Abstract
Loss/alteration of Smell and taste sensation is common in CoViD-19 infection. We conducted present study to find out the frequency, onset and severity of these lost sensations and their recovery in mild, moderate and severe COVID 19 positive patients in our setup. A questionnaire based study on 574 COVID-19 positive patients admitted in a dedicated COVID hospital between September–November, 2020 were followed up until their sensations recovered completely or maximum for two months. Fever was the most common symptom reported. Loss of smell and taste sensation is seen in 200 (34.84%) and 269 (46.86%) patients respectively; 163 (28.4%) developed both. Males were affected significantly more than females (p = 0.030 and 0.027). Approximately 1/4th patients [49 (24.5%) and 55 (20.45%)] reported loss of smell and taste sensation as their first symptom. Most common taste sensation lost was salty 191 (71.0%). Loss of smell sensation is seen maximally in mild cases and the difference among mild, moderate and severe cases is statistically significant (p = 0.00001); while the difference in loss of taste among all three grades of severity is statistically insignificant (p = 0.0770). Most of the patients [smell (142; 71%) and taste (198; 73.6%)] recovered after 2 weeks of onset of lost sensations while 96.5% (193/200) and 98.1% (264/269) patients reported complete recovery of smell and taste sensations after two months of onset. Present study shows that high percentage of COVID-19 positive patients develop loss of either one or both of smell and taste sensations but recovery is fast and complete in most of them.
Similar content being viewed by others
Introduction
COVID-19 was characterised by the World Health Organisation (2020) as a global pandemic and health emergency on March 11, 2020, which led to a world wide concern [1]. The disease is caused by a new coronavirus (SARS-CoV-2) from a potential bat origin [2] and from which viral genome was rapidly characterized [3]. In January 2020, angiotensin-converting enzyme 2 (ACE2) was identified as the functional receptor for SARS-CoV-2, present in multiple human organs including the central nervous system [4]. Viral entry occurs, after the proteolytic cleavage of the spike (S) protein by the transmembrane protease TMPRSS2 [5, 6]. Interestingly, ACE2 and TMPRSS2 show an extremely high expression in characteristic cells of the nasal epithelium, goblet, and ciliated cells. These cells are the candidates as loci of original viral infection and possible reservoirs for dissemination; in addition, SARS-CoV-2 is an enveloped virus that does not require cell lysis for viral release. Thus, the virus might exploit existing secretory pathways in nasal goblet cells for low-level, continuous release at the early stage with no overt pathology [7].
Smell impairment was first observed among other neurologic manifestations of COVID-19 in hospitalised patients [8] and subsequently has been reported to be a common symptom in patients with mild disease [9, 10]. There are many known causes of acquired smell loss include URTI by respiratory viruses (adenovirus, rhinovirus, coronavirus, influenza), traumatic brain injury, upper airway inflammation (rhinitis, rhinosinusitis), and neurodegenerative (Parkinson and Alzheimer) diseases while minor causes are intracranial/ sinonasal tumors, drugs, exposure to toxic substances, irradiation, or iatrogenic factors [11]. The onset of smell and/or taste loss is often abrupt in COVID infection and, unlike other upper respiratory tract infections, it often occurs in the absence of nasal obstruction [12, 13] The prevalence of loss of smell widely ranged from 10% to over 80% in European countries [14]. A systematic review and meta-analysis reported a pooled prevalence of 52.7% and 43.9% for olfactory and gustatory dysfunction, respectively in patients with COVID-19 infection [15].
L A Vaira C Hopkins et al. reported that within the first 4 days of COVID-19 symptom onset, 84.8% of patients had chemo sensitive dysfunction. Specifically, severe olfactory and gustatory disorders affected 60.9% and 40.6 percent of patients, respectively [16]. Another study by Vikas gupta et al. reported that out of 387 COVID-19 patients, 167 (43.15%) patients had olfactory disfunction, 153 (39.53%) patients had gustatory disfunction and 105 (27.1%) patients had both [17].
In a study done by Valeria Dell Era et al. 49.5% and 50.4% patients declared full recovery of smell and taste respectively at 14th day of infection, with a median recovery time of ten days [18]. Vikas Gupta et al. reported that they found complete recovery of smell and taste in 96.4% and 96.73% patients respectively by 8 weeks of onset [17].
