Abstract
Although COVID-19 disease primarily affects the respiratory system, it has been seen in many studies that it causes thromboembolic (TE) events in many tissues and organs. So that, to prevent TE can reduce mortality and morbidity. In this context, this study aimed to investigate the relationship between the previous use of warfarin or other new direct oral anticoagulants (OAC) and mortality in patients hospitalized with a diagnosis of COVID-19 before hospitalization. A total of 5575 patients who were diagnosed with COVID-19 were hospitalized and started treatment between March 21 and November 30, 2020 were included in the study. The primary outcome was in-hospital all-cause mortality. A retrospective cohort study design was planned. Patients were followed up until death or censoring on November 30, 2020. The candidate predictors for primary outcome should be clinically and biologically plausible, and their relationships with all-cause death should be demonstrated in previous studies. We considered all candidate predictors included in the model in accordance with these principles. The main candidate predictor was previous OAC use. The primary analysis method was to compare the time to deaths of patients using and not using previous OAC by a multivariable Cox proportional hazard model (CPHM). In the CPHM, previous OAC use was found to be associated with a significantly lower mortality risk (adjusted hazard ratio 0.62, 95% CI 0.42–0.92, p = 0.030). In hospitalized COVID-19 patients, in patients who previously used anticoagulantswas associated with lower risk of in-hospital death than in those who did not.
Similar content being viewed by others
Highlights
-
COVID-19 primarily affects the respiratory system, it causes thrombo-embolic events in many tissues and organs. Coagulopathy occurs in many patients with Covid-19.
-
We found that in hospitalized COVID-19 patients, in patients who previously used anticoagulants was associated with lower risk of in-hospital death than in those who did not.
Introduction
The COVID-19 disease caused by the SARS-CoV-2 virus was first seen in Wuhan, China, on December 1, 2019 and was recognized as a pandemic by the World Health Organization (WHO) on March 11, 2020 [1].It immediately drew attention from all over the world with its rapid clinical progression and high mortality, especially in patients with advanced age and chronic diseases. Although COVID-19 primarily affects the respiratory system, many studies have shown that it causes thromboembolic (TE) events in many tissues and organs [2, 3].
The mechanisms considered to be primarily responsible for the development of TE events are hypercoagulopathy and endothelial damage due to hypoxia and excessive inflammation. COVID-19 infection causes the excessive release of cytokines as a result of increased viral load and creates a sepsis-like clinical presentation [4, 5].In addition, overactivation in the coagulation cascade by the inflammatory mediators released induces thrombus development. The increase in sympathetic activity and neurohormonal irregularities occurring in the clinical course also causes hypercoagulability [6].
Anticoagulants are currently used in the prophylaxis and treatment of thromboembolism due to various diseases, especially atrial fibrillation (AF) [7]. Although oral forms, such as warfarin and new-generation anticoagulants, are widely used, parenteral forms are also used in daily practice.
As coagulopathy caused by COVID-19 is difficult to control, it is important to better understand what treatments have been studied so far and which ones show better results for hospitalized patients. In this context, this study aimed to investigate the relationship between the previous use of warfarin or other new direct oral anticoagulants (DOAC) and mortality in patients hospitalized with a diagnosis of COVID-19 before hospitalization.
Methods
A total of 5,575 patients who were diagnosed with COVID-19 by polymerase chain reaction (PCR) and/or computed tomography (CT), werehospitalized, and started treatment between March 21 and November 30, 2020 were included in thestudy. This workwas performedin accordance with the Helsinki Declaration and with the approval of the local ethics committee. Patient complaints, previousmedical history, history of drug use, clinical and demographic characteristics, and hematological and biochemical parameters were recorded. Patients whose PCR and CT were not compatible with COVID-19, who were found to have a different clinical diagnosis explaining their current clinical status, and who were recently hospitalized and received parenteral anticoagulation were excluded from the study.
