Abstract
Keywords
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic has infected millions of people and caused more than 1 million deaths worldwide, leading to the immediate demand for vaccination (1, 2). The inactivated Sinopharm’s China National Biotec Group developed the Sinopharm COVID-19 vaccine to prevent the COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS CoV 2). The vaccine has been tolerable and immunogenic in healthy people, with 2 doses injected 21 days apart (3). Sinopharm COVID-19 vaccination is universal, and its complications are not entirely known. The Sinopharm COVID-19 vaccine caused a branch retinal vein occlusion (BRVO) three weeks after the second dose. This event could have been coincidental to its use, although there are conditions, which can suspect the relationship in a natural cause-and-effect association.
2. Case Presentation
A 75-year-old female with sudden blurred vision in her left eye was referred to our clinic. She was non-obese, without a history of smoking, diabetes mellitus, or hypertension, and had no pathological or family history. Three weeks prior, she had received the second dose of the Sinopharm COVID-19 vaccine. Her vision reduced from the third day after vaccination, but she ignored it, and her vision reduction has progressed to blindness. The patient was PCR negative for SARS-Cov2.
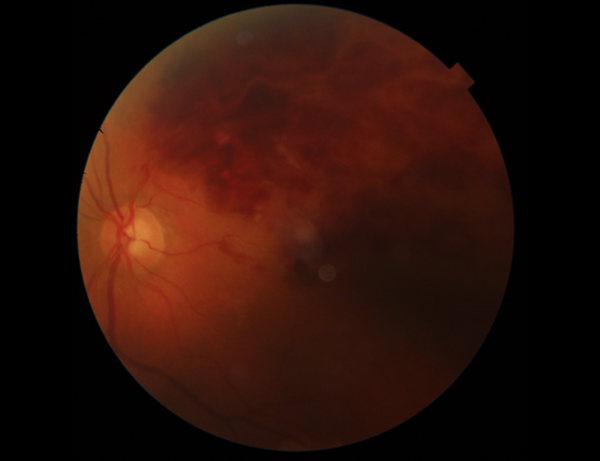
Her best-corrected visual acuity (BCVA) was 1m counting finger in the left eye and 10/10 in the right eye. The fundus examination demonstrated superior hemiretinal hemorrhage in the left eye Figure 1.
Fundus examination showed superior hemiretinal hemorrhage in the left eye.
The fluorescein angiography (FA) was performed on admission indicating the superior arcade blockage due to retinal and pre-retinal hemorrhage, dilation, tortuosity of the superior vein, vascular staining, and macular edema in the left eye. Fluorescein angiography of the right eye was normal. Topcon macular optical coherence tomography (OCT) was performed on the same day, showing a cystoid macular edema (CME), increased macular retinal thickness, and serous retinal detachment (central retinal thickness (CRT): 649 µm, average retinal thickness: 567.1 µm, and total volume 16.03 mm3). Optical coherence tomography of the right eye was normal.
The left eye was diagnosed with vaccine-induced CRVO. In addition, CBC panel, d-dimer, antithrombin III, homocysteine, coagulation protein C and S, pre and postprandial glycemia, lipid profile, creatinine, and ambulatory monitoring of blood pressure, were asked. In the end, blood reports showed no abnormalities.
Antithrombotic treatment of 80 mg/d low-dose entrocoated ASA was administered, accompanied by an intravitreal Aflibercept (Eylea) injection monthly and ongoing, starting from 05/09/2021.
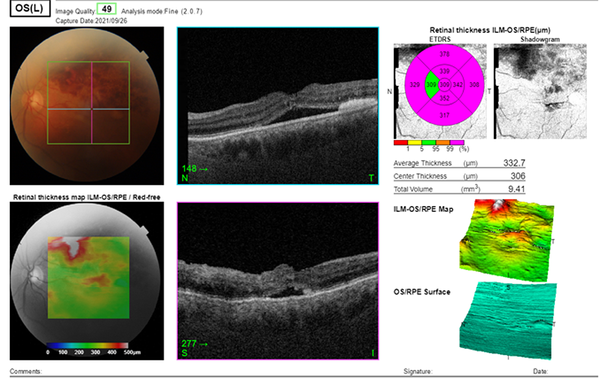
At the three-week follow-up, superior hemiretinal hemorrhage decreased on the fundus photograph, and the BCVA of the left eye improved to 2/10. Macular edema, retinal hemorrhage, and height of serous retinal detachment decreased on OCT Figure 2.
Macular edema, retinal hemorrhage, and height of serous retinal detachment decreased on OCT in the three-week follow-up.
