Abstract
Background
To investigate the temporal characteristics of clinical variables of hospital-acquired acute kidney injury (AKI) in COVID-19 patients and to longitudinally predict AKI onset.
Methods
There were 308 hospital-acquired AKI and 721 non-AKI (NAKI) COVID-19 patients from Stony Brook Hospital (New York, USA) data, and 72 hospital-acquired AKI and 303 NAKI COVID-19 patients from Tongji Hospital (Wuhan, China). Demographic, comorbidities, and longitudinal (3 days before and 3 days after AKI onset) clinical variables were used to compute odds ratios for and longitudinally predict hospital-acquired AKI onset.
Results
COVID-19 patients with AKI were more likely to die than NAKI patients (31.5% vs 6.9%, adjusted p < 0.001, OR = 4.67 [95% CI 3.1, 7.0], Stony Brook data). AKI developed on average 3.3 days after hospitalization. Procalcitonin was elevated prior to AKI onset (p < 0.05), peaked, and remained elevated (p < 0.05). Alanine aminotransferase, aspartate transaminase, ferritin, and lactate dehydrogenase peaked the same time as creatinine, whereas d-dimer and brain natriuretic peptide peaked a day later. C-reactive protein, white blood cell and lymphocyte showed group differences − 2 days prior (p < 0.05). Top predictors were creatinine, procalcitonin, white blood cells, lactate dehydrogenase, and lymphocytes. They predicted AKI onset with areas under curves (AUCs) of 0.78, 0.66, and 0.56 at 0, − 1, and − 2 days prior, respectively. When tested on the Tongji Hospital data, the AUCs were 0.80, 0.79, and 0.77, respectively.
Conclusions
Time-locked longitudinal data provide insight into AKI progression. Commonly clinical variables reasonably predict AKI onset a few days prior. This work may lead to earlier recognition of AKI and treatment to improve clinical outcomes.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) [1, 2] caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved into a global pandemic with devastating morbidity and mortality. The widespread outbreaks with recent spikes across the globe and the likelihood of recurrences have strained and will continue to strain healthcare resources. COVID-19 infection causes disproportional inflammatory responses and cytokine storm that often result in multiple organ failure. Acute kidney injury (AKI) has become a hallmark of COVID-19 infection and is associated with high incidence of critical illness and mortality in COVID-19 patients [1,2,3,4,5,6,7,8,9,10,11,12]. Kidneys could be damaged directly by the virus per se or indirectly by host-immune responses (i.e., hypoxia, thrombosis, cytotropic and cytokine-mediated immune responses, amongst others) [13,14,15]. Whether direct damage of the kidney and whether renal infection is common in patients with less severe COVID-19 is not yet established [13].
Although a few studies have found some clinical variables, usually at hospital admission, to be associated with high likelihood of in-hospital AKI [16,17,18], predicting AKI using only clinical variables at admission is likely inaccurate because patients came into hospitals at different stages of disease severity or they might already have community-acquired AKI. To our knowledge, there has been no systematic evaluation of the temporal characteristics of clinical variables leading up to in-hospital AKI in COVID-19 and how these temporal characteristics are judiciously used to inform clinical decision-making. The association of AKI evolution with respect to other clinical laboratory variables in the context of COVID-19 disease remains poorly understood. The ability to recognize AKI early and anticipate AKI development could improve patient management that include hemodynamic support, renal replacement therapy, and avoid non-steroidal anti-inflammatory drugs, nephrotoxins, and contrast [6,7,8,9,10,11,12], ultimately improving clinical outcomes.
The goal of this study was thus to investigate the temporal characteristics of clinical variables associated with hospital-acquired AKI in COVID-19 patients and to predict hospital-acquired AKI onset. We analyzed the temporal progression of different clinical variables with time lock to AKI onset and developed a mathematical model to predict hospital-acquired AKI at different days prior to onset. Association of treatments (invasive mechanical ventilation, anticoagulant, and steroids) with hospital-acquired AKI was also explored. This study was done using data from two hospital systems. To our knowledge, this is the first systematic documentation of the longitudinal clinical variables leading up to AKI onset and the first longitudinal prediction of AKI onset in COVID-19.
Methods
Study population and data collection
Our study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD). All methods were carried out in accordance with relevant guidelines and regulations, including those of stated in the “Declaration of Helsinki.” Data came from two sites: The Stony Brook University Hospital (Stony Brook, New York, USA) data were used for training, while the Tongji Hospital (Wuhan, China) data were used for testing purposes.