As limited data is available on prevalence, severity, and recovery pattern of involvement of smell and taste sensation in mild, moderate, and severe SARS-CoV-2 patients from India, present study is planned to estimate the same in patients from western part of India.
Methodology
This prospective, analytical questionnaire base study was conducted from September 2020 to January 2021. It recruited adult patients (18 years or more) who tested positive and treated for SARS-CoV-2 RNA by RT-PCR on nasopharyngeal and throat swabs, admitted in a dedicated COVID hospital in western India between September to November, 2020. Enrolled patients were followed up for two months at pre-defined intervals.
Pregnant women, children (< 18 years), patients with history of previous smell and|or taste disorders, allergic rhinitis, rhino sinusitis and dementia (who cannot report functional symptoms) were excluded from study. Patients who did not complete two months of follow-up were also excluded from the analysis.
The study was conducted with the approval of ethical committee of the hospital and informed consent was obtained verbally for telephonic interview. Patients were allowed to leave the interview in between also if they wish so.
A questionnaire was developed which was validated by two subject experts and revised as per their feedback. It was administered telephonically by principal investigator in native language and responses were recorded in Google form simultaneously.
Information was collected regarding age, gender, medical history (Co-morbidities and risk factors) general symptoms (fever, cough, difficulty in breathing, headache, body ache, abdominal pain, loose motion, vomiting, weakness and loss of appetite), ENT symptoms with special references to reduced, complete loss or altered sensation of taste and smell, their duration and recovery pattern separately. Information regarding altered smell and taste sensations before COVID infection, past medical or surgical conditions or co-medications that can cause altered smell or taste sensation, history of nasal allergy and smoking habit was also collected.
Patients were enrolled in the study at the time of discharge from the hospital. Subsequent calls were made after 2 weeks, one month and two month after discharge.
Statistical analysis: spread sheet was generated from Google forms and continuous variables were expressed as mean and standard deviation (SD). Categorical variables were expressed as numbers and percentages. strength of association for categorical variables has been calculated by chi square test.
Results
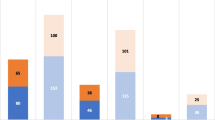
We recruited 605 patients; out of those, 31 patients were excluded from the final analysis as they could not be followed up completely (lost to follow-up; 5.12%). The mean (SD) age of 574 patients analyzed was 46.60 (14.63)years; Male: female ratio was found to be 2.1:1. Out of all the admitted patients, 176 (30.7%) received oxygen therapy through mask, 49 (8.54%) were admitted in Intensive Care Unit, and 11 (1.9%) received oxygen therapy through non-invasive ventilation. None were treated by invasive ventilation. Fever was the most common presenting symptom. All the general symptoms and ENT symptoms are summarized in Table 1. Patients presented with various co-morbidities are depicted in Fig. 1. Figure 2 is showing various symptoms patients were suffering with even after 14 days of illness. Of all the respondents, 20 (3.5%) were regular smoker.
Two hundred seventy nine (48.6%) of 574 respondents, reported a chemosensory deficit, out of which, 207 (36.1%) had sudden onset while 72 (12.5%) had gradual loss of sensations.
Two hundred (34.84%) patients reported a change in smell perception while 269 patients (46.86%) reported change in taste perception. 163 (28.4%) patients reported alteration in both smell and taste sensations. Their onset, type of involvement and recovery pattern is shown in Table 2. Those who had altered taste sensation, 191 (71.0%) lost the salty taste sensation, 108 (40.15%) lost sweet sensation, 87 (32.34%) lost bitter and 60 (22.30%) lost sour sensation. Many patients had loss of more than one taste sensation. After two months follow-up, 07 (3.5%) patients reported that their smell sensation is not recovered while 05(1.9%) patients had incomplete recovery from taste sensation (Table 2).
Loss of sensations was seen more in males as compared to females. Male: female ration for altered smell and taste sensation was 1.63:1 (124 vs. 76/200) and 1.72:1 (170 vs. 99/269) which was statistically significant (p value of 0.030 and 0.027) respectively.
Among 200 patients who experience altered smell sensation, 40 (20%) patients reported nasal blockage and 20 (10%) patients reported running of nose.
For sub-group analysis, we divided patients into mild, moderate, and severe groups as per clinical guidelines of ICMR and Ministry of Health and Family Welfare, Government of India, to compare frequency and recovery pattern of smell and taste dysfunctions among these groups.
