Oral Squamous Cell Carcinomas in Patient with Graft Versus-Host Disease Following Allogenic Bone Marrow Transplantation: A Case Report during Mutant COVID-19 Pandemic
A B S T R A C T
Aplastic anaemia is a severe haematological disorder characterized by an inadequate number of hematopoietic stem cells, resulting in pancytopenia, formed by a hypocellular bone marrow. Disorders of this nature are widely treated with haematopoietic stem cell transplantation (HSCT). A potential chronic complication following (HSCT) is the growth of secondary malignancies. Notably, patients suffering from chronic graft versus host disease (cGvHD) secondary to HSCT have been shown to be more susceptible to oral squamous cell carcinoma (OSCC). Here, we present a rare case of a 30-year-old Libyan woman treated with HSCT for aplastic anaemia, with subsequent complications of cGvHD and OSCC after few months of HSCT. These carcinomatous lesions were detected in the buccal gingiva and retromolar pad area at the age of 31. The present case report emphasizes the connection between oral cGvHD and OSCC, and the potential appearance of OSCC after HSCT at any time of patient life. Thus, closer follow-up is mandatory for all patients treated with HSCT who developed cGvHD, and efficient cGvHD prevention and therapeutic approaches are needed.
Keywords
Haematopoietic stem cell transplant, graft versus host disease, oral squamous cell carcinoma, OSCC
Introduction
Aplastic anaemia is a severe haematological illness characterized by the hypocellular bone marrow, leading to an inadequate amount of hematopoietic stem cells, in turn resulting in poor erythrocyte, granulocytes, and platelets production (pancytopenia) [1]. In severe cases of this condition, it is typical for neutrophil and platelet counts to fall below 500/μL and 20,000/μL, respectively. Aplastic anaemia appears to be associated with a geographic variation in its prevalence. For example, it is rare in countries of the west, with an average incidence of 2 persons per million, while appearing more prevalent across Asia and countries of the developing world [1]. In Libya, only few patients have been diagnosed and registered either due to a lack of diagnostic tools or wrong diagnosis altogether. Characteristically, the cause of this disease is unknown, but a link with certain drugs, benzene exposure, insecticides, viruses, and hepatitis have been reported [2]. The aetiology, however, generally remains unknown in half of the cases of aplastic anaemia. General weakness and tiredness, contusions, nosebleeds, and gingival bleeding are primary symptoms of aplastic anaemia. There is a greater risk of infections associated with complications related to persistent pancytopenia. In patients with aplastic anaemia, sepsis caused by both bacterial and fungal infections are the major reasons for expiration. Clinically, involvement of the mouth is commonly reported, but without sufficient details about frequency, risk factors, and significance of these oral conditions [1]. Haematopoietic stem cell transplantation (HSCT) is the treatment of choice for patients with aplastic anaemia associated with a significant success rate [3, 4]. A frequent complication from allogeneic transplantation is Graft versus host disease (GvHD) due to an immunologic reaction from grafting immunocompetent cells to an immunodeficient, with multiple organ involvement [5]. It has been reported that about 25-40% of survivors who lived for a long period of time after HSCT showed GvHD [6]. Skin, liver, gastrointestinal tract, lungs, and eyes are the most affected body organs in systemic GvHD. However, approximately 80% of cases show oral manifestations including atrophy, erythema, lichenoid lesions and dryness and pain in the oral cavity [5]. GvHD causes a significant deterioration in the quality of life of survivors, and it is likely to happen when the recipient (host) obtains a graft from an unrelated donor, or when there is considerable difference in the age between the donor and recipient [7].
The survival of patients cured of their original malignancy is influenced by the emergence of secondary cancers, described as a potentially significant long-term complication following HSCT. Secondary malignancies such as leukemia in donor-derived cells, Hodgkin disease, non-Hodgkin’s lymphoma, and granulocytic sarcoma are frequently reported [8]. The significant danger of developing oral squamous cell carcinoma (OSCC) and squamous cell carcinoma of the skin as secondary solid tumors has been described in many recent studies, although they remain less frequent [8]. We report a rare case of a Libyan female patient who was diagnosed by her haematologist in 2017 as a case of aplastic anaemia disease at the age of 28 years, complicated by GvHD after HSCT performed 28th August 2019. The importance of this case report lies on several points: firstly, the occurrence of this carcinoma as a late complication of bone marrow transplantation on a background of GvHD after a short period of time estimated totally by about one year and few months. Secondly, there has been the challenge associated with the management of such a case at the time of a worldwide pandemic due to Coronavirus (COVID-19). Thirdly, it is a challenging case of a medically compromised patient with multiple systemic diseases.
