Coronavirus Infection of the Central Nervous System: Animal Models in the Time of COVID-19
- Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis, Davis, CA, United States
Naturally occurring coronaviral infections have been studied for several decades in the context of companion and production animals, and central nervous system involvement is a common finding, particularly in cats with feline infectious peritonitis (FIP). These companion and production animal coronaviruses have many similarities to recent human pandemic-associated coronaviruses such as SARS-CoV, MERS-CoV, and SARS-CoV2 (COVID-19). Neurological involvement is being increasingly recognized as an important clinical presentation in human COVID-19 patients, often associated with para-infectious processes, and potentially with direct infection within the CNS. Recent breakthroughs in the treatment of coronaviral infections in cats, including neurological FIP, have utilized antiviral drugs similar to those currently in human COVID-19 clinical trials. Differences in specific coronavirus and host factors are reflected in major variations in incidence and mechanisms of CNS coronaviral infection and pathology between species; however, broad lessons relating to treatment of coronavirus infection present within the CNS may be informative across species.
Introduction
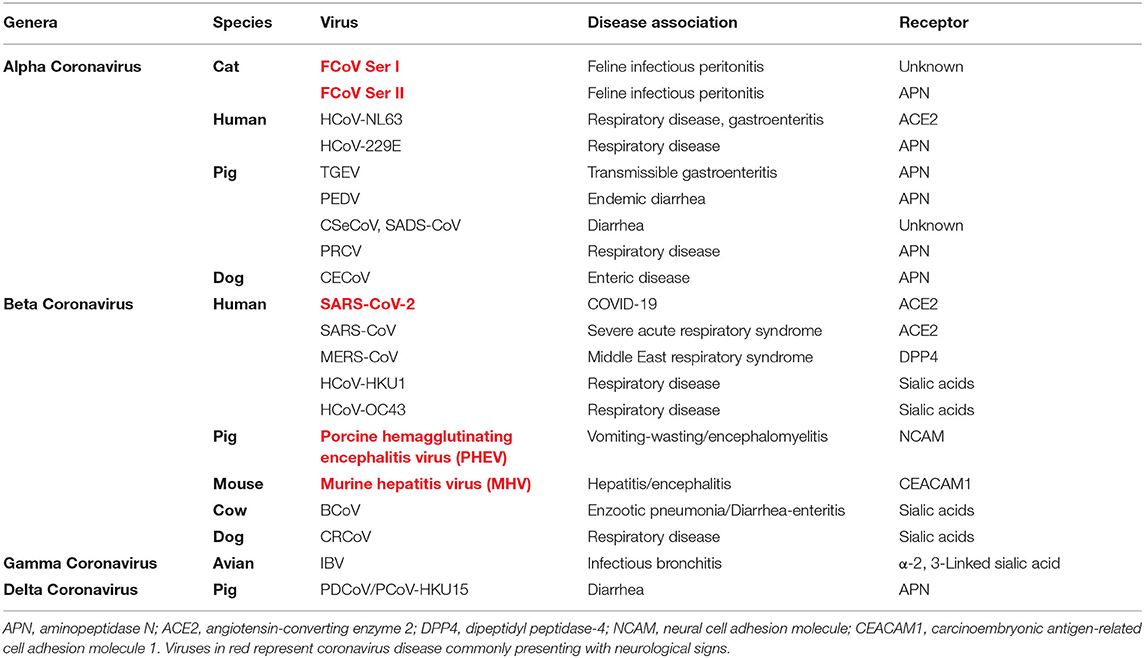
The Coronaviridae family of viruses are single-stranded RNA viruses found in a variety of species including cats, dogs, horses, mice, birds, pigs, bats, camels, whales, and humans (1). Coronaviruses are grouped into four genera; alpha, beta, gamma, and delta (Table 1) and viral particles contain four main structural proteins, namely, spike (S), envelope (E), membrane (M), and nucleocapsid (N) with specific coronaviruses also having a unique set of accessory proteins (2, 3). The distinctive trimeric spike protein (S) is primarily responsible for recognition of cellular receptors associated with viral binding and potentially internalization of host target cells (2–4). Many key receptors interacting with the spike proteins have been defined for the known coronaviruses (Table 1).
Coronavirus infections typically affect the respiratory or gastrointestinal tracts; however, coronavirus-related neurological disease is receiving increased attention as the COVID-19 (SARS-CoV-2) pandemic progresses. Neurological manifestations of COVID-19 infections in humans have become more widely recognized as a significant component of clinical disease (5–16); however, coronavirus involvement of the nervous system is not unique to the SARS-CoV-2.
Several coronaviruses have been associated with neurological disease as a common clinical presentation (Table 1), including feline infectious peritonitis (FIP), porcine hemagglutinating encephalitis virus, murine hepatitis virus (MHV), and currently with SARS-CoV2 virus in COVID-19 patients. Less commonly, the human respiratory disease coronaviruses HCoV-299E and OC43 have been demonstrated in brains of multiple sclerosis (17–21) and encephalitis patients (22–24). Coronavirus-associated encephalitis has been reported in children (25), and sporadic neurological disease has been reported in human Middle Eastern Respiratory syndrome (MERS) and SARS-CoV patients (26–33) although in a relatively limited manner compared to SARS-CoV-2 patients (27, 28, 34).
Mechanism of CNS Entry
Several mechanisms of entry of coronaviruses into the CNS have been postulated and vary depending on the specific coronavirus, host factors, viral dose, and site of infection. Mechanisms are incompletely or poorly understood in many species; however, hematogenous spread via capillary endothelial cells, retrograde axonal transport via olfactory, pulmonary vagal and enteric neurons, exosomes, and entry via macrophage/monocytic cells have been suggested as potential mechanisms (35–41). Porcine hemagglutinating encephalitis virus has been shown to infect the CNS via retrograde transport in peripheral nerves from primary sites of replication (40, 42), and a similar mechanism of entry has been shown for neurotropic strains of MHV (43) and in a SARS mouse disease model (37).