Stony Brook data
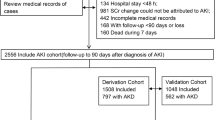
The Stony Brook data came from Stony Brook University Hospital (Stony Brook, NY), a 624-bed facility, for exempted informed consent and HIPAA waiver from the Institutional Research Board (IRB 2020-00207). Figure 1 shows the patient selection. Our COVID-19 Persons Under Investigation Registry of patients through the Emergency Department consisted of 6,678 persons clinically suspected of COVID-19 infection from February 7, 2020 to June 30, 2020, of which 2892 were tested positive using real-time polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on a nasopharyngeal swab specimen. Data of these cohort have been used previously for studies of different research questions [19,20,21,22,23,24,25,26,27]. All COVID-19 patients in this study were in-patient.
AKI was defined by KDIGO criteria [28, 29] with either a 0.3 mg/dl increase in serum creatinine within 48 h or 1.5 times the lowest reading during hospitalization due to lack of data prior to hospitalization, which served as a baseline [11, 30]. Patients in our cohort did not declare ESRD and, therefore, to our knowledge, we excluded all ESRD patients on dialysis. Non-AKI COVID-19 patients were those whose blood creatinine level did not rise above the threshold defined by KDIGO criteria during the entire time of their hospital stays.
Exclusion included patients with no creatinine reading (N = 73), < 18 years old (N = 23), still in the hospital at the time of this analysis (N = 21) and had community-acquired AKI (N = 295) defined as patients who had AKI within 24 h of admission by KDIGO standards [28, 29]. The final sample size used in this analysis was 1029 patients meeting the inclusion/exclusion criteria. Urine output was not used due unavailable urine data.
Demographics and chronic comorbidities, longitudinal vital signs, laboratory blood tests, and blood gases were extracted from electronic medical records. Demographic data included age, gender, ethnicity and race. Chronic comorbidities included smoking, diabetes, hypertension, asthma, chronic obstructive pulmonary disease (COPD), coronary artery disease, heart failure, cancer, immunosuppression and chronic kidney disease. Longitudinal laboratory tests included creatinine (Cr), procalcitonin (PROCAL), aspartate transaminase (AST), alanine aminotransferase (ALT), ferritin (FERR), lactate dehydrogenase (LDH), white blood cell count (WBC), C-reactive protein (CRP), lymphocytes count (LYMPH), D-dimer (DDIM), brain natriuretic peptide (BNP), estimated glomerular filtration rate (eGFR), and albumin concentration. Longitudinal vital signs included heart rate (HR), diastolic blood pressure (DBP), systolic blood pressure (SBP), respiratory rate (RR), pulse oxygen saturation (SpO2), and temperature (temp). Longitudinal blood gas variables include pO2, pCO2, and pH. In addition, we also collected the treatment onset of invasive mechanical ventilation (IMV), prophylactic and therapeutic anticoagulants and steroids relative to AKI onset.
We time-locked the time-series laboratory test variables to the day of AKI onset. Laboratory test variables were plotted across time with time lock (t = 0) to AKI onset, along with data 3 days before and 3 days after AKI onset. For comparison, time-series data for NAKI patients were time locked (t = 0) to 3 days after ED admission, along with data 3 days before and 3 days after that time point. The 3 days post-ED admission was chosen because COVID-19 patients on average developed AKI 3.3 days after ED admission (see Results). For ease of comparison, individual laboratory test values were also normalized to the NAKI group at t = 0 for individual patients.
Note that many patients developed AKI after 1, 2 or 3 days post-admission and thus these patients did not contribute to − 3, − 2, or − 1 day prior to onset. Removing data of patients with onset of 1, 2 or 3 days post-admission did not alter the temporal characteristics of the time courses of all clinical variables.
Tongji Hospital data
The Tongji Hospital (Wuhan, China) data were obtained from Jan 10, 2020 to Feb 24, 2020 (N = 485, of which 375 met the inclusion and exclusion criteria) with approval of their institutional review board and waiver of informed consent [27, 31]. This dataset was used for “testing.”
Prediction model
We built logistic regression models to predict outcomes at each day prior to AKI onset. Prediction was performed using individual variables and combination of a few top predictors, and at a few time points prior to AKI onset. We time-locked to the day of onset. We performed prediction up to 3 days prior because AUC performance dropped close to chance at and beyond 3 days prior. Prediction performance was evaluated by the area under the curve (AUC) of the receiver operating characteristic (ROC) curve.