Case Report
A 31-year-old Libyan woman; mother of two children aged 7 years and 5 years. She presented with a history of aplastic anaemia (AA), diagnosed in 2017. She was treated with several courses of antibiotics to control infections and received blood transfusion every 3 months to correct haemoglobin level which had dropped to 5-6 g/dl with low platelet and low white blood cell counts. In July 2017, her bone marrow aspirate and biopsy showed hypoplastic bone marrow, hyperplasia of thrombocytopoiesis cells, and 20% cellularity. She was treated with steroids, erythropoietin, folic acid, and danazol for 5 months. On December 18, 2017, bone marrow biopsy was performed and revealed severely hypoplastic bone marrow with 10% cellularity. Cyclosporine was therefore added to her medication for 3 months, and she had her last dose on May 5, 2018. May 7, 2018, her haemoglobin level dropped to 6 g/dl, she received 1 unit Packed red blood cells (PRBC). May 21, 2018, her bone marrow, biopsy, and aspirate showed unilateral hypocellular bone marrow with features compatible with Myelodysplastic syndrome (MDS) with multilineage and hypoplastic. Results of her blood investigations are shown in (Table 1). Ultrasound Scan for her abdomen, Echocardiogram and Doppler did not reveal any significant findings. A diagnosis of hypoplastic MDS (Fanconi anaemia) was arrived at, and she was enrolled for a reduced-intensity conditioning (RIC) allogeneic bone marrow transplantation (ALLO-BMT).
Table 1: Showing results of blood investigation performed prior to HSCT.
|
Test |
Result |
|
Flow cytometry |
No Paroxysmal nocturnal hemoglobinuria
(PNH) clone |
|
Fluorescence in situ hybridization
(FISH) for MDS |
Negative |
|
Cytogenetic |
Pseudodiploid clone with 1q triplication |
|
NGS: BCOR. Chromosomal breakage test |
Positive |
The patient was made to undergo ALLO-BMT with CY/FLU/ATC/TBI conditioning. 28 August 2019, an allogenic bone marrow transplant was performed, the matched donor being her younger sister aged 16 years at operation time with age difference of 14 years between the two sisters. She shared a similar blood group with the donor, different RH (patient B positive, donor B negative) and same Cytomegalovirus (CMV) status, DEP test negative for the donor.
A few months post-allogenic bone marrow transplantation, the therapeutic procedure was complicated by the onset of severe cGvHD with clinical manifestations including chest infection, acute kidney injury, mucositis, epistaxis, and gum bleeding, BK cystitis, mouth GvHD and skin GvHD. Post-transplant evaluation using bone marrow, biopsy, and aspirate revealed unilateral normocellular bone marrow with trilineage differentiation and maturation. No morphologic evidence of significant dysplasia. Chimerism: 98% >>>97%. Bone marrow cytogenetic: normal. Repeated bone marrow; final diagnosis: bone marrow, aspirate, and biopsy: slightly hypocellular bone marrow with trilineage hematopoiesis. Last chimerism: 96%. Her skin GvHD and mouth GvHD improving on tapering steroid and Cellcept 1000 mg twice a day.
On January 05, 2021, the patient was referred to us at the National Cancer Institute of Misrata where new blood investigations have been requested for her, and results are shown in (Table 2).