S protein interaction with cell surface receptors (Table 1) is a major determinant of virus virulence and tropism allowing cell binding; subsequent cleavage of the bound spike protein by cellular proteases such as transmembrane serine protease 2 (TMPRSS2) allows internalization by direct fusion with the plasma membrane or use of endocytic mechanisms. Specific coronavirus target receptors have been shown to be variably expressed in a variety of infected CNS cell types (36, 44, 45); however, virus–host interactions are complex as not all infected cells necessarily express a single receptor, additional mechanisms such as receptor independent fusion can occur (46), and binding and entry may utilize similar or different receptors for some viruses (47). Major receptors for the CNS-tropic coronaviruses have been defined in most species, including angiotensin-converting enzyme 2 (ACE2) utilized by human coronaviruses HCoV-NL63, SARS-CoV, and SARS-CoV-2; however, the specific mechanism by which the pre-dominant Type I pathogenic feline coronaviruses attach and enter host cells is poorly defined (48–50).
Mechanism of CNS Disease
Viral-mediated CNS damage may arise due to direct effects of viral replication within target cells and as a consequence of the vigorous inflammatory response that may have both positive anti-viral and potentially negative secondary effects (51, 52). Profound activation of inflammatory and immune cascades driven by a variety of cytokines and chemokines, including IL6, CXCL10, IL1, IFNγ, and TNFα have been documented in CNS coronavirus infections in a variety of species (11, 25, 37, 51, 53–55). Secondary immune-mediated mechanisms of pathology have also been described relating to the presence of viral antigens and antibody-mediated type III hypersensitivity vasculitis (56, 57). Although poorly defined, coronavirus CNS infections may also result in more chronic disease, as is seen with some strains of MHV (51, 56), and human coronavirus infection has been implicated in the pathogenesis of chronic conditions including Parkinson's disease, multiple sclerosis, and peripheral neuropathies (7, 12, 17, 19, 58).
Clinical and pathological findings in the most commonly affected species with CNS-associated coronavirus diseases is quite variable and likely reflects the variability in cellular tropism, mechanism of infection, and-immune mediated characteristics of disease in the different species. Para-infectious mechanisms, with neurological consequences secondary to extra-CNS disease factors such as sepsis and vascular disease, may also be important when the CNS is not the primary target organ as is the case for COVID-19 patients with acute respiratory disease (5, 7, 8, 59, 60).
Feline Infectious Peritonitis
Feline infectious peritonitis virus is a pathotype of the feline enteric coronavirus (FECV) arising through specific mutations in key viral genes [reviewed in (38)]. Feline infectious peritonitis is named for the more commonly presenting effusive “wet” form of the disease, with a less common “dry” form characterized by granulomatous disease in the absence of marked inflammatory exudation into body cavities (57). Both FECV and FIP biotypes exit as one of two serotypes (61, 62). Type I is the more common serotype and possibly more likely to cause disease (62–64), while type II represents a recombinant between feline and canine enteric coronaviruses (65). Neurological involvement with FIP is well-documented (57, 66–70), occurs in ~30–40% of cats presenting with the non-effusive form of the disease (57), and is almost universally fatal (57).
Coronavirus infections resulting in FIP do not generally infect primary CNS cells. Pathogenic transformation of the FECV to the FIP biotype involves a marked alteration of tropism from apical epithelial enteric cells to internalization and replication within macrophages/monocytes (71, 72) that pre-dominantly represents the infected cell population within the CNS. Histopathology reflects the pre-dominant immune-mediated perivasculitis mechanism of disease with a lymphoplasmacytic infiltrate and variable presence of macrophages and neutrophils, often perivascular and typically centered around the leptomeninges and ependyma. Lesions particularly affect the caudal brainstem with perivascular oriented meningitis, periventricular and superficial encephalitis, and choroiditis with secondary hydrocephalus (57, 66, 67, 70).
Mouse Hepatitis Virus
Unlike FIP virus, MHV is capable of infecting ependymal cells, astrocytes, microglia, oligodendrocytes, and neurons (56, 73). Depending on specific virus and mouse strain as well as route of infection, a variety of neuropathologies are seen with MHV infection, from acute encephalitis to a more chronic encephalomyelitis and demyelinating disease (56). Mixed inflammation with a significant neutrophilic component is typically present often centered around the choroid plexus, ependyma, and meninges (51, 74, 75).
Porcine Hemagglutinating Encephalomyelitis Virus
In contrast to MHV, PEHV causes a non-suppurative encephalomyelitis with lymphoplasmacytic cuffing involving the gray matter of the cerebrum and neuronal degeneration of the brainstem and trigeminal ganglia (42). Viral infection is restricted to the neuronal perikaryon following spread from primary sites of replication via the peripheral nervous system (40, 76).
Human CNS Coronavirus Infection
Detailed reports of cell tropism and histopathological lesions in human patients with coronavirus-associated neurological disease are lacking. SARS-CoV and HCoV-OC43 have been reported in cerebral neurons from autopsy specimens using immunohistochemistry and in situ hybridization (23, 32, 33, 77), and coronavirus has been similarly reported in unspecified cells from MS patients (17, 20). Neuronal degeneration, gliosis, and cerebral edema were the most consistent findings reported in SARS patients where histopathology of the brain was described (32, 33) and involvement of brainstem neurons has been proposed as a component of respiratory failure seen in patients (78, 79). Findings in COVID-19 patients are limited and variable. The most common underlying mechanisms of CNS involvement in COVID-19 patients remain to be defined (10, 80, 81), and direct evidence of virus in the CNS is limited. However, SARS-CoV-2 virus has been demonstrated specifically in the CSF (6, 80, 82, 83) and in brain tissue in up to 36% of COVID-19 patients examined at autopsy (59, 60, 84, 85). Variable neuropathological findings have been reported including subcortical white matter vascular and demyelinating lesions (86), lymphocytic meningoencephalitis with prominent neuronal loss (79), and hypoxic injury (60). Neuroimaging with MRI in 37 patients was similarly variable with common findings including signal abnormalities in the medial temporal lobe, multifocal white matter hyperintensities, and extensive white matter microhemorrhages (80).
