocytopenia have been associated with bleeding events in patients with COVID-19 receiving ECLS (24) and we aimed for a platelet count >80 G/L as recommended in the ELSO guidelines (23). However, a decrease in platelets and thrombocytopenia during ECLS are frequently observed, and the underlying mechanisms of these events are multifactorial (25). Besides that, ECMO runs were longer in patients from the second wave (Table 3). Therefore, low platelet levels before ECMO removal are reasonable. COVID-19 is associated with a hypercoagulable state; hence, guidelines suggested to target anticoagulation at the higher end of normal ECMO parameters (8) during the period of our study. However, we followed the same anticoagulation regimen (described in the Methods section) in all patients. Furthermore, the administration of PRBC units during ECMO therapy was comparable and even higher in patients of the first wave. This may indicate that bleeding in the first wave was less frequent and simultaneously more severe, or the classification of major bleedings established by the ISTH is excessively stringent. Besides that, hemolysis and decreased red cell lifespan is caused by artificial surface and shear stress from the ECMO circuit. Therefore, PRBCs are administered frequently to maintain appropriate Do2:Vo2 ratios and hemoglobin levels. As a result, PRBC units for incidental bleedings cannot stand out in a statistical comparison.
Selection of ARDS patients for ECMO was conducted according to the ELSO guidelines (12). During the study period, we did not change our protocol for ARDS patients as we reported positive results during the first wave. Prone positioning was used in all patients before and on ECMO, however, in some patients we needed to stop because of severe complications. As many studies reported positive results with dexamethasone in mechanically ventilated COVID-19 patients (26), the use of steroids increased from 47% to 75% in the second wave (Table 1). Steroids were commonly administered early after hospital admission, and a survival benefit of steroids has been reported in many studies (27). Therefore, the second wave of had more patients who did not respond to steroid therapy and were selected for ECMO therapy. This finding supports the idea of worse outcomes in patients who did not respond to adjuvant therapies (16). Furthermore, some studies suggested that steroids could induce a delayed SARS–CoV-2 clearance from the airway and worsen survival outcomes (28), however, more evidence is needed.
Even though our common approach for ARDS patients did not change, our data revealed some significant differences which need to be discussed. In patients of the first wave, we reported significantly lower pH prior ECMO (Table 1). In general, this indicates more severe illness and Raasveld et al. reported that COVID-19 non-survivors were more acidotic prior to the initiation of ECMO (29). An explanation for this dubious finding can be acidosis due to a therapeutic modality, which is known to have a protective effect against ventilator-associated lung injury (30) in the absence of right ventricular failure (31). The second epidemic wave was managed with significantly lower PEEP values. Adjustment of appropriate PEEP values was conducted according to lung compliance, and esophageal titration was used in some cases. The difference in PEEP values was small; nevertheless, this suggests that lung compliance, which is associated with mortality in patients with ARDS on ECMO, was less during the second wave (32). We also reported significantly higher BMI values in patients of the second wave, which could have further impaired lung compliance. However, the association between obesity and ECLS mortality remains unclear (33,34). Hemolysis is common in patients receiving ECMO; however, we found significantly higher pfHb levels in patients of the second wave. Omar et al. reported that high pfHb levels (<50 mg/dL, 24 h after initiation of ECMO) can be used as an independent risk factor for mortality in patients receiving support (35). In the present study, the levels of pfHb were already exceeding this limit prior to the initiation of ECMO therapy. High levels of pfHb are associated with multiorgan failure (36,37) and may have influenced outcomes. Numerous studies added further knowledge regarding the appropriate utilization of ECMO and found interesting prognostic factors in patients with COVID-19. Old age (>65 years), immunosuppression, need for VA-ECMO, and presence of common comorbidities (hypertension, diabetes, and obesity) are associated with poor ECMO outcomes (14). The present study included only four patients aged >65 years, one patient receiving immunosuppressive medication, and one patient who received VA-ECMO. However, comorbidities were present in the majority of patients (Table 1).