Table 2: Results of blood investigations before taking the oral incisional
biopsy.
|
Test |
Result |
Test |
Result |
|
HbA1C |
4.14 |
WBC |
9.2 x 103/μl |
|
CA19.9 |
35.1 U/ml |
RBC |
4.10 x 106/μl |
|
CEA |
3.60 ng/ml |
HGB |
14.3 g/dL |
|
Blood urea |
74 mg/dl |
HCT |
42.3% |
|
Blood Creatinine |
0.46 mg/dl |
MCV |
103.2 fL |
|
Serum potassium |
3.28 mmol/L |
MCH |
34.9 pg |
|
Blood Sodium |
138 mmol/L |
MCHC |
33.8 g/dL |
|
ESR |
7 mm/2hrs |
PLT |
139 x 103/μl |
|
CRP Titer |
14.0 mg/l |
LYM% |
19.1% |
|
Serum Creatinine |
0.7 mg/dl |
MXD% |
9.3% |
|
Total bilirubin |
0.6 mg/dl |
NEUT% |
71.6% |
|
S.G.O.T |
25 U/I |
LYM# |
1.8 x 103/μl |
|
S.G.P.T |
30 U/I |
MXD# |
0.9 x 103/μl |
|
ALK.Phosphat |
261 U/I |
NEUT# |
6.5 x 103/μl |
|
Mg |
2.1 mg/dl |
RDW-SD |
56.5 fL |
|
Vitamin B12 |
427 bg/ml |
RDW-CV |
14.7% |
|
Folic Acid |
8.9 |
RDW |
14.5 fL |
|
HBsAg by Elisa |
Negative |
MPV |
11.3 fL |
|
HCV Ab by Elisa |
Negative |
P-LCR |
35.5% |
|
HIV by Elisa |
Negative |
PCT |
0.16 |
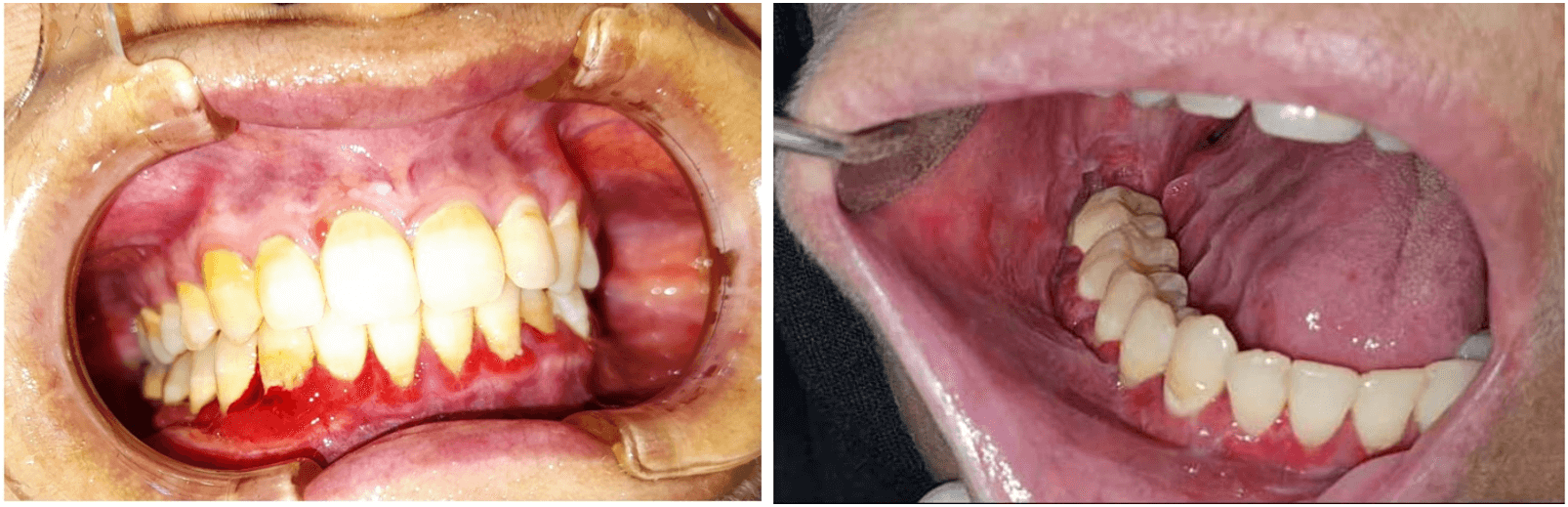
She was first seen at the Maxillofacial Unit at Misrata medical centre on January 12, 2021 for oral evaluation. The patient complained of mouth soreness, unpleasant discharge, discomfort, and pain in the posterior part of the lower right jaw. Clinical examination of the oral cavity revealed severely inflamed, erythematous, and ulcerated gingiva. The buccal mucosa, bilaterally, showed red and white lichenoid-type lesions (Figure 1), and a plaque-like lesion on the right lateral side of the tongue. Examination of the teeth revealed the presence of grade III mobility of lower molars on the right side. The possibility of epithelial dysplasia was considered. Two incisional biopsies, on the right retro-molar area and on the right buccal gingiva, were performed. Apart from the chlorhexidine mouthwash prescribed, no extra treatment was provided.