Mild disease group includes patients (n = 338) with upper respiratory tract symptoms with or without fever without shortness of breath/hypoxia and admitted in COVID ward for observation and symptomatic treatment. Moderate disease group (n = 176) includes patients with breathlessness and SpO2 90% to 93% on room air and required oxygen by face mask. Severe disease group included 60 patients who presented with breathlessness, SpO2 < 90% on room air and respiratory rate > 30/minute. They were admitted in HDU/ICU and required high flow oxygen or non invasive ventilation. A comparative table (Table 3) shows that the loss of smell sensation is seen maximally in mild cases and the difference among mild, moderate and severe cases are statistically significant (p value 0.00001); while the difference in loss of taste sensation among all three grades of severity is statistically insignificant (p value 0.0770).
Discussion
This prospective analytical study was undertaken to assess the involvement of smell and taste sensation, its duration, and recovery pattern in RTPCR positive COVID-19 patients, and also to compare these amongst mild, moderate and severe cases.
In present study, 34.84% (200/574) patients reported alteration/loss of smell, while 46.86% (269/574) patients reported alteration/loss of taste sensation. Larco et al. reported that the frequency of anosmia in COVID-19 affected persons ranged between 22 and 68% and frequency of taste dysfunction from 20 to 33% [19]. Another series of 417 patients with COVID-19 reported the involvement of smell and taste sensation in 85.6% and 88.0% respectively which is much higher than reported in present study [20]. Paolo Boscolo-Rizzo et al. reported prevalence of smell and taste dysfunction in 66.3% patients in their case series of 202 patients [21] Mullol et al. showed a high variability of smell or taste dysfunction ranging from 5 to 98% depending on the methodology used in the study and country [22]. A meta analysis based on data from 24 studies on 8438 patients reported prevalence of olfactory and gustatory dysfunction in 41⋅0% and 38⋅2%, respectively [23]. Another study done on 718 patients reported that 101 (14%) patients experienced either altered smell or taste, with 52 (7%) experiencing both altered smell and taste. Seventy-seven (10.7%) patients had altered smell and 76 patients had altered taste sensation (10.5%) [24].
We observed a clear male predominance in our study as 62% (124/200) male and 38% (76/200) female patients reported altered smell sensation while 63.1% (170/269) male and 36.8% (99/269) female patients reported alteration in taste sensation which is in contrast with other studies presenting female predominance [25].
Out of 60 patients with severe disease, altered smell was found in 30% (18) patients and altered taste was seen in 50% (30) patients with 100% recovery in altered smell and 93.33% in altered taste within 2 months. Andrea mazzatenta et al. conducted a study on 100 patients with severe CoViD-19 infection who were on assisted breathing with oxygen therapy, reported that smell impairment occured in 95% of patients, while 47% showed taste dysfunction [26].
There is paucity of data among severely affected patients as the description of anosmia and dysgeusia may seem unnecessary when clinicians deal with critically ill patients (Yang et al. 2020) [27]. Furthermore, details of symptoms are difficult to obtain when patients are critical.
Present study reports 68% (136/200) patients developed onset of loss of smell and 67.66% (182/269) patients develop altered taste sensation within seven days of onset of illness. A study done by Claire hopkins et al. found that 60% (229/382) and 94.8% patients reported onset of anosmia and reduced sense of taste respectively in less than 1 week [28].
Gluseppe mercante et al. found a significant association between nasal obstruction (38.2%) and runny nose (40.2%) with severe reduction of taste and smell [29] in our study only 20% (40/200) patients reported nasal blockage and 10% (20/200) patients reported running of nose. In 2007, Suzuki et al. demonstrated that coronavirus may be detected in the nasal discharge of patients with olfactory dysfunction. They observed that some patients with normal acoustic rhinometry did not recover their olfaction, suggesting that nasal inflammation and related obstruction were not the only etiological factors underlying the olfactory dysfunction in viral infection. This could explain the presence of olfactory dysfunction without nasal congestion [30].