Parenteral anticoagulation treatments were given at therapeutic doses topatients who had previously used DOAC. Parenteral anticoagulation was initiated at the time of the next dose in DOAC users and when INR < 2 for warfarin users. The parenteral anticoagulation treatment dose of patients who do not use OAC was arranged according to the Ministry of Health guidelines byestimated glomerular filtration rate (eGFR), weight, and clinical status of the disease.
Definitions
Hypertension (HT) was defined as a systolic blood pressure > 140 mmHg and/or a diastolic blood pressure > 90 mmHg or the use of an antihypertensive drug [8]. Diabetes mellitus (DM) was defined according to the current American Diabetes Association guidelines [9]. A history of coronary artery disease (CAD) was defined as patients who had invasive or non-invasive imaging studies showing evidence of coronary artery disease. Renal failure was defined as creatinine clearance below 60 ml/min.The Cockroft–Gault equation was used to calculate the eGFR.
PCR test A combined swab sample was taken in accordance with the specified procedures in all patients admitted to the emergency room or infection outpatient clinics [10].
CT: In the COVID-19 guide published by the Ministry of Health, the findings are classified as typical, indeterminate, atypical, and negative in the table of CT findings and reporting recommendations [11].
The patients were diagnosed with COVID-19 based on their current complaints, contact histories, physical examination findings, blood parameters, imaging findings, and PCR test results. In light of the updated scientific data, the treatments of the patients were also updated, and their treatments were planned and started in accordance with the algorithms and guidelines of the Ministry of Health [12]. Drug doses and durations were arranged by relevant clinicians according to the clinical characteristics and comorbid conditions of the patients [13, 14].
Analysis of blood samples
Blood samples were taken from the antecubital vein by creating a slight venous stasis in the upper arm.The samples were placed in tubes with potassium EDTA for complete blood count.Hemoglobin, hematocrit, platelet, and white blood cell counts were determined by the electrical impedance method with an automatic blood counting device (BeckmanCoulter LH 780). The biochemical parameters were measured by standard laboratory methods.
Statistics
For all statistical analyses, we used R-software version3.5.1 (R statistical software, Institute for Statistics and Mathematics, Vienna, Austria). Numerical variables were presented as median and interquartile ranges. Categorical data were shown as percentages and n. The primary outcome was in-hospital all-cause mortality. A retrospective cohort study design was planned. Patients were followed up until death or censoring on November 30, 2020.
The candidate predictors (main candidate predictor and adjustment variables) for primary outcome should be clinically and biologically plausible, and their relationships with all-cause death should be demonstrated in previous studies. We considered all candidate predictors included in the model in accordance with these principles. The main candidate predictor wasprevious OAC use. The primary analysis method wasto compare the time to deaths of patients using and not using previous OAC by a multivariable Cox proportional hazard model.In addition to previous OAC use, age, gender, HT, DM, heart failure (HF), CAD, eGFR, albumin, CRP, D-dimer, hemoglobin, platelet count, LDH, and oxygen saturation variables were also included in the model. We retained all candidate predictors in the model and did not remove any of these predictors based on statistical significance. The associations between candidate predictors and all-cause death were quantified by the adjusted hazard ratio with a 95% confidence interval. Two more models were created for sensitivity analysis: (i) propensity score (PS) adjusted multivariable Cox proportional hazard and (ii) mixed Cox proportional hazard model after PS matching. To obtain the PS, we fit the multivariable logistic regression analysis with the previous OAC as the outcome conditional on the following covariates: age, gender, HT, DM, HF, CAD, pulmonarythromboembolism eGFR, albumin, CRP, D-dimer, hemoglobin, platelet count, LDH, oxygen saturation, troponin, procalcitonin, ferritin,and white blood cell. The nearestneighbor 2:1 matching algorithm was used with calipers of 0.2 SDs of the logit of the PS. When creating both the primary analysis and the PS score, numerical variables were included in the models with a restricted cubic spline (4 knots) transformation. Due to the non-collapsibility of the hazard ratio, to obtain a more precise hazard ratio, the PS-adjusted multivariable Cox proportional hazard model included age, gender, and PS with previous OAC use, while themixed Cox proportional hazard model after PS matching model included age and gender with previous OAC use.