Statistical analysis
All statistical analyses were performed using Python packages (Sklearn and Statsmodels, Python Software Foundation, Wilmington, DE) and SAS software (Analytics Software and Solution, Cary, NC). Frequencies and percentages for categorical variables between the AKI and NAKI groups were compared using χ2 tests. Continuous variables, expressed as median (IQR), were compared between groups using nonparametric Mann–Whitney U tests. Mortality rates were compared between groups with χ2 tests adjusted with covariates. Differences between AKI and NAKI group for clinical variables in time series graphs was analyzed via linear mixed model and least squares means test with Tukey’s adjustment. P values < 0.05 were considered statistically significant unless otherwise specified. P values and odd ratios for mortality were adjusted for age, ethnicity, and significant comorbidities.
Results
Stony Brook data
In the Stony Brook data, the general floor cohort, had 197 AKI and 681 NAKI patients (22.4% AKI prevalence), with the mortality rate of 14.7% and 6.0%, respectively. The ICU cohort had 111 AKI and 40 NAKI patients (73.5% AKI prevalence), with the mortality rate of 61.3% and 22.5%, respectively. Overall, AKI patients had a markedly higher mortality rate than NAKI patients (31.5% vs 6.9%, adjusted p < 0.001, adjusted OR of 4.67 [95% CI 3.1, 7.0]. The overall AKI prevalence rate was 23.0%.
Table 1 summarizes patient demographics and comorbidities of the Stony Brook data. The median age of hospital-acquired AKI cohort was older than that of the NAKI cohort (p < 0.001). Sex was not significantly different between groups. Ethnicity (p = 0.003), but not race (p = 0.66), was significantly different between groups. Smoking, diabetes, hypertension, coronary artery disease, cancer, chronic kidney disease (p < 0.05), but not asthma, COPD, and immunosuppression (p > 0.05), were significantly between groups. AKI patients generally had more comorbidities.
The majority of patients developed AKI within a few days of hospital admission, averaging 3.3 days (median = 2 days). There were 36.2%, 21.4%, 15.5%, 6.6%, 5.3%, 3.6%, 3.3%, and 8.2% of COVID-19 patients developed AKI after 1, 2, 3, 4, 5, 6, 7, > 7 days of hospitalization, respectively (Fig. 2).
Figure 3 depicts the time series of laboratory tests relative to AKI onset of the Stony Brook data. Laboratory values were normalized to the NAKI group at t = 0 by individual patients. In the NAKI cohort, Cr remained low and time invariant. In the AKI cohort, Cr spiked 4.5 times from baseline at t = 0 and returned toward baseline but above NAKI level.
Time courses of normalized laboratory tests of HAKI and NAKI COVID-19 patients of the Stony Brook data. Normalization was relative to No AKI at t = 0 for individual patient. For HA-AKI patients, t = 0 represents day of AKI onset (Cr first peaked), and for No AKI patients 3 days after hospital admission. Abbreviations: Cr, creatinine. BNP, brain natriuretic peptide. ALT, alanine aminotransferase. AST, aspartate transaminase. PROCAL, procalcitonin. CRP, C-reactive protein. LDH, lactate dehydrogenase. WBC, white blood cell. DBP, diastolic blood pressure. SBP, systolic blood pressure. FERR, ferritin. LYMPH, lymphocyte count. DDIM, d-dimer. HR, heart rate. RR, respiratory rate. SpO2, pulse oxygen saturation. PO2, arterial oxygen pressure. PCO2, arterial carbon dioxide pressure. The * represents a significant difference based on the linear mixed model in mean measures between two groups at each time point. Error bars are SEM
Procalcitonin was elevated one day prior to AKI onset, peaked the same time as Cr and remained elevated. AST, ALT, ferritin, LDH showed similar temporal patterns as Cr. CRP, WBC, lymphocyte, eGFR showed divergence between groups starting − 2 days prior. Note that lymphocyte counts and eGFR of AKI were below those of NAKI, in contrast to most other variables. d-Dimer elevated − 1 day prior but peaked a day later than Cr. BNP peak lagged Cr by 1 day.
Most vitals and blood gases (HR, DBP pressure, SBP, SpO2, temperature, pO2, pCO2 and pH) and albumin were similar between groups across these time points, except that RR was consistently elevated and pH was consistently lower in the AKI group.