We observed that most of patients with altered smell 96.5% (193/200) and taste 98.1% (264/269) sensation recovered to normal after two months of onset. A study done by Jerome R. Lechien et al. showed 79.5% of patients recovered normal smell sense within 2 months following the onset of loss of smell [31]. Pradipt ranjan sahoo et al. reported that 92% patients regained olfaction and 96% regained taste sensation, on follow-up at the 2 weeks of the PCR positivity [24]. The varied time taken for the recovery in different patients may be due to the various pathophysiological mechanisms causing damage to neuronal tract. Studies have suggested that COVID-19 could be neuro invasive in the central structures of the olfactory system [32]. Mao et al. [33] suggested 3 possible means of entry of SARS-CoV-2 into the brain; via olfactory nerves in the nasal cavity, through interaction with angiotensin-converting enzyme-2 (ACE-2) receptor in the brain, and through cytokine storm-induced blood–brain barrier disruption. This variability of the virus’s potential entry into and damage to peripheral and central structures of the olfactory system may result in varied presentations.
Fever (81.9%) was most common symptom found in our study followed by cough (49.5%), body aches (40.8%) and dyspnea (29.6%). kadhiresan jayashree et al. reported fever (30%) as most common symptom followed by headache (18%) and cough (18%) in their COVID-19 positive patients [34].
The strength of present study is the adequate sample size of patients. We have also compared the frequency, duration and recovery of smell and taste alterations among mild, moderate and severe cases of COVID19 positive patients which to the best of our knowledge, no study has compared and published the data. It has got its limitations too: it was self reported loss of sensations rather than their objective assessment. Secondly, we lost 31 (5.12%) patients who could not complete all the follow-ups but even this is well within the acceptable range.
Conclusions: The present study confirms the high prevalence of olfactory and gustatory disorders in COVID-19 infection. Smell and taste loss may be used as indicators of potential infection and early identification may help to reduce the risk of spread, especially in asymptomatic /pre symptomatic cases. It also concludes that majority of patients recovers completely within two months of onset of altered sensations.
References
Speth MM (2020) Olfactory dysfunction and sinonasal symptomatology in covid-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg 163(1):114–120
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W et al (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579(7798):270–273
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H et al (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395(10224):565–574
Joaquim M, Isam A et al (2020) the Loss of Smell and Taste in the COVID -19 Outbreak: a Tale of Many Countries. Curr Allergy Asthma Rep. https://doi.org/10.1007/s11882-020-00961-1
Matsuyama S, Nagata N, Kazuya S, Kawase M, Takeda M, Taguchi F (2010) Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J Virol 84:12658–12664
Hoffmann M, Kleine W H, Kruger N, Muller M, Drosten C, Pohlmann, S. The novel coronavirus 2019. (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv [Preprint]. (2020). doi: https://doi.org/10.1101/2020.01.31.929042.
Sungnak W, Huang N, Beavin C, Berg M, Lung (2020) Biological Network HCA. SARS-CoV-2 entry genes are most highly expressed in nasal goblet and ciliated cells within human airways. Nat Med 26:681–7
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with Coronavirus Disease 2019 in Wuhan. JAMA Neurol China. https://doi.org/10.1001/jamaneurol.2020.1127
Lechien JR, Chiesa-Estomba CM, DeSiati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-020-05965-1
Spinato G, Fabbris C, Polesel J et al (2020) Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 323(20):2089–2090. https://doi.org/10.1001/jama.2020.6771
Hummel T, Whitcroft KL, Andrews P, Altundag A, Cinghi C, Costanzo RM et al (2017) Position paper on olfactory dysfunction. Rhinol Suppl 54:1–30
Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S (2020) Loss of smell and taste in 2013 european patients with mild to moderate COVID-19. Ann Intern Med 173(8):672–675. https://doi.org/10.7326/m20-2428
Gengler I, Wang JC, Speth MM, Sedaghat AR (2020) Sinonasal pathophysiology of SARS-CoV-2 and COVID-19: a systematic review of the current evidence. Laryngoscope Investig Otolaryngol 5(3):354–359. https://doi.org/10.1002/lio2.384
