Abstract
Since COVID-19 was first reported, different neurological complications have been acknowledged, but their description is constantly evolving. We report a case of concurrent tonic pupil and trochlear nerve palsy in this context. A 62-year-old man reported a 5-day history of binocular vertical diplopia and blurred vision in his left eye, noticing that his left pupil was dilated. He had suffered a flu-like syndrome 2 weeks before. Clinical exam showed a right trochlear nerve palsy and a left mydriatic pupil. MRI, X chest ray, and analytical results were normal. Antibodies for SARS-CoV-2 were positive (low IgM and high IgG titers). Antiganglioside antibodies were negative. A 0.125% pilocarpine test confirmed Adie’s pupil diagnosis. The patient was treated with a tapered prednisone dose with resolution of his diplopia but no change in Adie’s pupil. This is the first case reporting Adie’s pupil as a postinfectious manifestation of COVID-19. An immune-mediated mechanism is presumed.
Similar content being viewed by others
Introduction
Since COVID-19 was recognized as a new disease in December 2019, different neurological symptoms have been reported such as headache, dizziness, consciousness impairment, and anosmia (Asadi-Pooya and Simani 2020). More recently, some cases of Guillain-Barre syndrome (GBS), mono or polyneuritis cranialis, and complete Miller-Fisher syndrome have broadened the neurological spectrum (Scheidl et al. 2020; Gutiérrez-Ortiz et al. 2020). Here, we report a case of a fourth cranial mononeuropathy coexisting with a contralateral tonic pupil developing 2 weeks after a SARS-CoV-2 infection. To our knowledge, this is the first case of tonic pupil reported in this particular context.
Case report
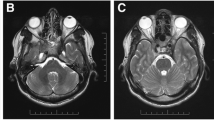
A 62-year-old man with an antecedent of hypertension attended our hospital reporting a 5-day history of binocular vertical diplopia and blurred vision in his left eye, noticing that his left pupil was dilated. No pain with ocular movements nor impairment in color vision was noticed. Three weeks prior, he had suffered a flu-like syndrome, including symptoms such as high fever, myalgia, intense coughing, and asthenia for 10 days. The general examination was normal. The neuro-ophthalmological exam revealed that ductions were apparently full and no evident ocular misalignment was found in primary gaze position, but an alternate cover test showed a subtle right hypertropia. Vertical diplopia was also noticed, which worsened in left gaze and was maximum at the down-and-left position, where right hypertropia was more evident in the alternate cover test. A double Maddox rod test revealed an excyclotorsion of the right eye, which was supported by a worsening of the diplopia with a head tilt towards the right shoulder and its improvement with a head tilt towards the left one. Overall, this was consistent with a right trochlear nerve palsy. Neither ptosis nor fatigability was noticed. In addition, he displayed a mydriatic left pupil of about 9 mm diameter, not reactive to light but slowly reactive to convergence. His right pupil was normal size showing a correct pupillary reflex. Old photos of the patient were reviewed, and we could confirm the presence of symmetrical pupils, thus ensuring that the tonic pupil was new. The remaining neurological exploration, including deep tendon reflexes and balance, was normal. A cranial CT and a cerebral MRI enhanced with gadolinium revealed no abnormalities, including no signs of orbital inflammation. Chest X-ray was negative. Blood tests were normal—including thyroid function, serologies for syphilis, HIV, and Lyme disease. An autoimmunity panel including antinuclear, antigliadin, antithyroid, and classic antineuronal antibodies was negative. Serological tests for SARS-CoV-2 were positive, including IgG antibodies at high titers and IgM antibodies at low titers. Antiganglioside antibodies in serum were negative (antiganglioside panel included IgG and IgM anti-GM1, anti-GM2, anti-GM3, anti-GM4, anti-GD1a, anti-GD1b, anti-GD2, anti-GD3, anti-GT1a, anti-GT1b, and anti-GQ1b). CSF analysis revealed no white cells with a slight elevation in protein count (48 mg/dl, limit 45 mg/dl) and normal glucose. Microbiological and pathological analyses of the CSF were normal. Oligoclonal bands analysis showed a “mirrored” type 4 pattern.