Table 2 shows the ORs of the top five individual variables for developing AKI for − 1 day prior of the Stony Brook data. Creatinine and procalcitonin concentrations had the largest ORs by far, followed by white blood cell count, lactate dehydrogenase and lymphocyte count.
We performed prediction of AKI onset at multiple time points. Figure 4 shows ROC curves of individual top variables and the combined five top variables at different days prior to AKI onset of the Stony Brook data. Figure 5 shows the prediction performance indices (AUCs) of individual top variables and the combined five top variables at different days prior to AKI onset of the Stony Brook data. AUCs were high at 0 and − 1 day before AKI onset, and decreased − 2 and − 3 days before AKI onset. The combined five top variables yielded the best AUC compared to individual predictors across all time points studied.
Table 3A summarizes the other performance metrics of the predictive model using the combined top five variables. The best model using the combined five top variables predicted AKI onset with AUC of 0.78, 0.66, and 0.56 at 0, − 1, and − 2 days prior, respectively.
For external validation, a logistic regression model trained on the Stony Brook hospital data were tested on the Tongji Hospital data using the significant variables: procalcitonin, creatinine, white blood cells, lactate dehydrogenase lymphocytes. Of the 375 patients, 201 survived and 174 died (46.4% mortality rate) and 72 had hospital-acquired AKI (19.2% AKI prevalence rate). The results are shown in Table 3B. The AUC for predicting AKI onset were 0.93, 0.80, and 0.88 at 0, − 1, and − 2 days prior to onset.
AKI onset association with treatments
We also explored the possible association of treatments with AKI onset in the Stony Brook data. More AKI patients had IMV (32.1% vs 2.4%, p < 0.001), noninvasive mechanical ventilation (12.0% vs 4.2%, p = 0.006), prophylactic (p < 0.001) and therapeutic anticoagulants (p = 0.002) and steroids (32.1% vs 9.6%, p = 0.006) than NAKI patients (Table 4). A total of 16.2% of AKI patients needed continuous renal replacement therapy (1.6%) or hemodialysis (9.4%), with 5.2% needing both.
We also analyzed the differences in onset times between AKI development and the initiation of IMV, anticoagulant, steroid and dialysis treatment (Fig. 6). For most patients, IMV was initiated − 1 day prior to AKI onset. Anticoagulant treatment was initiated − 1 to − 3 days prior to AKI onset. Steroid treatment was initiated − 2 to 0 days before AKI onset but were comparatively more spread out. For all treatments, treatment initiation day is not significantly associated with mortality (p > 0.05).
Histogram of the treatment initiation prior to AKI onset for A invasive mechanical ventilation, B anticoagulants and C steroids of the Stony Brook data. For example, most of the patients were treated with invasive mechanical ventilation − 1 day prior to AKI onset. For all treatments, treatment initiation day is not significantly associated with mortality (p > 0.05)
The eGFR was 78.3 ± 5.5 (SEM) mL/min/1.73m2 for AKI and 99.1 ± 1.9 mL/min/1.73m2 for NAKI patients for survivors at discharge.
Discussion
This study investigated the temporal characteristics of clinical variables associated with AKI development during hospitalization in COVID-19 patients at two hospitals. The key findings are: (1) COVID-19 patients with hospital-acquired AKI acquired it within 3.3 days after hospitalization on average, and patients with hospital-acquired AKI showed a markedly higher mortality rate than NAKI patients. (2) Creatinine peaked 4.5 times from baseline and returned toward baseline but remained above NAKI level. (3) Procalcitonin elevated prior to AKI onset, peaked the same time as creatinine and remained elevated. AST, ALT, ferritin, LDH peaked the same time as Cr, whereas d-dimer and BNP lagged by a day. CRP, WBC, lymphocyte and eGRF showed differences between groups starting − 2 days prior. (4) Abnormal creatinine and procalcitonin had the highest odds ratios for developing AKI, followed by white blood cells, lactate dehydrogenase and lymphocytes. (5) The best predictive model using the combined five top variables predicted AKI onset with AUCs of 0.78, 0.66, and 0.56 at 0, − 1, and − 2 days prior, respectively (Stony Brook Hospital data). When tested on an external validation data (Tongji Hospital data), the AUCs were 0.93, 0.80, and 0.88 at 0, − 1, and − 2 days prior to onset, respectively.