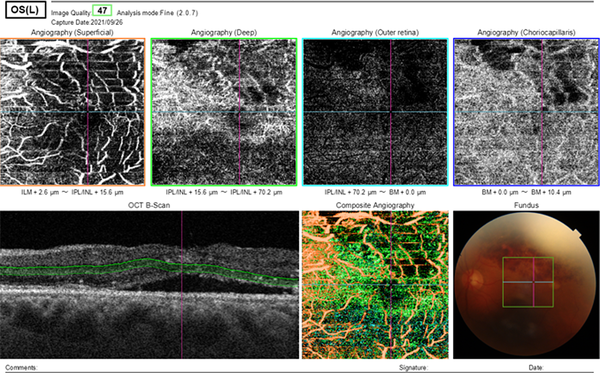
Optical coherence tomography angiography (OCTA) of the left eye showed decreasing in vessel density and disruption in the foveal avascular zone, vascular tortuosity, dilation, and telangiectasia, and capillary non-perfusion in the superficial capillary plexus (SCP), and the deep capillary plexus (DCP). Dark irregular areas without vessel signal in OCTA correspond to CME Figure 3.
OCTA of the left eye showed decreased vessel density and disrupted foveal avascular zone, vascular tortuosity, dilation and telangiectasia, and capillary non-perfusion in SCP and DCP. Dark irregular areas without vessel signal in OCTA correspond to CME.
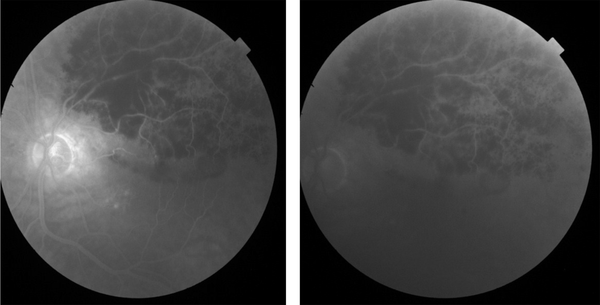
Fluorescein angiography of the left eye showed decreasing vessel tortuosity and dilation, vascular staining, and retinal blockage due to lowering corresponding retinal vessels (Figure 4).
FA of the left eye showed decreasing vessel tortuosity and dilation, vascular staining, and retinal blockage due to the lowering of corresponding retinal vessels.
3. Discussion
Many thromboembolic adverse events have been reported after COVID-19 vaccinations, including systemic inflammation, platelet dysfunction, and endothelial dysfunction (4), although there is evidence of ophthalmic complications.
To the best of our knowledge, there are no reported cases in the world literature reporting BRVO related to COVID-19 vaccination. Cardiovascular diseases, hypertension, increased body mass index, glaucoma, diabetes mellitus, and hematologic disorders are common risk factors for BRVOs (5-7). Our patient had no previous history of any of these situations.
This old-aged woman experienced vision reduction from the third day after the second dose of the Sinopharm COVID-19 vaccine, but she ignored the condition. The vision reduction progressed to blindness, and she was referred three weeks after vaccination. The pathophysiology, in this case, is currently unclear, and causality may be revealed in future studies.
3.1. Conclusions
In general, the present case may show a Sinopharm COVID-19 vaccination-induced BRVO. Therefore, BRVO should be added to the possible ophthalmic complications after Sinopharm COVID-19 vaccination. After vaccinations, patients with symptomatic eye conditions ought to consult an ophthalmologist. In the end, further research and observations are warranted.
References
-
1.
Carli G, Nichele I, Ruggeri M, Barra S, Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern Emerg Med. 2021;16(3):803-4. [PubMed ID: 33687691]. [PubMed Central ID: PMC7940863]. https://doi.org/10.1007/s11739-021-02685-0.
-
2.
Daniel SJ. Education and the COVID-19 pandemic. Prospects (Paris). 2020;49(1-2):91-6. [PubMed ID: 32313309]. [PubMed Central ID: PMC7167396]. https://doi.org/10.1007/s11125-020-09464-3.
-
3.
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21(1):39-51. [PubMed ID: 33069281]. [PubMed Central ID: PMC7561304]. https://doi.org/10.1016/S1473-3099(20)30831-8.
-
4.
Neri P, Pichi F. COVID-19 and the eye immunity: lesson learned from the past and possible new therapeutic insights. Int Ophthalmol. 2020;40(5):1057-60. [PubMed ID: 32314322]. [PubMed Central ID: PMC7167536]. https://doi.org/10.1007/s10792-020-01389-2.
-
5.
The Eye Disease Case-control Study Group. Risk Factors for Branch Retinal Vein Occlusion. Am J Ophthalmol. 1993;116(3):286-96. https://doi.org/10.1016/s0002-9394(14)71345-5.
-
6.
Johnston RL, Brucker AJ, Steinmann W, Hoffman ME, Holmes JH. Risk factors of branch retinal vein occlusion. Arch Ophthalmol. 1985;103(12):1831-2. [PubMed ID: 4074173]. https://doi.org/10.1001/archopht.1985.01050120065021.
-
7.
Rehak J, Rehak M. Branch retinal vein occlusion: pathogenesis, visual prognosis, and treatment modalities. Curr Eye Res. 2008;33(2):111-31. [PubMed ID: 18293182]. [PubMed Central ID: PMC2430176]. https://doi.org/10.1080/02713680701851902.