Clinical neurological signs associated with the COVID-19 SARS-CoV-2 virus are variable and have been commonly associated with sequelae secondary to systemic effects of COVID-19 infection as well as primary viral effects on the CNS and peripheral nervous system. Common presentations include encephalopathy with delirium/psychosis, inflammatory CNS syndromes, ischemic strokes, peripheral neurological disorders including Guillain–Barre syndrome, and an/hyposmia and dys/hypogeusia (altered sense of smell and taste) (5, 6, 8–11, 13–16). As with other CNS coronaviral infections, the proposed pathological mechanisms include secondary inflammatory syndromes, secondary immune-mediated syndromes, neurological consequences of systemic disease including sepsis, hypoxia, and hypercoagulability, and direct neuronal/glial cell injury.
Treatment
Data relating specifically to treatment of naturally occurring CNS coronavirus infections is extremely limited in humans, domestic, and production animals. Therapeutic approaches are generally similar regardless of organ systems affected; however specific issues relating to the blood–brain barrier/blood–CSF barrier limitations on drug delivery and pronounced neurological effects due to secondary inflammation need to be considered. The variable pathogenesis and clinical aspects of coronavirus disease in non-human species means that translational therapeutic studies in these animals may have some limitations. However, CNS coronaviral infections in domestic cats (FIP), in particular, may be translationally valuable given both the severity of disease presentation and the individualized approach to treatment in a companion vs. production or research setting. Recent data relating to treatment of both non-CNS and CNS FIP with antiviral drugs may have relevance to specific aspects of ongoing trials in SARS-CoV-2 patients. Interestingly, domestic and big cats are susceptible to SARS-CoV-2 infection, consistent with expression of ACE2 viral receptor in these species (87, 88), although associated clinical CNS disease has not been reported (89, 90).
Management of coronavirus infections consists of a variety of preventative and therapeutic approaches based on pathogenic mechanisms of the targeted coronaviruses as well as species-specific aspects of clinical disease. Several reviews of therapeutic aspects of coronavirus infections are available and discuss the main arms of disease management relating to prevention, husbandry, vaccination, antiviral drugs, and modulation of immune/inflammatory aspects of coronavirus infections in humans (91–94) and domestic animals (57, 95–98).
Preventative
Preventative management, beyond husbandry, and environmental management of disease outbreaks is centered around vaccination. The value of vaccination depends on both severity of the disease and efficacy/longevity of the vaccines developed. Development of effective vaccines for human coronavirus infections, particularly SARS-CoV-2 (COVID-19), is an ongoing priority (91, 92). Inactivated and live attenuated vaccines have been shown to provide protective immunity in several domestic species (98); however, the value of vaccination has to be balanced against expense and prevalence of disease. Immunological sequelae following coronavirus infection appears to play a major role in disease progression, particularly in the CNS, and adverse events associated with vaccination must be considered in this context. Immunity to FIP is largely cell mediated, and humoral immunity with systemic antibodies to FIP virus may exacerbate disease by enhancing viral uptake and replication in macrophages and by stimulating a vascular-oriented Arthus-type hypersensitivity reaction (57, 99). An intranasal temperature-sensitive mutant FIP vaccine generating a local IgA response has been shown to have efficacy; however, its value in the clinical setting is questionable (57).
Anti-inflammatory/Immunomodulatory Therapies
Dexamethasone is one of the few therapies that has been shown to have a beneficial effect in COVID-19 patients (100), although the pros and cons of anti-inflammatory vs. immunosuppressive effects have been debated with COVID 19 as with other coronaviruses such as SARS-CoV and MERS-CoV. Use of corticosteroids and intravenous immunoglobulin therapy for non-specific inflammatory and potential immune-mediated aspects of CNS disease have been anecdotally reported in neurological COVID-19 (8). Non-specific anti-inflammatory drugs such as corticosteroids, cyclophosphamide, and cyclosporine have anecdotally been associated with amelioration of signs in FIP CNS disease but are not curative (57, 66, 95–97). More targeted inhibition of specific cytokines such as TNFα have shown mixed therapeutic benefits in systemic FIP (101–103), and poor responses have generally been seen with the use of interferons α, β, and omega (57, 96, 97).
Antivirals—Lessons From Feline Trials
A wide spectrum of antiviral drugs has been developed targeting most aspects of the coronavirus life cycle [reviewed in (92)], including neutralizing antibodies (convalescent plasma or monoclonal), fusion and viral protease inhibitors, nucleoside analogs, host protease and receptor inhibitors, and lipidomic reprogramming drugs. The nucleoside analogs ribavirin, NHC (β-D-N4-hydroxycytidine), and remdesivir/GS-5734 have activity against a variety of RNA viruses including coronaviruses. Chloroquine/hydroxychloroquine is an antimalarial and autoimmune drug that can block viral infection by increasing endosomal pH (required for virus-cell fusion) and can also interfere with glycosylation of cellular receptors. Remdesivir and chloroquine can inhibit SARS-CoV-2 in vitro (104) and are in trials for COVID-19 patients. There is currently no evidence for a beneficial effect of chloroquine/hydroxychloroquine in COVID-19 patients (105), and chloroquine had only modest effects in cats with experimentally induced FIP, and toxicity with elevations of serum alanine aminotransferase has been noted (106).