Figure 1: Intraoral photographs for the lesions at time of presentation.
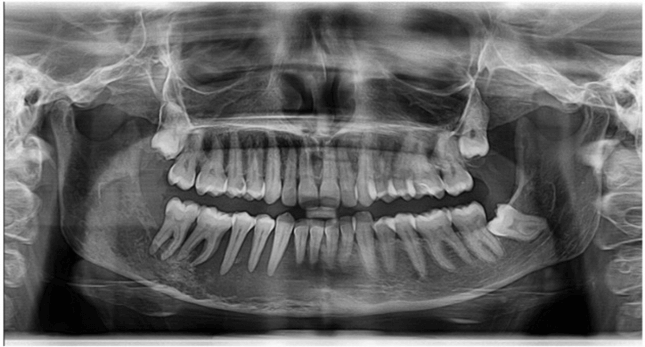
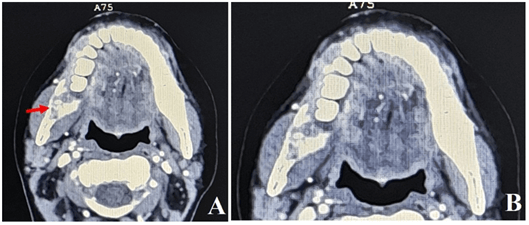
The microscopic examination of the specimens showed overlying squamous epithelium with ulceration, and invasive malignant tumor composed of nests of malignant squamous epithelium. The tumor cells exhibit moderate nuclear pleomorphism with frequent mitosis and the presence of keratin pearls. The histopathologic examination also showed the presence of fibrous desmoplasia. No fungal infection was reported. The final diagnosis was moderately differentiated squamous cell carcinoma (G2). The patient presented two weeks later with continuing discomfort in her mouth. On examination, the oral lesions showed essentially identical features as the first examination with good healing of the surgical biopsy site. Clinical examination of head and neck lymph nodes revealed no indication of their involvement. Orthopantomography (OPG) examination showed right-sided invasive bone destructive lesion with osteolytic nature (Figure 2). Computerized tomography scan revealed the presence of an ill-defined osteolytic bone destructing lesion (Figure 3), with soft tissues enhancing components on the medial and lateral mandibular aspects, measuring 5.8 x 2.7 x 1.8 cm, mainly seen at the right mandibular body, extending beyond the symphysis menti of the left side. Multiple bilateral enlarged cervical lymph nodes at levels II and III, the largest one seen at right submandibular region measuring 10 x 5 mm. The nasopharynx, oropharyngeal and laryngeal air spaces appeared normal. No glottic, supra, or infra glottis mass lesions were observed. Intact cartilaginous skeleton of the larynx. Normal appearance of thyroid glands, submandibular, parotid salivary glands. Abdomen and pelvis CT scan revealed the presence of right labial soft tissue mass lesion measuring 3.5 x 2.5 cm.
Figure 2: Orthopantomograph showing presence of ill-defined osteolytic destructive lesion related to lower teeth on the right side.
Figure 3: Computerized tomography scan revealed presence of an ill-defined osteolytic bone destructive lesion with soft tissue enhancing components on the medial and lateral mandibular aspects, mainly seen at the right mandibular body (Red arrow) in (A). (B) is a magnification of A.