A diluted 0.125% pilocarpine test was performed. There was a mild constriction of the left pupil after a first drop, and a stronger effect with a second one after 15 min, becoming both pupils equal in size, thus confirming the diagnosis of tonic pupil (Fig. 1).
Patient with anisocoria pre- and post-instillation of pilocarpine 0.125%. The right pupil (a) shows a normal constriction to light. Conversely, the left pupil (b) is mydriatic and non-reactive. After the instillation of pilocarpine 0.125%, the left pupil shows a strong constriction (c), becoming both pupils equal in size (d)
The patient was treated with a tapering dose of oral prednisone for 4 weeks, resulting in progressive improvement until resolution of his diplopia. The tonic pupil remained unchanged.
Discussion
This case provides some further evidence of peripheral nervous system pathogenicity of SARS-CoV-2.
Adie’s pupil is a tonic, mydriatic pupil, due to an aberrant regeneration of parasympathetic nerve fibers after damage to the ciliary ganglion. As a result, fibers intended for the ciliary body will instead innervate the iris sphincter muscle (Moeller and Maxner 2007). This fact justifies the absence of photomotor reflex but a tonic response of the pupil to the near vision. Most of the cases are idiopathic, either isolated or associated to generalized areflexia (i.e., Holmes-Adie syndrome). Nevertheless, often times, they have also been related to autoimmune diseases, paraneoplastic entities, connective tissue diseases, and postinfectious conditions (i.e., syphilis, Lyme’s disease, or herpes virus family) (Moeller and Maxner 2007).
Regarding the cases of cranial neuropathies and GBS reported in COVID-19 (Scheidl et al. 2020; Gutiérrez-Ortiz et al. 2020), some have been described concurring with the systemic symptoms but others beginning days to weeks after their complete resolution. On this basis, both a direct invasion of the nerves by the virus and a delayed immune-mediated mechanism have been hypothesized.
In our case, several facts lead us to interpret this case as an immuno-mediated manifestation of COVID-19. Firstly, SARS-CoV-2 is largely known to generate a strong systemic inflammatory response which is thought to be responsible for part of the systemic damage. Additionally, a latency of several weeks is particularly characteristic in immune-mediated peripheral nervous damage, in order to develop cross immunity with different host epitopes. Furthermore, the presence of a mirrored pattern of oligoclonal bands supports the presence of a systemic immune reaction reaching intrathecal space by means of damage to the blood-CSF barrier (Matà et al. 2006). Lastly, the improvement with corticosteroids also points to an immune mechanism.
Indeed, we cannot exclude the possibility of a vasculopathic origin of the fourth nerve palsy (with or without the contribution of SARS-CoV-2 by microvascular inflammation and thrombosis) since our patient suffered from hypertension as a vascular risk factor, and the improvement period within several weeks is also typical in this condition. Nevertheless, the concurrence with a new-onset tonic pupil and the improvement just after steroid initiation make us feel this etiology more unlikely.
In conclusion, we report an exceptional case of trochlear mononeuropathy and tonic pupil occurring shortly after a SARS-CoV-2 infection, with a presumable immune-mediated mechanism. Further reports are needed to properly delineate the complete clinical picture of peripheral and central neurological implications of COVID-19.
References
Asadi-Pooya AA, Simani L (2020 Jun 15) Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 413:116832
Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S et al (2020) Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. https://doi.org/10.1212/WNL.0000000000009619
Matà S, Galli E, Amantini A, Pinto F, Sorbi S, Lolli F (2006) Anti-ganglioside antibodies and elevated CSF IgG levels in Guillain-Barré syndrome. Eur J Neurol 13(2):153–160
Moeller JJ, Maxner CE (2007) The dilated pupil: an update. Curr Neurol Neurosci Rep 7(5):417–422
Scheidl E, Canseco DD, Hadji-Naumov A, Bereznai B (2020) Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst 25:204–207
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ordás, C.M., Villacieros-Álvarez, J., Pastor-Vivas, AI. et al. Concurrent tonic pupil and trochlear nerve palsy in COVID-19. J. Neurovirol. 26, 970–972 (2020). https://doi.org/10.1007/s13365-020-00909-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13365-020-00909-1