Recent trials using antiviral drugs in clinical FIP have been extremely encouraging that treatment and potential cures are a realistic goal, including for CNS disease. Screening of large numbers of antiviral compounds to identify individual and combinations of drugs shows promise for future effective FIP therapies (107) and may address concerns relating to development of resistance with single drug regimens (108, 109). However, monotherapy with the nucleoside analog GS-441524 (Gilead Sciences Inc.) and a 3C-like antiviral protease inhibitor (Anivive Life Sciences Inc.) have already shown efficacy in experimental and naturally acquired non-CNS FIP (108, 110–112), although limitations associated with drug access across the blood–brain barrier resulted in CNS relapses, particularly with protease inhibitor therapy (108, 112). Cat pharmacokinetic data for GS-441524 showed that CSF concentrations of GS-441524 were ~20% of plasma levels (111) and that doses five times those shown to effectively treat non-CNS FIP (2–4 mg/kg) would be necessary to achieve 1 μM concentrations consistent with the in vitro 50% effective concentration (EC50) to prevent coronavirus cytopathic effects. Subsequent pilot data from cats presenting with CNS FIP supported these data with resolution of disease signs and apparent cures with dosing up to 10 mg/kg (Figure 1) (113). GS-441524 is a 1′-cyano-substituted adenine C-nucleoside ribose analog that inhibits viral RNA synthesis once it has been tri-phosphorylated intracellularly. Remdesivir (GS-5734) is a monophosphate prodrug of GS-441524 with the phosphate masked by McGuigan prodrug moieties designed to promote release of the monophosphorylated analog intracellularly and to overcome the perceived rate-limiting first phosphorylation step. Remdesivir has been given emergency use authorization for treatment of SARS-CoV-2 with encouraging if limited preliminary results (114–116). Given the efficacy of GS-441524 in the treatment of FIP, it has been suggested that there may be advantages to the use of the parent (GS-441524), rather than the prodrug (remdesivir) in human trials (117). Remdesivir appears to be rapidly metabolized in the serum to GS-441524 rather than entering cells intact (118, 119), and GS-441524 can be present in the serum at concentrations 1,000-fold higher than remdesivir (118). In vitro comparison of antiviral efficacy of remdesivir and GS-441524 against SARS-CoV and MERS-CoV showed similar EC50 values, and GS-441524 values were lower in some cases than the EC50 values reported in feline CRFK cells (Crandel Reese Feline Kidney Cells) infected with FIP virus (109, 111). GS-441524 serum levels in humans would more likely exceed these EC50 values based on published data (117), and similarities to cat in vitro data together with the encouraging clinical efficacy in cat FIP (111–113) would support the investigation of GS-441524 for use in human coronaviral disease, including CNS infections. Current dosing of remdesivir in COVID-19 trials is 200 mg loading followed by 100 mg (114, 115), equivalent to 1.5–3 mg/kg for a 70-kg human. These doses fall within the range shown to be effective in treating non-CNS FIP in cats (111, 112); however, the increased doses necessary to treat CNS FIP infections (8–10 mg/kg) in cats (113) would be equivalent to 560–700 mg for a 70-kg human. GS-441524 appears to have a high therapeutic index and minimal adverse effects at all doses of GS441524 reported in cats (2–10 mg/kg) (111–113). CNS blood–brain, blood–CSF barrier pharmacokinetic limitations are likely to be similar between cats and humans, and experience with FIP suggests that dose escalation of remdesivir (or GS-441524) may be necessary to optimize clinical efficacy in humans if targeting of coronavirus within the CNS is a specific therapeutic goal.
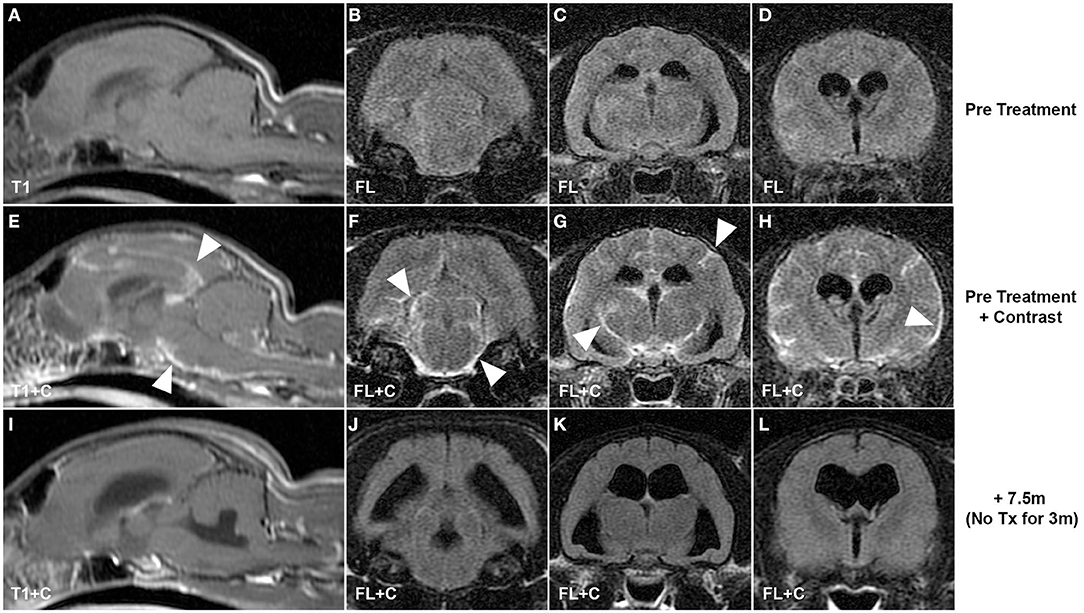
Figure 1. CNS coronavirus infection (FIP) in a cat presenting with neurological deficits and treated with GS-441524, the parent nucleoside of remdesivir. Pre-contrast (A–D) and post-contrast T1-weighted and fluid-attenuated inversion recovery pre-treatment MRI sequences (E–H) reveal multifocal leptomeningeal lesions (arrowheads) typical of CNS FIP. Resolution of clinical signs was incomplete using drug dosing typically effective in non-CNS disease (4 mg/kg); however, increased dosing (10 mg/kg) resulted in resolution of clinical signs and resolution of MR lesions on images acquired 7.5 months after initiation of treatment and 3 months after completion of treatment (I–L). T1, T1-weighted; FL, fluid-attenuated inversion recovery; +C, contrast (gadopentetate dimeglumine, “Magnevist”).