The patient was referred to the medical oncology department to provide the necessary management. Regarding medical management of this patient, immunosuppressive agents, including steroid discontinued and then referred to gynaecologist to take biopsy from genital growth to exclude the presence of another potential tumor. Moreover, due to lack of oncological guidelines in the management of such a patient, and the peculiarities of the oral lesion, the patient was referred to radiotherapy department. Additionally, there was also the concern and challenge that; stoppage of immunosuppressive therapy may result in worsening of GVHD with multisystem involvement.
Discussion
The risk of developing secondary cancers in HSCT patients is considerably high, with a greater than ten times increased frequency of oral, oesophageal, and thyroid cancers [6, 8]. It has been reported that in people who are 10 years old at the time of HSCT, the risk reaches its maximum figure and continues to be high in people of age group between 10-29 years old, while it diminishes in people who are above 30 years [3]. In patients with a primary diagnosis of aplastic anaemia and Fanconi anaemia, the risk of solid cancer was stated to be higher, whereas other reports have mentioned the presence of higher risks for patients with a primary diagnosis of acute and chronic leukemia [8, 9]. The development of secondary solid tumors peaks between eight and nine years after HSCT [6]. In Libya, few aplastic anaemia cases have been registered and this may be due to lack of facilities for proper diagnosis. Also, stem cell transplant is not readily available locally and patients are not usually able to travel overseas for such procedures; therefore, SCC secondary to GvHD is extremely rare.
In our case, the patient was aged 28 years at the time she was diagnosed with aplastic anaemia and did undergo bone marrow transplantation when she was 30 years and 4 months. Few months later, she had the manifestation of GvHD and in the space of one year, starting with the appearance of the GvHD manifestation, she developed at least two OSCCs. We were not able to confirm histopathologically the clinical changes in the buccal mucosa on both sides because of her general condition at time of biopsy. The risk factors involved in the development of secondary tumors in patients treated with HSCT have been investigated by many researchers. Occurrence of new malignant lesions are broadly considered to be associated with chemotherapy agents or irradiation for pre-transplant cytotoxic therapy [6]. No relationship has been established between cytotoxic therapy and the risk of solid cancer in this case [3]. Risk factors including smoking, alcohol consumption and older age are found unrelated in patients treated with HSCT. In parallel with these data, our patient had never smoked and did not consume alcohol or other hazardous habits considered to be known risk factors. Blood investigation results of the three viral screening performed by Elisa tests were negative. Viral screening routinely done before any stem cell transplant to avoid flare-up of HCV or HBV.
Chronic GvHD (cGvHD) is thought to be a possible risk factor for the development of secondary cancers after HSCT. OSCCs have been identified by many researchers in cases with widespread cGvHD with its chronic inflammation affecting the skin and mucosa [10]. Some authors have reported two cases that developed OSCCs, one of them, having previously had cGvHD in the buccal mucosa [10, 11]. The other OSCC appeared in the gingiva at a site of previous lichenoid mucositis. Moreover, an association between cGvHD and cancer has been demonstrated in different anatomical locations. For instance, cutaneous SCC and melanoma have developed on skin previously affected by GvHD [12]. Also, reports described pulmonary tissue previously diagnosed with GvHD-related lung disease had been affected by pulmonary mucoepidermoid carcinoma [13]. In accordance with the literature, our patient has been affected by the GvHD after receiving the HSCT therapy and developed OSCCs in at least two oral sites in addition to a labial growth as the gynaecological examination and CT scan studies revealed. It is very important to exclude SCC in genitalia because of the multifocality nature of the disease.
cGvHD of the oral cavity manifests as lesions that are clinically and histologically similar to oral lichen planus, a moderately common oral cavity condition with limited but substantial risk for malignancy. Both conditions are of similar pathogenesis, where T-lymphocytes chronically target the epithelium of the oral cavity. Such chronic immunologic damage to the oral mucosa by T cells is thought to lead to some form of malignant transformation. For the management of cGvHD, therefore, HSCT recipients must use immunosuppressive medications for prolonged periods of time, and several reports have stressed the association between the development of solid cancers and long-term use of immunosuppressive drugs [3, 6]. The relationship between chronic inflammation and immunosuppression state that results from therapy are not totally clear; however, immunosuppression can hinder tissue repair in the field of chronic inflammation, such as cGvHD, thus raising the risk of malignant tumor development. In the current case, the patient has used immunosuppressive drugs, and this is in agreement with the above-mentioned data but the period of elapsing between HSCT and the development of OSCCs as secondary solid tumors was shorter than that stated in the literature which is a relatively short period of time compared with reported peaks between 8 and 9 years after HSCT [6]. The difference in age between the donor and recipient is an important factor and as described in the literature can result in HSCT therapy failure, even if both donor and recipients are very close relatives [7].