The strength of this investigation is that the clinical course before and after the initiation of ECMO is accurately presented and important details are reported. As this was a single-center study, we can ensure that the groups were comparable and received the same treatment. Although our results add further knowledge to the management of patients with COVID-19, the present study has some limitations. One of the main disadvantages is the small sample size, which reduced power and increased the margin of error in our study. Furthermore, the probability for false positive findings is high due to a wide range of variables and small number of patients. Multiple comparison correction was considered but common methods, such as Bonferroni correction, would eliminate all significant values of the results. As an early single center analysis, we presented all possible differences with a low threshold. This approach prevented the occurrence of false negatives values which could have been important in the future. Retrospective studies are associated with a potential risk for selection bias, and results are dependent on accurate recordkeeping. For MV parameters (e.g., PEEP) and laboratory values, we only analyzed specific time points which may not reflect longer periods of time and, thus, lead to misconceptions.
Conclusions
In a single-center study on ECMO-supported patients with COVID-19, we reported an overall survival rate of 41% in the first year. Similar to various registries, we observed less favorable outcomes during the second wave. Lessons learned from our experience are that direct communication with referring hospitals and early initiation of ECMO remains challenging. We identified two topics for further analysis of patients with COVID-19 receiving ECMO. Studies involving large sample sizes are warranted to identify predictors of mortality, as well as the influence of different SARS-CoV-2 mutations or subtypes on the clinical course and ECMO outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/jtd-21-971
Data Sharing Statement: Available at https://dx.doi.org/10.21037/jtd-21-971
Peer Review File: Available at https://dx.doi.org/10.21037/jtd-21-971
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/jtd-21-971). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the local ethics board of RWTH Uniklinik Aachen (No. 20-085) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8:506-17. [Crossref] [PubMed]
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020;323:2052-9. [Crossref] [PubMed]
- Armstrong RA, Kane AD, Kursumovic E, et al. Mortality in patients admitted to intensive care with COVID-19: an updated systematic review and meta-analysis of observational studies. Anaesthesia 2021;76:537-48. [Crossref] [PubMed]
- Tzotzos SJ, Fischer B, Fischer H, et al. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care 2020;24:516. [Crossref] [PubMed]
- Marullo AG, Cavarretta E, Biondi Zoccai G, et al. Extracorporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiol 2020;68:368-72. [Crossref] [PubMed]
- World Health Organization. Clinical management of COVID-19: interim guidance, 27 May 2020. Geneva: World Health Organization; 2020. Contract No.: WHO/2019-nCoV/clinical/2020.5
- Ramanathan K, Antognini D, Combes A, et al. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med 2020;8:518-26. [Crossref] [PubMed]
- Shekar K, Badulak J, Peek G, et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: A Consensus Document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J 2020;66:707-21. [Crossref] [PubMed]
- Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet 2020;396:1071-8. [Crossref] [PubMed]
- Ramanathan K, Shekar K, Ling RR, et al. Extracorporeal membrane oxygenation for COVID-19: a systematic review and meta-analysis. Crit Care 2021;25:211. [Crossref] [PubMed]
- Combes A, Peek GJ, Hajage D, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med 2020;46:2048-57. [Crossref] [PubMed]
- Badulak J, Antonini MV, Stead CM, et al. Extracorporeal Membrane Oxygenation for COVID-19: Updated 2021 Guidelines from the Extracorporeal Life Support Organization. ASAIO J 2021;67:485-95. [Crossref] [PubMed]
- ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Huang S, Zhao S, Luo H, et al. The role of extracorporeal membrane oxygenation in critically ill patients with COVID-19: a narrative review. BMC Pulm Med 2021;21:116. [Crossref] [PubMed]
- Schulman S, Kearon CSubcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4. [Crossref] [PubMed]
- Broman LM, Eksborg S, Coco VL, et al. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med 2021;9:e80-1. [Crossref] [PubMed]
- COVID-19 Cases on ECMO in the ELSO Registry. Accessed July 28, 2021. Available online: https://www.elso.org/Registry/FullCOVID19RegistryDashboard.aspx
- Survey of Critically ill COVID-19 patients in Japan, managed by the Japan ECMOnet for COVID-19. Accessed July 28, 2021. Available online: https://crisis.ecmonet.jp
- Steimer DA, Hernandez O, Mason DP, et al. Timing of ECMO Initiation Impacts Survival in Influenza-Associated ARDS. Thorac Cardiovasc Surg 2019;67:212-5. [Crossref] [PubMed]
- Karagiannidis C, Strassmann S, Merten M, et al. High In-Hospital Mortality Rate in Patients with COVID-19 Receiving Extracorporeal Membrane Oxygenation in Germany: A Critical Analysis. Am J Respir Crit Care Med 2021;204:991-4. [Crossref] [PubMed]
- Karagiannidis C, Windisch W, McAuley DF, et al. Major differences in ICU admissions during the first and second COVID-19 wave in Germany. Lancet Respir Med 2021;9:e47-8. [Crossref] [PubMed]
- Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 2016;6:97. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. Guidelines for Adult Respiratory Failure. August 2017.