Results
A total of 5575 patients were enrolled in the study. The median age was 64 (IQR 51–74), and 2801 patients were male (50.2%). Among the patients, 451 (8.1%) were using previous OAC. The baseline characteristics of the patients using and not using OAC are shown in Table 1. Among the 451 patients using OAC, 383 were using DOAC, and 68 were using warfarin. Among the 383 patients using DOAC, 178 were using rivaroxaban, 164 were using apixaban, 29 were using edoxaban, and 12 were using dabigatran.Previous OAC patients had significantly more comorbidity, were older, and had higher levels of inflammatory biomarkers than those who did not use OAC.After PSmatching, a good balance was observed between the groups that used the previous AC and those that did not (Table2). The median follow-up time was 4 days (8–12) in previous OAC patients and7days (4–11) in patients who did not receive previous OAC (overall median follow-up 7 days, IQR 4–11). A total of 4,961 (88.9%) patients were discharged alive, and 616 (10.1%) died at follow-up. Amongthe 616 patients who died, 62 had received OAC, and 554 had not received OAC (previous OAC patients were 62/451 = 14%, previous OAC was 554/5124 = 11%, unadjusted hazard ratio = 1.08, 95% CI 0.83–1.40, p = 0.575). Table2 shows the clinical characteristics of the patients who died and survived during follow-up.
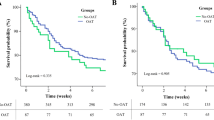
In the adjusted multivariable Cox proportional hazard model, previous OAC use was found to be associated with a significantly lower mortality risk (adjusted hazard ratio 0.62, 95% CI 0.42–0.92, p = 0.030) (Fig. 1). In the PS-based secondary analyses of both the PS-adjusted multivariable Cox proportional hazard model (adjusted hazard ratio 0.72, 95% CI 0.54–0.95, p = 0.018) and the multivariable mixed Cox proportional hazard model made in the propensity matched population (adjusted hazard ratio 0.65, 95% CI 0.47–0.88, p = 0.030), previous OAC use was associated with a significantly lower mortality risk.
In the subgroup analyses, a significant interaction was found between previous OAC use and sex, CAD, HF, and eGFR (interaction p < 0.05). The lower risk of mortality with the previous use of AC was more apparent in males than in females. The lower risk of mortality with previous OAC use was more evident in those with HF than in those without, while the lower risk of mortality with previous use of OAC was more evident in those without CAD than in those with CAD. The risk of mortality with previous OAC use was similar when the eGFR was < 100 compared with previous OAC use, whereas the use of previous OAC was associated with higher mortalitywhen it was > 100 (Fig. 2).
Discussion
This study found that previous use of OAC associated with lower mortality in patients hospitalized due to COVID-19.
COVID-19 is a viral infection that causes a sepsis-like clinical presentation in severe cases, causing systemic inflammation and hypercoagulopathy. Although the main mechanism of hypercoagulopathy in COVID-19 is not clearly understood, micro- and macro-embolisms have been detected in some postmortem studies [15,16,17]. Inflammation causes vascular damage as a result of endothelial dysfunction and hypercoagulopathy. Occasionally, disseminated intravascular coagulation and venoarterial TE develop secondary to an excessive inflammatory response. Pulmonary and extrapulmonary microvascular thrombosis can cause acute lung injury, progression of the disease, and subsequent multiorgan dysfunction [18]. This situation causes serious morbidity and even mortality. Therefore, parenteral or oral anticoagulant therapy is recommended to prevent TE events.
Parenteral anticoagulants are used for TE prophylaxis in COVID-19 both during treatment and after discharge. In addition to the anticoagulant effects of these agents, antiviral, anti-inflammatory, and cytoprotective effects have also been observed [19]. DOAC (e.g., dabigatran, rivaroxaban, apixaban, edoxaban), especially factor Xa inhibitors, may be considered as another option in the treatment of these patients due to their similar effects [20,21,22]. In our study, some patients used warfarin and DOAC. We consider our findings on mortality to be due to the effects of DOAC.