In the present case, the patient has received the bone marrow from her younger sister because she was the only family member who was compatible. However, the fourteen years difference in age between the two sisters could be one of the reasons behind the HSCT failure and development of GvHD in our patient. Regarding the medical management of this patient, immunosuppressive agents, including steroid discontinued and then referred to gynaecologist to take the biopsy from genital growth to exclude another potential tumor. Moreover, due to the lack of oncological guidelines in the management of such patient, and the peculiarities of the oral lesion making her a poor candidate for surgery, she was referred to radiotherapy department. Additionally, the challenge is that; stoppage of immunosuppressive therapy may result in worsening of GvHD with multisystem involvement. Furthermore, another factor that had a strong influence on our case management, besides the above-mentioned points, is the pandemic of Coronavirus (COVID-19). Many surgical procedures in public and private practice have been limited to very restricted emergency cases only. Also, treatment of such case is not readily available locally and patient is not able to travel overseas because of the tough restrictions applied by the health authorities during the pandemic of COVID-19 and the presence of different mutations of coronavirus.
Conclusion and Recommendations
A potential risk to have oral squamous cell carcinoma is linked to HSCT patients with cGvHD at high risk at any time of their age after HSCT. The known link between GvHD of oral cavity mucosa and OSCC should be a warning to doctors to pay attention to closer follow-up and the biopsy of suspected lesions for these patients. Policies for avoiding cGvHD in patients with symptomatic GvHD should be related to the production of more successful and less carcinogenic treatments. Increased indications and patients undergoing allogenic stem cell transplant may predict an increase in the incidence of secondary cancers and needs further studies and clinical trials and development of special guidelines of management.
Article Info
Article Type
Case ReportPublication history
Received: Mon 10, May 2021Accepted: Mon 31, May 2021
Published: Fri 18, Jun 2021
Copyright
© 2023 Khaled Saleh Ben Salah. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Hosting by Science Repository.DOI: 10.31487/j.JDOA.2021.01.01
Author Info
Khaled Saleh Ben Salah Abdulfattah A Elturki Yuossef Swaisi Fatma M. Emaetig Mohamed A. Elfagieh Firas K. Abdulmalik Yaser O. Howayw Mohamed A. Harrama Eltaher Elshagmani Ebrahim H. ElMahjoubi Matthew E. Agwae Eleni Nikolopoulou Abdulhakim W Zaggut
Corresponding Author
Khaled Saleh Ben SalahDepartment of Pathology, National Cancer Institute (NCI), Misrata, Libya
Figures & Tables
Table 1: Showing results of blood investigation performed prior to HSCT.
|
Test |
Result |
|
Flow cytometry |
No Paroxysmal nocturnal hemoglobinuria
(PNH) clone |
|
Fluorescence in situ hybridization
(FISH) for MDS |
Negative |
|
Cytogenetic |
Pseudodiploid clone with 1q triplication |
|
NGS: BCOR. Chromosomal breakage test |
Positive |
Table 2: Results of blood investigations before taking the oral incisional
biopsy.