GS-441524 is not approved or available for clinical veterinary use limiting the potential for expanded and regulated clinical studies necessary to support approval in clinical veterinary practice. Unapproved sources of GS-441524 have become available online to owners of FIP cats, and FIP advocacy groups have collated observational data relating to outcomes in these “owner-treated” animal cohorts. Data arising from unverified drug sources and owner reported outcomes have major limitations; however, against a historical background of almost universal fatality in cases of CNS FIP, some clinically relevant data may be available. Advocacy group treatment regimens, based on published data (111–113), typically recommend a minimum 12-week course of treatment, with 4- to 6-mg/kg doses for non-CNS FIP treatment and 8- to10-mg/kg doses for CNS disease cases. Cure or remission is defined as no evidence of clinical disease >12 or <12 weeks, respectively, after completion of treatment. Data from an FIP advocacy group (personal communication) detailing owner outcomes from 110 cats with neurological signs and presumptive FIP treated with unapproved GS-441524 drug showed the following: 57/110 (52%) in remission, 22/110 (20%) cured, 9/110 (8%) died or euthanized, and 7/110 (6%) with relapsed CNS disease. Fifteen cats (14%) presented with non-CNS disease but relapsed with CNS signs following treatment. Sequential dose data was available for five cats that relapsed with CNS disease; initial doses ranged from 5 to 7 mg/kg, and four of five cats were subsequently cured with one in remission following dose escalation to 10–16 mg/kg. These uncontrolled data are supportive of the efficacy previously documented in four cats treated with GS-441524 (113) and of the necessity of increased dosing for optimal treatment of CNS infections. A striking aspect of GS-441524 treatment of FIP is the dramatic (often <36 h) improvement in clinical signs following adequate dosing (112, 113). Resolution of gross neuropathology in this time period is unlikely, and it is possible that decreased production of inflammatory cytokines, known to be a significant component of CNS coronaviral pathology, may be responsible for this rapid clinical improvement. Whether similar clinical correlates will be present with treatment of human coronaviral infections with GS-441524 or remdesivir remains to be seen.
Naturally occurring coronaviral infections in companion and production animals have many similarities to human pandemic-related diseases such as SARS, MERS, and COVID-19, although species and virus-specific factors described above mean that broad translation of therapeutic data across species will have major limitations. However, findings relating to basic treatment-related factors such as blood–brain barrier effects on therapeutic drug penetration to the CNS are likely to be relevant across species. It is currently unclear to what degree viral infection of the CNS impacts the clinical outcome in COVID-19 patients and how it may influence therapeutic practice; however, advances in the treatment of previously fatal coronavirus infections in cats with antiviral nucleoside analog drugs, particularly in the context of CNS infection, is encouraging that similar approaches may be efficacious in other species.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
PD conceived and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Data for FIP cats treated with unapproved sources of GS-441524 was provided by the FIP Warriors advocacy group.
References
1. Woo PC, Lau SK, Huang Y, Yuen KY. Coronavirus diversity, phylogeny and interspecies jumping. Exp Biol Med. (2009) 234:1117–27. doi: 10.3181/0903-MR-94
2. Cavanagh D. The coronavirus surface glycoproteins. In: Siddell SG, editor. The Coronaviridae. New York, NY: Plenum Press (1995). p. 73–113.
3. Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol. (2015) 1282:1–23. doi: 10.1007/978-1-4939-2438-7_1
4. Collins AR, Knobler RL, Powell H, Buchmeier MJ. Monoclonal antibodies to murine hepatitis virus-4 (strain JHM) define the viral glycoprotein responsible for attachment and cell–cell fusion. Virology. (1982) 119:358–71. doi: 10.1016/0042-6822(82)90095-2
5. Roman GC, Spencer PS, Reis J, Buguet A, Faris MEA, Katrak SM, et al. The neurology of COVID-19 revisited: a proposal from the Environmental Neurology Specialty Group of the World Federation of Neurology to implement international neurological registries. J Neurol Sci. (2020) 414:116884. doi: 10.1016/j.jns.2020.116884
6. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
7. Montalvan V, Lee J, Bueso T, De Toledo J, Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. (2020) 194:105921. doi: 10.1016/j.clineuro.2020.105921
8. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. (2020). doi: 10.1093/brain/awaa240
9. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
10. Helms J, Kremer S, Merdji H, Clere-Jehl R, Schenck M, Kummerlen C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
11. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology. (2020):201187. doi: 10.1148/radiol.2020201187
12. Cilia R, Bonvegna S, Straccia G, Andreasi NG, Elia AE, Romito LM, et al. Effects of COVID-19 on Parkinson's disease clinical features: a community-based case-control study. Mov Disord. (2020) 35:1287–92. doi: 10.1002/mds.28170
13. Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
14. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID-19) and anosmia. JAMA Neurol. (2020) 77:1028–29. doi: 10.1001/jamaneurol.2020.2125
15. Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. (2020) 91:889–91. doi: 10.1136/jnnp-2020-323586
16. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barre syndrome associated with SARS-CoV-2. N Engl J Med. (2020) 382:2574–6. doi: 10.1056/NEJMc2009191
17. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. (2000) 74:8913–21. doi: 10.1128/jvi.74.19.8913-8921.2000
18. Burks JS, DeVald BL, Jankovsky LD, Gerdes JC. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. (1980) 209:933–4. doi: 10.1126/science.7403860
19. Dessau RB, Lisby G, Frederiksen JL. Coronaviruses in brain tissue from patients with multiple sclerosis. Acta Neuropathol. (2001) 101:601–4. doi: 10.1007/s004010000331
20. Murray RS, Brown B, Brian D, Cabirac GF. Detection of coronavirus RNA and antigen in multiple sclerosis brain. Ann Neurol. (1992) 31:525–33. doi: 10.1002/ana.410310511
21. Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology. (1992) 191:502–5. doi: 10.1016/0042-6822(92)90220-j
22. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. (2004) 113:e73–6. doi: 10.1542/peds.113.1.e73
23. Morfopoulou S, Brown JR, Davies EG, Anderson G, Virasami A, Qasim W, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. (2016) 375:497–8. doi: 10.1056/NEJMc1509458
24. Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis. (2020) 52:419–22. doi: 10.1080/23744235.2020.1729403
25. Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. (2016) 59:163–9. doi: 10.1159/000453066
26. Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. (2015) 43:495–501. doi: 10.1007/s15010-015-0720-y
27. Algahtani H, Subahi A, Shirah B. Neurological complications of Middle East respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. (2016) 2016:3502683. doi: 10.1155/2016/3502683
28. Kato V, Laure B, Harald C. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg. (2020). doi: 10.1007/s13760-020-01412-4. [Epub ahead of print].
29. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. (2004) 10:342–4. doi: 10.3201/eid1002.030638
30. Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. (2003) 49:2108–9. doi: 10.1373/clinchem.2003.025437
31. Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am. (2019) 33:869–89. doi: 10.1016/j.idc.2019.07.001
32. Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. (2005) 41:1089–96. doi: 10.1086/444461
33. Gu J, Gong E, Zhang B, Zheng J, Gao Z, Zhong Y, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. (2005) 202:415–24. doi: 10.1084/jem.20050828
34. Desforges M, Le Coupanec A, Stodola JK, Meessen-Pinard M, Talbot PJ. Human coronaviruses: viral and cellular factors involved in neuroinvasiveness and neuropathogenesis. Virus Res. (2014) 194:145–58. doi: 10.1016/j.virusres.2014.09.011
35. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
36. Toljan K. Letter to the editor regarding the viewpoint “evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism”. ACS Chem Neurosci. (2020) 11:1192–4. doi: 10.1021/acschemneuro.0c00174
37. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. (2008) 82:7264–75. doi: 10.1128/JVI.00737-08
38. Pedersen NC. An update on feline infectious peritonitis: virology and immunopathogenesis. Vet J. (2014) 201:123–32. doi: 10.1016/j.tvjl.2014.04.017
39. Tammer R, Evensen O, Lutz H, Reinacher M. Immunohistological demonstration of feline infectious peritonitis virus antigen in paraffin-embedded tissues using feline ascites or murine monoclonal antibodies. Vet Immunol Immunopathol. (1995) 49:177–82. doi: 10.1016/0165-2427(95)05459-j
40. Andries K, Pensaert MB. Virus isolated and immunofluorescence in different organs of pigs infected with hemagglutinating encephalomyelitis virus. Am J Vet Res. (1980) 41:215–8.
41. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. (2020) 77:1018–27. doi: 10.1001/jamaneurol.2020.2065
42. Mora-Diaz JC, Pineyro PE, Houston E, Zimmerman J, Gimenez-Lirola LG. Porcine hemagglutinating encephalomyelitis virus: a review. Front Vet Sci. (2019) 6:53. doi: 10.3389/fvets.2019.00053
43. Perlman S, Jacobsen G, Afifi A. Spread of a neurotropic murine coronavirus into the CNS via the trigeminal and olfactory nerves. Virology. (1989) 170:556–60. doi: 10.1016/0042-6822(89)90446-7
44. Chen DS, Asanaka M, Yokomori K, Wang F, Hwang SB, Li HP, et al. A pregnancy-specific glycoprotein is expressed in the brain and serves as a receptor for mouse hepatitis virus. Proc Natl Acad Sci USA. (1995) 92:12095–9. doi: 10.1073/pnas.92.26.12095
45. Ramakrishna C, Bergmann CC, Holmes KV, Stohlman SA. Expression of the mouse hepatitis virus receptor by central nervous system microglia. J Virol. (2004) 78:7828–32. doi: 10.1128/JVI.78.14.7828-7832.2004
46. Gallagher TM, Buchmeier MJ, Perlman S. Cell receptor-independent infection by a neurotropic murine coronavirus. Virology. (1992) 191:517–22. doi: 10.1016/0042-6822(92)90223-c
47. Szczepanski A, Owczarek K, Bzowska M, Gula K, Drebot I, Ochman M, et al. Canine respiratory coronavirus, bovine coronavirus, and human coronavirus OC43: receptors and attachment factors. Viruses. (2019) 11:328. doi: 10.3390/v11040328
48. Jaimes JA, Whittaker GR. Feline coronavirus: insights into viral pathogenesis based on the spike protein structure and function. Virology. (2018) 517:108–21. doi: 10.1016/j.virol.2017.12.027
49. Dye C, Temperton N, Siddell SG. Type I feline coronavirus spike glycoprotein fails to recognize aminopeptidase N as a functional receptor on feline cell lines. J Gen Virol. (2007) 88:1753–60. doi: 10.1099/vir.0.82666-0
50. Hohdatsu T, Izumiya Y, Yokoyama Y, Kida K, Koyama H. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch Virol. (1998) 143:839–50. doi: 10.1007/s007050050336
51. Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host-virus stand-off. Nat Rev Microbiol. (2006) 4:121–32. doi: 10.1038/nrmicro1343
52. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
53. Skinner D, Marro BS, Lane TE. Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunol. (2019) 32:25–37. doi: 10.1089/vim.2018.0073
54. Foley JE, Rand C, Leutenegger C. Inflammation and changes in cytokine levels in neurological feline infectious peritonitis. J Feline Med Surg. (2003) 5:313–22. doi: 10.1016/S1098-612X(03)00048-2
55. Foley JE, Leutenegger C. A review of coronavirus infection in the central nervous system of cats and mice. J Vet Intern Med. (2001) 15:438–44. doi: 10.1892/0891-6640(2001)015<0438:arocii>2.3.co;2
56. Schaumburg CS, Held KS, Lane TE. Mouse hepatitis virus infection of the CNS: a model for defense, disease, and repair. Front Biosci. (2008) 13:4393–406. doi: 10.2741/3012
57. Pedersen NC. A review of feline infectious peritonitis virus infection: 1963–2008. J Feline Med Surg. (2009) 11:225–58. doi: 10.1016/j.jfms.2008.09.008
58. Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord. (1992) 7:153–8. doi: 10.1002/mds.870070210
59. Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. (2020) 173:268–77. doi: 10.7326/M20-2003
60. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, et al. Neuropathological features of COVID-19. N Engl J Med. (2020) 383:989–92. doi: 10.1056/NEJMc2019373
61. Hohdatsu T, Sasamoto T, Okada S, Koyama H. Antigenic analysis of feline coronaviruses with monoclonal antibodies (MAbs): preparation of MAbs which discriminate between FIPV strain 79-1146 and FECV strain 79-1683. Vet Microbiol. (1991) 28:13–24. doi: 10.1016/0378-1135(91)90096-x
62. Pedersen NC, Black JW, Boyle JF, Evermann JF, McKeirnan AJ, Ott RL. Pathogenic differences between various feline coronavirus isolates. Adv Exp Med Biol. (1984) 173:365–80. doi: 10.1007/978-1-4615-9373-7_36
63. Benetka V, Kubber-Heiss A, Kolodziejek J, Nowotny N, Hofmann-Parisot M, Mostl K. Prevalence of feline coronavirus types I and II in cats with histopathologically verified feline infectious peritonitis. Vet Microbiol. (2004) 99:31–42. doi: 10.1016/j.vetmic.2003.07.010
64. Kummrow M, Meli ML, Haessig M, Goenczi E, Poland A, Pedersen NC, et al. Feline coronavirus serotypes 1 and 2: seroprevalence and association with disease in Switzerland. Clin Diagn Lab Immunol. (2005) 12:1209–15. doi: 10.1128/CDLI.12.10.1209-1215.2005
65. Herrewegh AA, Smeenk I, Horzinek MC, Rottier PJ, de Groot RJ. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J Virol. (1998) 72:4508–14.