- Durak K, Kersten A, Grottke O, et al. Thromboembolic and Bleeding Events in COVID-19 Patients receiving Extracorporeal Membrane Oxygenation. Thorac Cardiovasc Surg 2021;69:526-36. [Crossref] [PubMed]
- Jiritano F, Serraino GF, Ten Cate H, et al. Platelets and extra-corporeal membrane oxygenation in adult patients: a systematic review and meta-analysis. Intensive Care Med 2020;46:1154-69. [Crossref] [PubMed]
- RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021;384:693-704. [Crossref] [PubMed]
- Ma S, Xu C, Liu S, et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther 2021;6:83. [Crossref] [PubMed]
- Liu J, Zhang S, Dong X, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest 2020;130:6417-28. [Crossref] [PubMed]
- Raasveld SJ, Delnoij TSR, Broman LM, et al. Extracorporeal Membrane Oxygenation in Patients With COVID-19: An International Multicenter Cohort Study. J Intensive Care Med 2021;36:910-7. [Crossref] [PubMed]
- Salmon AA, Hotchkiss JR. Hypercapnic acidosis in ARDS: a tolerated side effect or an important therapeutic modality? Crit Care 2007;11:304. [Crossref] [PubMed]
- Repessé X, Vieillard-Baron A. Hypercapnia during acute respiratory distress syndrome: the tree that hides the forest! J Thorac Dis 2017;9:1420-5. [Crossref] [PubMed]
- Kim HS, Kim JH, Chung CR, et al. Lung Compliance and Outcomes in Patients With Acute Respiratory Distress Syndrome Receiving ECMO. Ann Thorac Surg 2019;108:176-82. [Crossref] [PubMed]
- Blum JM, Stentz MJ. The Known Unknowns of Obesity and Extracorporeal Membrane Oxygenation. Anesth Analg 2020;131:751-3. [Crossref] [PubMed]
- Galvagno SM Jr, Pelekhaty S, Cornachione CR, et al. Does Weight Matter? Outcomes in Adult Patients on Venovenous Extracorporeal Membrane Oxygenation When Stratified by Obesity Class. Anesth Analg 2020;131:754-61. [Crossref] [PubMed]
- Omar HR, Mirsaeidi M, Socias S, et al. Plasma Free Hemoglobin Is an Independent Predictor of Mortality among Patients on Extracorporeal Membrane Oxygenation Support. PLoS One 2015;10:e0124034 [Crossref] [PubMed]
- Vermeulen Windsant IC, Hanssen SJ, Buurman WA, et al. Cardiovascular surgery and organ damage: time to reconsider the role of hemolysis. J Thorac Cardiovasc Surg 2011;142:1-11. [Crossref] [PubMed]
- Rother RP, Bell L, Hillmen P, et al. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 2005;293:1653-62. [Crossref] [PubMed]