Le Bon SD, Pisarski N, Verbeke J et al (2020) Psychophysical evaluation of chemosensory functions 5 weeks after olfactory loss due to COVID-19: a prospective cohort study on 72 patients. Eur Arch Oto-Rhino-Laryngol 278(1):101–108
Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T (2020) The prevalence of olfactory and gustatory dysfunction in covid-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 163(1):3–11. https://doi.org/10.1177/0194599820926473
Vaira LA, Hopkins C, Petrocelli M et al (2020) Smell and taste recovery in coronavirus disease patients: a 60-day objective and prospective study. J Laryngol Otol. https://doi.org/10.1017/S0022215120001826
Gupta V, Lohith BR et al (2021) Olfactory and gustatory dysfunction in covid-19 patients from northern india: a cross-sectional observational study. Indian J Otolaryngol Head Neck Surg. https://doi.org/10.1007/s12070-021-02391-5
Dell’Era V, Farri F, Garzaro G, Gatto M, Aluffi Valletti P, Garzaro M (2020) Smell and taste disorders during COVID-19 outbreak: a cross-sectional study on 355 patients. Head & Neck. https://doi.org/10.1002/hed.26288
Carrillo-Larco RM, Altez-Fernandez C. Anosmia and dysgeusia in COVID-19: a systematic review. In: Wellcome Open Research. vol. 5. F1000 Research Ltd; 2020. https://doi.org/10.12688/wellcomeopenres.15917.1
Lechien JR, Chiesa-Estomba CM, De Siati DR et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277(8):2251–2261. https://doi.org/10.1007/s00405-020-05965-1
Paolo Boscolo-Rizzo MD, Daniele B (2020) Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg 146(8):729–732. https://doi.org/10.1001/jamaoto.2020.1379
Mullol J, Alobid I, Mariõ-Sańchez F et al (2020) The loss of smell and taste in the COVID-19 outbreak: a tale of many countries. Curr Allergy Asthma Rep 20(10):61
Agyeman AA, Lee Chin K, Landersdorfer CB, Liew D, Ofori-Asenso R (2020) Smell and taste dysfunction in patients with COVID-19: a systematic review and meta-analysis. Mayo Clin Proc 95(8):1621–1631. https://doi.org/10.1016/j.mayocp.2020.05.030
Sahoo PR, Sahu M et al (2021) Evolution of olfactory and gustatory dysfunctions in COVID-19 patients in India. European Arch Oto-Rhino-Laryngol. https://doi.org/10.1007/s00405-020-06563-x
Mercante G, Ferreli F et al (2020) Prevalence of taste and smell dysfunction in coronavirus disease. JAMA Otolaryngol Head Neck Surg 146(8):723–728
Mazzatent A, Neri G, D’Arde D et al (2020) Smell and taste in severe covid-19: self-reported vs testing. Front Med. https://doi.org/10.3389/fmed.2020.589409
Yang X, Yu Y, Xu J, Shu H, Xia J’an, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single- centered, retrospective, observational study. Lancet Respir Med [Internet] 2020; (February). doi:https://doi.org/10.1016/S2213-2600(20)30079-5
Hopkins C, Surda P, Whitehead E, Kumar BN (2020) Early recovery following new onset anosmia during the COVID-19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg. https://doi.org/10.1186/s40463-020-00423-8
Mercante G, Ferrel F, Virgilio AD et al (2020) Prevalence of taste and smell dysfunction in coronavirus disease 2019. JAMA Otolaryngol Head Neck Surg. 146(8):723–728
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, Mur- akami S (2007) Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 117(2):272–277
Lechien JR, Journe F, Hans S (2020) Severity of anosmia as an early symptom of covid-19 infection may predict lasting loss of smell. Front Med. https://doi.org/10.3389/fmed.2020.582802
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92(6):552–555. https://doi.org/10.1002/jmv.25728
Mao L, Jin H, Wang M, Hu Y, Chen S, He Q et al (2020) Neurologic manifestations of hospitalized patients with coro- navirus disease 2019 in Wuhan. China JAMA Neurol 77(6):683–690. https://doi.org/10.1001/jamaneurol.2020.1127
Jeyashree K, Raju M, Ponnaiah M (2021) Self-reported and clinically identified loss of smell and taste among persons tested for COVID-19 in Chennai, southern India, July-August 2020: a cross sectional study. Clin Epidemiol Glob Health Jul-Sep. https://doi.org/10.1016/j.cegh.2021.100718
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedure performed in the study were in accordance with the ethical standards of the institution.
Informed Consent
Informed consent was obtained from all individual participant in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Goyal, R., Kapoor, A., Goyal, M.K. et al. Alteration of Smell and Taste Sensations in Covid-19 Positive Patients: A Prospective Cohort Study in Western India. Indian J Otolaryngol Head Neck Surg 73, 371–377 (2021). https://doi.org/10.1007/s12070-021-02670-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12070-021-02670-1