66. Kline KL, Joseph RJ, Averill DR. Feline infectious peritonitis with neurologic involvement—clinical and pathological findings in 24 cats. J Am Anim Hosp Assoc. (1994) 30:111–8.
67. Kornegay JN. Feline infectious peritonitis—central nervous-system form. J Am Anim Hosp Assoc. (1978) 14:580–4.
68. Marioni-Henry K, Vite CH, Newton AL, Van Winkle TJ. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med. (2004) 18:851–8. doi: 10.1892/0891-6640(2004)18<851:podots>2.0.co;2
69. Foley JE, Lapointe JM, Koblik P, Poland A, Pedersen NC. Diagnostic features of clinical neurologic feline infectious peritonitis. J Vet Intern Med. (1998) 12:415–23.
70. Rissi DR. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J Vet Diagn Invest. (2018) 30:392–9. doi: 10.1177/1040638718755833
71. Pedersen NC. Morphologic and physical characteristics of feline infectious peritonitis virus and its growth in autochthonous peritoneal cell cultures. Am J Vet Res. (1976) 37:567–72.
72. Rottier PJ, Nakamura K, Schellen P, Volders H, Haijema BJ. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J Virol. (2005) 79:14122–30. doi: 10.1128/JVI.79.22.14122-14130.2005
73. Wang FI, Hinton DR, Gilmore W, Trousdale MD, Fleming JO. Sequential infection of glial cells by the murine hepatitis virus JHM strain (MHV-4) leads to a characteristic distribution of demyelination. Lab Invest. (1992) 66:744–54.
74. Weiner LP. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. (1973) 28:298–303. doi: 10.1001/archneur.1973.00490230034003
75. Zhou J, Stohlman SA, Hinton DR, Marten NW. Neutrophils promote mononuclear cell infiltration during viral-induced encephalitis. J Immunol. (2003) 170:3331–6. doi: 10.4049/jimmunol.170.6.3331
76. Meyvisch C, Hoorens J. An electron microscopic study of experimentally-induced HEV encephalitis. Vet Pathol. (1978) 15:102–13. doi: 10.1177/030098587801500112
77. Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. (2004) 203:622–30. doi: 10.1002/path.1560
78. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. (2020) 92:552–5. doi: 10.1002/jmv.25728
79. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. (2020) 395:e109. doi: 10.1016/S0140-6736(20)31282-4
80. Kremer S, Lersy F, de Seze J, Ferre JC, Maamar A, Carsin-Nicol B, et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. (2020):202222. doi: 10.1148/radiol.2020202222
81. Al-Harthi L, Campbell E, Schneider JA, Bennett DA. What HIV in the brain can teach us about SARS-CoV-2 neurological complications? AIDS Res Hum Retroviruses. (2020). doi: 10.1089/AID.2020.0161. [Epub ahead of print].
82. Xiang PPP, Xu XM, Gao LL. First case of 2019 novel coronavirus disease with encephalitis. China XivT. (2020) 202003:00015.
83. Zhou L, Zhang M, Wang J, Gao J. Sars-Cov-2: underestimated damage to nervous system. Travel Med Infect Dis. (2020) 36:101642. doi: 10.1016/j.tmaid.2020.101642
84. Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol. (2020) 92:699–702. doi: 10.1002/jmv.25915
85. Puelles VG, Lutgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. (2020) 383:590–2. doi: 10.1056/NEJMc2011400
86. Reichard RR, Kashani KB, Boire NA, Constantopoulos E, Guo Y, Lucchinetti CF. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. (2020) 140:1–6. doi: 10.1007/s00401-020-02166-2
87. Damas J, Hughes GM, Keough KC, Painter CA, Persky NS, Corbo M, et al. Broad host range of SARS-CoV-2 predicted by comparative and structural analysis of ACE2 in vertebrates. Proc Natl Acad Sci USA. (2020) 117:22311–22. doi: 10.1073/pnas.2010146117
88. Chiocchetti R, Galiazzo G, Fracassi F, Giancola F, Pietra M. ACE2 expression in the cat and the tiger gastrointestinal tracts. Front Vet Sci. (2020) 7:514. doi: 10.3389/fvets.2020.00514
89. Shi J, Wen Z, Zhong G, Yang H, Wang C, Huang B, et al. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS-coronavirus 2. Science. (2020) 368:1016–20. doi: 10.1126/science.abb7015
90. Halfmann PJ, Hatta M, Chiba S, Maemura T, Fan S, Takeda M, et al. Transmission of SARS-CoV-2 in domestic cats. N Engl J Med. (2020) 383:592–4. doi: 10.1056/NEJMc2013400
91. Conte C, Sogni F, Affanni P, Veronesi L, Argentiero A, Esposito S. Vaccines against coronaviruses: the state of the art. Vaccines (Basel). (2020) 8:309. doi: 10.3390/vaccines8020309
92. Tse LV, Meganck RM, Graham RL, Baric RS. The current and future state of vaccines, antivirals and gene therapies against emerging coronaviruses. Front Microbiol. (2020) 11:658. doi: 10.3389/fmicb.2020.00658
93. Behzadi MA, Leyva-Grado VH. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and Middle East respiratory syndrome coronavirus infections. Front Microbiol. (2019) 10:1327. doi: 10.3389/fmicb.2019.01327
94. Li CC, Wang XJ, Wang HR. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov Today. (2019) 24:726–36. doi: 10.1016/j.drudis.2019.01.018
95. Addie D, Belak S, Boucraut-Baralon C, Egberink H, Frymus T, Gruffydd-Jones T, et al. Feline infectious peritonitis. ABCD guidelines on prevention and management. J Feline Med Surg. (2009) 11:594–604. doi: 10.1016/j.jfms.2009.05.008
96. Hartmann K, Ritz S. Treatment of cats with feline infectious peritonitis. Vet Immunol Immunopathol. (2008) 123:172–5. doi: 10.1016/j.vetimm.2008.01.026
97. Pedersen NC. An update on feline infectious peritonitis: diagnostics and therapeutics. Vet J. (2014) 201:133–41. doi: 10.1016/j.tvjl.2014.04.016
98. Tizard IR. Vaccination against coronaviruses in domestic animals. Vaccine. (2020) 38:5123–30. doi: 10.1016/j.vaccine.2020.06.026