In the review written by Harenberet al.,the clinical course of patients with severe COVID-19 and a history of non-valvular AF or deep vein thrombosis and using OAC was milder [23]. Denas et al. analyzed 4697 patients over 65 years and found that all-cause mortality was lower in COVID-19 patients who received chronic OAC treatment [24]. Similar results were observed in our study. In the presentstudy, groups with similar comorbidities were formed by PS matching, and factors that could affect the results were minimized.
In a Swedishregistry study by Flam et al., no positive or negative effects of DOAC on mortality were found [18].In this study, only patients with AF and using DOAC were included in the sample. However, unlike our study, both those using DOAC and those using warfarin were included in the analysis. Although the majority of the population used OAC with the diagnosis of AF, those who used OAC for any indication other than AF were also included in the analysis.
In addition, our results could not be generalized to all COVID-19 patients in the general population because of selection conditions based on hospitalized patients and this selection bias may lead to an artifactual association between previous use of OAC and primary outcomes due to those receiving OAC may be being treated better or healthier than those who need OAC but not on this therapy.
The hospital admission period of COVID-19 patients is sometimes late, and TEevents may develop during this time. This situation aggravates the clinical picture and has a negative effect on in-hospital mortality. In light of the findings of our study, we consider that in-hospital mortality may be observed less in patients who previously used OAC.
Conclusion
In hospitalized COVID-19 patients,in patients who previously used anticoagulantswas associated with lower risk of in-hospital death than in those who did not. However, these findings should be interpreted carefully because of unmeasured confounding and supported by further research.
Limitations
Our study has some limitations. Primarily due to the nature of retrospective studies, the results may be biased because of measured and unmeasured confounding. Although measured confounding wasreduced by regression analysis and propensity matching, the results should be interpreted with caution because of unmeasured confounding and residual confounding. The fact that the patients were taken from a single center andthe relatively low number of patients can also be considered limitations. Moreover, the interaction of other drugs used by patients with anticoagulant drugs was not detected.
Data availability
Available.
References
WHO (2020) Coronavirus disease (COVID-19) pandemic
Vinayagam S, Sattu K (2020) SARS-CoV-2 and coagulation disorders in different organs. Life Sci 260:118431. https://doi.org/10.1016/j.lfs.2020.118431
Kruse JM, Magomedov A, Kurreck A, Munch FH, Koerner R, Kamhieh-Milz J et al (2020) Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care 24(1):676. https://doi.org/10.1186/s13054-020-03401-8
Song P, Li W, Xie J, Hou Y, You C (2020) Cytokine storm induced by SARS-CoV-2. Clin Chim Acta 509:280–287. https://doi.org/10.1016/j.cca.2020.06.017
Barnes GD, Burnett A, Allen A, Blumenstein M, Clark NP, Cuker A et al (2020) Thromboembolism and anticoagulant therapy during the COVID-19 pandemic: interim clinical guidance from the anticoagulation forum. J Thromb Thrombolysis 50(1):72–81. https://doi.org/10.1007/s11239-020-02138-z
Lippi G, Sanchis-Gomar F, Favaloro EJ, Lavie CJ, Henry BM (2021) Coronavirus disease 2019-associated coagulopathy. Mayo Clin Proc 96(1):203–217. https://doi.org/10.1016/j.mayocp.2020.10.031
Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al (2020) ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. https://doi.org/10.1093/eurheartj/ehaa612