Our incidence of AKI and mortality odds ratio for patients with hospital-acquired AKI is in general agreement with the literature [6,7,8,9,10,11,12]. Procalcitonin elevated prior to AKI onset, peaked the same time as Cr and remained elevated. Elevated procalcitonin is known to be a sign of a serious infection, including sepsis [32] and its elevation prior to AKI onset suggests it might be an early predictor of AKI in COVID-19. AST, ALT, ferritin, LDH peaked the same time as Cr. Elevated AST, ALT and ferritin are suggestive of liver damage. Increased levels in AST and ALT in COVID-19 are associated with the need for escalated care and higher rate of mortality [33]. Elevated ferritin is indicative of inflammation and cytokine storm, amongst others [34]. Lactate dehydrogenase is a marker of cell death and multi-organ failure [35,36,37]. Taken together, these temporal profiles are consistent with multi-organ failure [35,36,37] that occurred at roughly the same time.
CRP, WBC and lymphocyte concentrations showed divergence between groups very early on starting − 2 days prior and these variables could have predictive values. CRP is made by the liver and elevated level of CRP is a sign of inflammation [38]. Elevated white blood cell count is indicative of cytokine and immune response, including in COVID-19 infection [39]. Lymphocyte count is known to be lower in more severe COVID-19 patients and is associated with high mortality rate [20, 24, 40,41,42] and is associated with hospital-acquired AKI [43].
Interestingly, d-dimer concentration, an indicator of blood clots [44], elevated − 1 day prior but peaked a day later than Cr concentration, suggesting that potential embolic event might be present early but peaked in the later stage [10]. d-Dimer has been reported to be associated with COVID-19 and high mortality. BNP, an indicator of heart damage, lagged Cr by 1 day. It is not surprising that most vitals and blood gases were not different between groups across time points because they were actively maintained within normal physiological ranges. The exceptions were RR which was consistently elevated and pH which was consistently lower in the AKI group, indicative of respiratory distress and acidosis.
Taken together, time-locked longitudinal data offered insights into disease pathophysiology. If the clinical variables at ED admission were averaged across patients without time lock, most of these clinically significant effects could likely be averaged out. It is important to emphasize that most of the clinical variables of NAKI patients were also abnormal compared to normative healthy controls.
Predictive models
Abnormal creatinine (OR = 8.08), procalcitonin (OR = 1.92), white blood cell count (OR = 1.35), lactate dehydrogenase (OR = 1.21), and lymphocyte count (OR = 0.79) at − 1 day prior were associated with higher likelihood of AKI development. The temporal progression of these clinical variables suggests some of these variables could predict AKI onset. We found that creatinine, procalcitonin, white blood cell, lactate dehydrogenase and lymphocyte levels individually predicted AKI onset moderately well, and their combination outperformed individual predictors. The overall prediction performance by AUC was good at day 0, and moderate at day − 1 and − 2. The moderate performance of the training dataset could be due to data asymmetry, low sensitivity, small sample size and unpredictability of AKI. Interestingly, AUCs of the validation data showed better performance than those of the training data. A possible explanation is that the Tongji Hospital cohort had worse disease severity where laboratory variables were more consistently abnormal. Further validation studies are needed. Both datasets had low sensitivity and accuracy, but high specificity. The low sensitivity could be due to data asymmetry and small sample size.
Although creatinine was used to define AKI onset, we decided to include it in the model for the purpose of comparison. While these clinical variables have previously been associated with critical illness and mortality in COVID-19 patients [45], this is the first time that these clinical variables were used to predict AKI onset in a longitudinal fashion. These findings could help frontline physicians to identify patients needing early interventions to prevent AKI. Taken together, these findings suggest that AKI is likely a result of multi-organ failure contributing to higher mortality rate. Time-locked longitudinal data can provide insight into AKI progression that may lead to earlier recognition of AKI, intervention and improvement in clinical outcomes.
Association with treatments
Significantly more AKI patients had IMV, anticoagulants and steroids than NAKI patients. For most patients, IMV was initiated − 1 day prior to AKI onset. Anticoagulant treatment was initiated − 1 to − 3 days prior to AKI onset. Although glomerular hemorrhage could lead to AKI [46], over-anticoagulation has also been associated with AKI [47, 48] and thus it is possible that higher dose of anticoagulant could unintentionally contribute to AKI in COVID-19 patients. The use therapeutic anticoagulants in COVID-19 patients warrant further study [48, 49]. Steroid treatment was initiated − 2 to 0 days before AKI onset but were comparatively more spread out. A recent post hoc analysis of a placebo-controlled, randomized trial suggests that intraoperative steroids might lower the risk of renal replacement therapy for AKI after cardiac surgery [50]. It is important to note that these observations are associations, not cause and effect. For example, IMV and higher dose of anticoagulant might be needed in more severe COVID-19 patients and thus they were also more likely to develop AKI in the hospital. Other explanations are possible.