99. Pedersen NC, Boyle JF. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am J Vet Res. (1980) 41:868–76.
100. Horby P, Lim WS, Emberson J, Mafham M, Bell J, Linsell L, et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv [Preprint]. (2020). doi: 10.1101/2020.06.22.20137273
101. Doki T, Takano T, Kawagoe K, Kito A, Hohdatsu T. Therapeutic effect of anti-feline TNF-alpha monoclonal antibody for feline infectious peritonitis. Res Vet Sci. (2016) 104:17–23. doi: 10.1016/j.rvsc.2015.11.005
102. Doki T, Toda M, Hasegawa N, Hohdatsu T, Takano T. Therapeutic effect of an anti-human-TNF-alpha antibody and itraconazole on feline infectious peritonitis. Arch Virol. (2020) 165:1197–206. doi: 10.1007/s00705-020-04605-7
103. Fischer Y, Ritz S, Weber K, Sauter-Louis C, Hartmann K. Randomized, placebo controlled study of the effect of propentofylline on survival time and quality of life of cats with feline infectious peritonitis. J Vet Intern Med. (2011) 25:1270–6. doi: 10.1111/j.1939-1676.2011.00806.x
104. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
105. Hernandez AV, Roman YM, Pasupuleti V, Barboza JJ, White CM. Hydroxychloroquine or chloroquine for treatment or prophylaxis of COVID-19: a living systematic review. Ann Intern Med. (2020). doi: 10.7326/M20-2496
106. Takano T, Katoh Y, Doki T, Hohdatsu T. Effect of chloroquine on feline infectious peritonitis virus infection in vitro and in vivo. Antiviral Res. (2013) 99:100–7. doi: 10.1016/j.antiviral.2013.04.016
107. Cook SE, Vogel H, Castillo D, Olsen M, Pedersen N, Murphy BG. A rational approach to identifying effective combined anticoronaviral therapies against feline coronavirus. bioRxiv [Preprint]. (2020). doi: 10.1101/2020.07.09.195016
108. Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, et al. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J Feline Med Surg. (2018) 20:378–92. doi: 10.1177/1098612X17729626
109. Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. (2018) 9:e00221. doi: 10.1128/mBio.00221-18
110. Kim Y, Liu H, Galasiti Kankanamalage AC, Weerasekara S, Hua DH, Groutas WC, et al. Reversal of the progression of fatal coronavirus infection in cats by a broad-spectrum coronavirus protease inhibitor. PLoS Pathog. (2016) 12:e1005531. doi: 10.1371/journal.ppat.1005531
111. Murphy BG, Perron M, Murakami E, Bauer K, Park Y, Eckstrand C, et al. The nucleoside analog GS-441524 strongly inhibits feline infectious peritonitis (FIP) virus in tissue culture and experimental cat infection studies. Vet Microbiol. (2018) 219:226–33. doi: 10.1016/j.vetmic.2018.04.026
112. Pedersen NC, Perron M, Bannasch M, Montgomery E, Murakami E, Liepnieks M, et al. Efficacy and safety of the nucleoside analog GS-441524 for treatment of cats with naturally occurring feline infectious peritonitis. J Feline Med Surg. (2019) 21:271–81. doi: 10.1177/1098612X19825701
113. Dickinson PJ, Bannasch M, Thomasy SM, Murthy VD, Vernau KM, Liepnieks M, et al. Antiviral treatment using the adenosine nucleoside analogue GS-441524 in cats with clinically diagnosed neurological feline infectious peritonitis. J Vet Intern Med. (2020) 34:1587–93. doi: 10.1111/jvim.15780
114. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19—preliminary report. N Engl J Med. (2020). doi: 10.1056/NEJMoa2007764. [Epub ahead of print].
115. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9
116. Pardo J, Shukla AM, Chamarthi G, Gupte A. The journey of remdesivir: from Ebola to COVID-19. Drugs Context. (2020) 9:2020-4-14. doi: 10.7573/dic.2020-4-14
117. Yan VC, Muller FL. Advantages of the parent nucleoside GS-441524 over remdesivir for COVID-19 treatment. ACS Med Chem Lett. (2020) 11:1361–6. doi: 10.1021/acsmedchemlett.0c00316
118. Williamson BN, Feldmann F, Schwarz B, Meade-White K, Porter DP, Schulz J, et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. Nature. (2020). doi: 10.1038/s41586-020-2423-5. [Epub ahead of print].
Keywords: GS-441524, remdesivir, SARS-CoV-2, feline infectious peritonitis (FIP), treatment
Citation: Dickinson PJ (2020) Coronavirus Infection of the Central Nervous System: Animal Models in the Time of COVID-19. Front. Vet. Sci. 7:584673. doi: 10.3389/fvets.2020.584673
Received: 17 July 2020; Accepted: 21 September 2020;
Published: 23 October 2020.
Edited by:
Andrea Tipold, University of Veterinary Medicine Hannover, GermanyReviewed by:
Wolfgang Baumgärtner, University of Veterinary Medicine Hannover, GermanyKaspar Matiasek, Ludwig Maximilian University of Munich, Germany
Copyright © 2020 Dickinson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Dickinson, pjdickinson@ucdavis.edu
 Peter J. Dickinson
Peter J. Dickinson