|
Test |
Result |
Test |
Result |
|
HbA1C |
4.14 |
WBC |
9.2 x 103/μl |
|
CA19.9 |
35.1 U/ml |
RBC |
4.10 x 106/μl |
|
CEA |
3.60 ng/ml |
HGB |
14.3 g/dL |
|
Blood urea |
74 mg/dl |
HCT |
42.3% |
|
Blood Creatinine |
0.46 mg/dl |
MCV |
103.2 fL |
|
Serum potassium |
3.28 mmol/L |
MCH |
34.9 pg |
|
Blood Sodium |
138 mmol/L |
MCHC |
33.8 g/dL |
|
ESR |
7 mm/2hrs |
PLT |
139 x 103/μl |
|
CRP Titer |
14.0 mg/l |
LYM% |
19.1% |
|
Serum Creatinine |
0.7 mg/dl |
MXD% |
9.3% |
|
Total bilirubin |
0.6 mg/dl |
NEUT% |
71.6% |
|
S.G.O.T |
25 U/I |
LYM# |
1.8 x 103/μl |
|
S.G.P.T |
30 U/I |
MXD# |
0.9 x 103/μl |
|
ALK.Phosphat |
261 U/I |
NEUT# |
6.5 x 103/μl |
|
Mg |
2.1 mg/dl |
RDW-SD |
56.5 fL |
|
Vitamin B12 |
427 bg/ml |
RDW-CV |
14.7% |
|
Folic Acid |
8.9 |
RDW |
14.5 fL |
|
HBsAg by Elisa |
Negative |
MPV |
11.3 fL |
|
HCV Ab by Elisa |
Negative |
P-LCR |
35.5% |
|
HIV by Elisa |
Negative |
PCT |
0.16 |
References
1. Young NS (1999) Acquired aplastic
anemia. JAMA 282: 271-278. [Crossref]
2. Brown KE, Tisdale J, Barrett AJ,
Dunbar CE, Young NS (1997) Hepatitis-associated aplastic anemia. N Engl J
Med 336: 1059-1064. [Crossref]
3. Curtis RE, Metayer C, Rizzo JD, Socié
G, Sobocinski KA et al. (2005) Impact of chronic GVHD therapy on the
development of squamous-cell cancers after hematopoietic stem-cell
transplantation: an international case-control study. Blood 105:
3802-3811. [Crossref]
4. Georges GE, Storb R (2016)
Hematopoietic stem cell transplantation for acquired aplastic anemia. Curr
Opin Hematol 23: 495-500. [Crossref]
5. Eggleston TI, Ziccardi VB, Lumerman H
(1998) Graft-versus-host disease. Case report and discussion. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod 86: 692-696. [Crossref]
6. Demarosi
F, Soligo D, Lodi G, Moneghini L, Sardella A et al. (2005)
Squamous cell carcinoma of the oral cavity associated with graft versus host
disease: report of a case and review of the literature. Oral Surg Oral Med
Oral Pathol Oral Radiol Endod 100: 63-69. [Crossref]
7. Bauters T, Bordon V, Van de Velde V,
Van Lancker S, Robays H et al. (2010) Highly effective treatment with
tacrolimus ointment in an adolescent with oral graft-versus-host disease. Pharm
World Sci 32: 350-352. [Crossref]
8. Kolb HJ, Socié G, Duell T, Van Lint
MT, Tichelli A et al. (1999) Malignant neoplasms in long-term survivors of bone
marrow transplantation. Late Effects Working Party of the European Cooperative
Group for Blood and Marrow Transplantation and the European Late Effect Project
Group. Ann Intern Med 131: 738-744. [Crossref]
9. Deeg HJ, Socié G, Schoch G, Henry-Amar
M, Witherspoon RP et al. (1996) Malignancies after marrow transplantation for
aplastic anemia and fanconi anemia: a joint Seattle and Paris analysis of
results in 700 patients. Blood 87: 386-392. [Crossref]
10. Otsubo H, Yokoe H, Miya T, Atsuta F,
Miura N et al. (1997) Gingival squamous cell carcinoma in a patient with
chronic graft-versus-host disease. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod 84: 171-174. [Crossref]
11. Abdelsayed RA, Sumner T, Allen CM,
Treadway A, Ness GM et al. (2002) Oral precancerous and malignant lesions
associated with graft-versus-host disease: report of 2 cases. Oral Surg Oral
Med Oral Pathol Oral Radiol Endod 93: 75-80. [Crossref]
12. Lishner M, Patterson B, Kandel R, Fyles G, Curtis JE et al. (1990) Cutaneous and mucosal neoplasms in bone marrow transplant recipients. Cancer 65: 473-476. [Crossref]
13. Sánchez J, Serrano J, Gómez P, Román J, Cosano A et al. (1997) Bronchial mucoepidermoid carcinoma after allogeneic bone marrow transplantation. J Clin Pathol 50: 969-970. [Crossref]