Finally, the eGFR was 78.3 ± 5.5 (SEM) mL/min/1.73m2 for AKI and 99.1 ± 1.9 mL/min/1.73m2 for NAKI patients for survivors at discharge, where normal eGFR for adults is > 90 mL/min/1.73m2 according to the National Kidney Foundation. This observation suggests kidney function had not yet returned to normal at discharge.
The strengths and novelties of our study include large sample size and the use of longitudinal variables with time-locked to AKI onset to characterize the progression of relevant clinical variables between AKI and non-AKI COVID-19 patients. The comparison of the temporal dynamics of various clinical variables is important because it reveals insights into leading indicators of AKI onset in the context of COVID-19, which has not been previously studied. We also developed and tested longitudinal predictive models to predict AKI onset a few days prior to onset using data from two hospitals, in contrast to essentially all published papers to date that reported AKI data in COVID-19 patients at a single time point at admission.
Limitations
This study had several limitations. We did not have data on proteinuria and hematuria, which have been associated with in-hospital mortality. Future studies should include urine analysis in the predictive modeling. As with all observational studies, other residual confounders may exist that were not accounted for in our analysis. Note that many patients developed AKI after 1 day post-admission and thus they did not contribute to − 3 and − 2 day prior to onset. Removing data of patients with onset of 1 or 2 days post-admission did not significantly affect the temporal characteristics of all time courses. Some of the performance indices of prediction were moderate and further testing on larger and independent datasets are needed. Future prospective studies are warranted.
Conclusions
This study compared the clinical profiles between COVID-19 patients with and without hospital-acquired AKI with respect to temporal progression of clinical variables, demographics, comorbidities, escalated care, mortality, and association with treatments. It also identified the top early predictors of in-hospital AKI and provided a mathematical model to predict AKI onset a few days prior, with cross-validation using an independent dataset from another hospital. This approach has the potential to provide frontline physicians with an objective tool to risk stratification and prediction of in-hospital AKI in COVID-19 patients in time-sensitive and potentially resource-constrained environments.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Shao M, Li X, Liu F, Tian T, Luo J, Yang Y. Acute kidney injury is associated with severe infection and fatality in patients with COVID-19: A systematic review and meta-analysis of 40 studies and 24,527 patients. Pharmacol Res. 2020;161:105107.
Oliveira CB, Lima CAD, Vajgel G, Campos Coelho AV, Sandrin-Garcia P. High burden of acute kidney injury in COVID-19 pandemic: systematic review and meta-analysis. J Clin Pathol. 2020;7:89.
Nadim MK, Forni LG, Mehta RL, Connor MJ Jr, Liu KD, Ostermann M, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–64.
Brienza N, Puntillo F, Romagnoli S, Acute TL, Injury K, in Coronavirus Disease. Infected patients: a meta-analytic study. Blood Purif. 2019;2020:1–7.
Farouk SS, Fiaccadori E, Cravedi P, Campbell KN. COVID-19 and the kidney: what we think we know so far and what we don’t. J Nephrol. 2020;33(6):1213–8.
Hamilton P, Hanumapura P, Castelino L, Henney R, Parker K, Kumar M, et al. Characteristics and outcomes of hospitalised patients with acute kidney injury and COVID-19. PLoS ONE. 2020;15(11):e0241544.
Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. AKI in hospitalized patients with and without covid-19: a comparison study. J Am Soc Nephrol. 2020;31(9):2145–57.
Ouyang L, Gong Y, Zhu Y, Gong J. Association of acute kidney injury with the severity and mortality of SARS-CoV-2 infection: A meta-analysis. Am J Emerg Med. 2021;43:149–57.
Trabulus S, Karaca C, Balkan II, Dincer MT, Murt A, Ozcan SG, et al. Kidney function on admission predicts in-hospital mortality in COVID-19. PLoS ONE. 2020;15(9):e0238680.
Wagner J, Garcia-Rodriguez V, Yu A, Dutra B, DuPont A, Cash B, et al. Elevated D-dimer is associated with multiple clinical outcomes in hospitalized Covid-19 patients: a retrospective cohort Study. SN Compr Clin Med. 2020;22:1–7.
Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–18.
Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151–60.
Khan S, Chen L, Yang CR, Raghuram V, Khundmiri SJ, Knepper MA. Does SARS-CoV-2 Infect the Kidney? J Am Soc Nephrol. 2020;31(12):2746–8.
Ahmadian E, Hosseiniyan Khatibi SM, Razi Soofiyani S, Abediazar S, Shoja MM, Ardalan M, et al. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev Med Virol. 2020;7:e2176.
Adapa S, Chenna A, Balla M, Merugu GP, Koduri NM, Daggubati SR, et al. COVID-19 pandemic causing acute kidney injury and impact on patients with chronic kidney disease and renal transplantation. J Clin Med Res. 2020;12(6):352–61.
Xia P, Wen Y, Duan Y, Su H, Cao W, Xiao M, et al. clinicopathological features and outcomes of acute kidney injury in critically ill Covid-19 with prolonged disease course: a retrospective cohort. J Am Soc Nephrol. 2020;31(9):2205–21.
Hectors SJ, Riyahi S, Dev H, Krishnan K, Margolis DJA, Prince MR. Multivariate analysis of CT imaging, laboratory, and demographical features for prediction of acute kidney injury in COVID-19 patients: a Bi-centric analysis. Abdom Radiol. 2020;7:87.
Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46(7):1339–48.
Zhu J, Shen B, Abbasi A, Hoshmand-Kochi M, Li H, Duong TQ. Deep transfer learning artificial intelligence accurately stages COVID-19 lung disease severity on portable chest radiographs. PLoS ONE. 2020;15(7):e0236621.
Zhao Z, Chen A, Hou W, Graham JM, Li H, Richman PS, et al. Prediction model and risk scores of ICU admission and mortality in COVID-19. PLoS ONE. 2020;15(7):e0236618.
Shen B, Hoshmand-Kochi M, Abbasi A, Glass S, Jiang Z, Singer AJ, et al. Initial chest radiograph scores inform COVID-19 status, intensive care unit admission and need for mechanical ventilation. Clin Radiol. 2021;76(6):473.
Musheyev B, Borg L, Janowicz R, Matarlo M, Boyle H, Singh G, et al. Functional status of mechanically ventilated COVID-19 survivors at ICU and hospital discharge. J Intensive Care. 2021;9(1):31.
Lu JQ, Musheyev B, Peng Q, Duong TQ. Neural network analysis of clinical variables predicts escalated care in COVID-19 patients: a retrospective study. PeerJ. 2021;9:e11205.
Li X, Ge P, Zhu J, Li H, Graham J, Singer A, et al. Deep learning prediction of likelihood of ICU admission and mortality in COVID-19 patients using clinical variables. PeerJ. 2020;8:e10337.
Lam KW, Chow KW, Vo J, Hou W, Li H, Richman PS, et al. Continued in-hospital ACE inhibitor and ARB Use in hypertensive COVID-19 patients is associated with positive clinical outcomes. J Infect Dis. 2020;222(8):1256–64.
Hou W, Zhao Z, Chen A, Li H, Duong TQ. Machining learning predicts the need for escalated care and mortality in COVID-19 patients from clinical variables. Int J Med Sci. 2021;18(8):1739–45.
Chen A, Zhao Z, Hou W, Singer AJ, Li H, Duong TQ. Time-to-death longitudinal characterization of clinical variables and longitudinal prediction of mortality in COVID-19 patients: a two-center study. Front Med. 2021;8:661940.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Ad-hoc working group of E, Fliser D, Laville M, Covic A, Fouque D, Vanholder R, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines on acute kidney injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrol Dial Transplant. 2012;27(12):4263–72.
Pelayo J, Lo KB, Bhargav R, Gul F, Peterson E, DeJoy IR, et al. Clinical characteristics and outcomes of community- and hospital-acquired acute kidney injury with COVID-19 in a US inner city hospital system. Cardiorenal Med. 2020;10(4):223–31.
Yan L, Zhang HT, Goncalves J, Xiao Y, Wang M, Guo Y, et al. An interpretable mortality prediction model for COVID-19 patients. Nat Machine Intelligence. 2020;2:283–8.
Kellum JA, Nadim MK, Forni LG. Sepsis-associated acute kidney injury: is COVID-19 different? Kidney Int. 2020;98(6):370–1372.