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M et al (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J 39(33):3021–3104. https://doi.org/10.1093/eurheartj/ehy339%JEuropeanHeartJournal
Chamberlain JJ, Rhinehart AS, Shaefer CF Jr, Neuman A (2016) Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 164(8):542–552. https://doi.org/10.7326/M15-3016
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan China. JAMA 323(11):1061–1069. https://doi.org/10.1001/jama.2020.1585
T.C. SAĞLIK BAKANLIĞI HALK SAĞLIĞI GENEL MÜDÜRLÜĞÜ.COVID-19 (SARS-CoV-2 ENFEKSİYONU) GENEL BİLGİLER, EPİDEMİYOLOJİ VE TANI 2020
T.C. SAĞLIK BAKANLIĞI HALK SAĞLIĞI GENEL MÜDÜRLÜĞÜ.COVID-19 (SARS-CoV-2 ENFEKSİYONU)ERİŞKİN HASTATEDAVİSİ. 2020
T.C. SAĞLIK BAKANLIĞI HALK SAĞLIĞI GENEL MÜDÜRLÜĞÜ. COVID-19 (SARS-CoV-2 ENFEKSİYONU) AĞIR PNÖMONİ, ARDS, SEPSİS VE SEPTİK ŞOK YÖNETİMİ. 2020
T.C. SAĞLIK BAKANLIĞI HALK SAĞLIĞI GENEL MÜDÜRLÜĞÜ. COVID-19 (SARS-CoV-2 ENFEKSİYONU) ANTİSİTOKİN-ANTİİNFLAMATUAR TEDAVİLER, KOAGÜLOPATİ YÖNETİMİ. 2020
Rico-Mesa JS, Rosas D, Ahmadian-Tehrani A, White A, Anderson AS, Chilton R (2020) The role of anticoagulation in COVID-19-induced hypercoagulability. Curr Cardiol Rep 22(7):53. https://doi.org/10.1007/s11886-020-01328-8
Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A et al (2020) Autopsy findings and venous thromboembolism in patients With COVID-19: a prospective cohort study. Ann Intern Med 173(4):268–277. https://doi.org/10.7326/M20-2003
Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN et al (2020) Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost 18(6):1517–1519. https://doi.org/10.1111/jth.14844
Flam B, Wintzell V, Ludvigsson JF, Martensson J, Pasternak B (2020) Direct oral anticoagulant use and risk of severe COVID-19. J Intern Med. https://doi.org/10.1111/joim.13205
Kipshidze N, Dangas G, White CJ, Kipshidze N, Siddiqui F, Lattimer CR et al (2020) Viral coagulopathy in patients With COVID-19: treatment and care. Clin Appl Thromb Hemost 26:1076029620936776. https://doi.org/10.1177/1076029620936776
Sutherland MR, Simon AY, Shanina I, Horwitz MS, Ruf W, Pryzdial ELG (2019) Virus envelope tissue factor promotes infection in mice. J Thromb Haemost 17(3):482–491. https://doi.org/10.1111/jth.14389
Sparkenbaugh EM, Chen C, Brzoska T, Nguyen J, Wang S, Vercellotti GM et al (2020) Thrombin activation of PAR-1 contributes to microvascular stasis in mouse models of sickle cell disease. Blood 135(20):1783–1787. https://doi.org/10.1182/blood.2019003543
Pryzdial ELG, Sutherland MR, Lin BH, Horwitz M (2020) Antiviral anticoagulation. Res Pract Thromb Haemost 4(5):774–788. https://doi.org/10.1002/rth2.12406
Harenberg J, Favaloro E (2020) COVID-19: progression of disease and intravascular coagulation - present status and future perspectives. Clin Chem Lab Med 58(7):1029–1036. https://doi.org/10.1515/cclm-2020-0502
Denas G, Gennaro N, Ferroni E, Fedeli U, Lorenzoni G, Gregori D et al (2020) Reduction in all-cause mortality in COVID-19 patients on chronic oral anticoagulation: a population-based propensity score matched study. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2020.12.024
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no conflict of interest to declare.
Consent for publication
Authors have agreed for the final version of this paper to be submitted for publication.
Ethical approval
From Erzurum Education and Research Hospital Ethical Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gülcü, O., Aksakal, E., Aydemir, S. et al. Association between previous anticoagulant use and mortality among hospitalized patients with COVID-19. J Thromb Thrombolysis 53, 88–95 (2022). https://doi.org/10.1007/s11239-021-02489-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-021-02489-1