Hundt MA, Deng Y, Ciarleglio MM, Nathanson MH, Lim JK. Abnormal liver tests in COVID-19: a retrospective observational cohort study of 1827 patients in a major US Hospital Network. Hepatology. 2020;72(4):1169–76.
Lorenz G, Moog P, Bachmann Q, La Rosee P, Schneider H, Schlegl M, et al. Title: Cytokine release syndrome is not usually caused by secondary hemophagocytic lymphohistiocytosis in a cohort of 19 critically ill COVID-19 patients. Sci Rep. 2020;10(1):18277.
Thierry AR, Roch B. SARS-CoV2 may evade innate immune response, causing uncontrolled neutrophil extracellular traps formation and multi-organ failure. Clin Sci (Lond). 2020;134(12):1295–300.
Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol. 2020;51(6):613–28.
Lim MA, Pranata R, Huang I, Yonas E, Soeroto AY, Supriyadi R. Multiorgan failure with emphasis on acute kidney injury and severity of COVID-19: systematic review and meta-analysis. Can J Kidney Health Dis. 2020;7:2054358120938573.
Mueller AA, Tamura T, Crowley CP, DeGrado JR, Haider H, Jezmir JL, et al. Inflammatory biomarker trends predict respiratory decline in COVID-19 patients. Cell Rep Med. 2020;78:100144.
Anurag A, Jha PK, Kumar A. Differential white blood cell count in the COVID-19: a cross-sectional study of 148 patients. Diabetes Metab Syndr. 2020;14(6):2099–102.
Tavakolpour S, Rakhshandehroo T, Wei EX, Rashidian M. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol Lett. 2020;225:31–2.
Naoum FA, Ruiz ALZ, Martin FHO, Brito THG, Hassem V, Oliveira MGL. Diagnostic and prognostic utility of WBC counts and cell population data in patients with COVID-19. Int J Lab Hematol. 2021;7:84.
Zhu JS, Ge P, Jiang C, Zhang Y, Li X, Zhao Z, et al. Deep-learning artificial intelligence analysis of clinical variables predicts mortality in COVID-19 patients. J Am Coll Emerg Phys Open. 2020;1:1364–73.
Wagner J, DuPont A, Larson S, Cash B, Farooq A. Absolute lymphocyte count is a prognostic marker in Covid-19: a retrospective cohort review. Int J Lab Hematol. 2020;42(6):761–5.
Guo H, Sheng Y, Li W, Li F, Xie Z, Li J, et al. Coagulopathy as a prodrome of cytokine storm in COVID-19-infected patients. Front Med. 2020;7:572989.
Mesas AE, Cavero-Redondo I, Alvarez-Bueno C, Sarria Cabrera MA, Maffei de Andrade S, Sequi-Dominguez I, et al. Predictors of in-hospital COVID-19 mortality: a comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS ONE. 2020;15(11):e0241742.
Glassock RJ. Anticoagulant-related nephropathy: it’s the real Mccoy. Clin J Am Soc Nephrol. 2019;14(6):935–7.
Brodsky S, Eikelboom J, Hebert LA. Anticoagulant-related nephropathy. J Am Soc Nephrol. 2018;29(12):2787–93.
Wijaya I, Andhika R, Huang I. The use of therapeutic-dose anticoagulation and its effect on mortality in patients with Covid-19: a systematic review. Clin Appl Thromb Hemost. 2020;26:1–9.
Mahajan P, Dass B, Radhakrishnan N, McCullough PA. COVID-19-associated systemic thromboembolism: a case report and review of the literature. Cardiorenal Med. 2020;10(6):462–9.
Scrascia G, Guida P, Rotunno C, de Luca Tupputi Schinosa L, Paparella D. Anti-inflammatory strategies to reduce acute kidney injury in cardiac surgery patients: a meta-analysis of randomized controlled trials. Artif Organs. 2014;38(2):101–12.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
JL: analyzed and edited paper. WH: analyzed and edited paper. TD: designed, supervised, wrote paper, and approved final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective single-center study was approved by the Institutional Research Board of Stony brook University (IRB 2020–00207) with an exemption for informed consent and a HIPAA waiver.
Conflict of interest
Authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lu, J.Y., Hou, W. & Duong, T.Q. Longitudinal prediction of hospital-acquired acute kidney injury in COVID-19: a two-center study. Infection 50, 109–119 (2022). https://doi.org/10.1007/s15010-021-01646-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-021-01646-1