Abstract
Sepsis and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, and its severe form coronavirus disease 2019 (COVID-19), represent the major medical challenges of the modern era. Therapeutic options are limited, mostly symptomatic, partially relying on antibodies and corticosteroids and, in the case of SARS-CoV-2 infection, supplemented by the antiviral drug remdesivir, and more recently by molnupiravir, nirmatrelvir/ritonavir, and the Janus kinase (JAK) inhibitors tofacitinib and baricitinib. Sepsis and severe SARS-CoV-2 infection/COVID-19 share many features at the level of pathophysiology and pro-inflammatory mediators, thus enabling a common disease management strategy. New ideas in successfully targeting the prognostic severity and mortality marker pentraxin 3 (PTX3) in sepsis and severe SARS-CoV-2 infection/COVID-19; the complement (C3/C3a/C3aR and C5/C5a/C5aR axis); tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 expression; IL-6-triggered expression of C5aR receptor in vascular endothelial cells; and release of anti-inflammatory IL-10 are still missing. Small molecules with lysosomotropic characteristics such as the approved drugs amitriptyline, desloratadine, fluvoxamine, azelastine, and ambroxol have demonstrated their clinical benefits in rodent models of sepsis or clinical trials in COVID-19; however, their exact mode of action remains to be fully elucidated. Addressing disease-relevant targets such as viral infection of host cells, shedding of toll-like receptors (TLRs), expression of pro-inflammatory mediators such as TNF-α, IL-1β, IL-6, PTX3, and the complement receptor C5aR, highlight the advantages of this multi-target approach in comparison to current standards. Rational drug repurposing of approved drugs or screening for active compounds with virtually exclusively lysosomotropic pharmacologic effects is a major opportunity to improve prophylaxis and treatment of sepsis and/or SARS-CoV-2 infection, and its severe form COVID-19.
Keywords
Sepsis, COVID-19, small molecules, lysosomotropism, drug repurposing, antibodies, lysosome, metabolitesIntroduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1] and its treatment has baffled researchers for nearly 3,000 years by now [2] and continues to be one of the major challenges facing medicine in the modern era. In late 2019, another global challenge emerged—the struggle against the coronavirus disease 2019 (COVID-19) pandemic and the disease-causing pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) posing serious challenges to health care systems worldwide. The severe progression COVID-19 of SARS-CoV-2 infection has stretched health systems across the world to their limit. COVID-19, initially a new poorly characterized and difficult-to-treat disease, has become a fairly well-characterized disease with a number of promising treatment options. In a variety of publications, the scientific community has rapidly gained knowledge about the clinical manifestation, pathophysiology, biomarkers, and diagnostic tests [3–16]. New vaccines and therapeutics have been established in clinical practice [16]; however, drug prophylaxis is still lacking.
The majority of COVID-19 patients hospitalized in the intensive care unit (ICU) meet Sepsis-3 criteria and exhibit infection-associated organ dysfunction [17]. Given the commonalities at the cellular and molecular level, there is a legitimate hope to improve treatment options and to derive new drugs. Small molecules play a minor part in the treatment options of sepsis available to date. Lipopolysaccharide (LPS) sequestrants, toll-like receptor 4 (TLR4) antagonists (e.g., TAK-242), C5a receptor antagonists (e.g., PMX53), inhibitors of macrophage migration inhibitory factor, inhibitors of histidine sensor kinase (QseC) signaling, A3 adenosine receptor agonists and A2A adenosine receptor antagonists, estrogen receptor β-agonists, and caspase inhibitors have been pre-clinically tested as effective [18] but have never received marketing authorization. Findings from cell culture experiments, animal studies, clinical trials, and observational studies of lysosomotropic drugs for other indications provide evidence for the key role of the (endo) lysosome as a target in treatment and prophylaxis of sepsis and COVID-19 that has received little attention to date [19–29]. Small molecules targeting the lysosome and signaling pathways involving the lysosome could therefore become valuable options to close the gap in treatment and prophylaxis of SARS-CoV-2 infection/COVID-19 [20, 30]. Given the common characteristics of sepsis and COVID-19 and the effects of lysosomotropic drugs on lysosome-dependent signaling pathways, the pivotal involvement of the lysosome in metabolism, endocytosis, and exocytosis renders it an interesting therapeutic target. We aim to highlight the benefits of lysosomotropic drugs and based on selection criteria, we suggest approved drugs that could be promising candidates in both diseases.
Pathways of SARS-CoV-2 entry into cells
SARS-CoV-2, a 30 kbp non-segmented positive sense single-stranded RNA (ssRNA) virus from the species Coronaviridae has been identified as the disease-causing pathogen of the COVID-19 causing an atypical interstitial pneumonia and diffuse alveolar damage, ending in a previously unknown acute respiratory distress syndrome (ARDS) and multi-organ failure in Wuhan, China [31, 32]. To enter cells of the airways and for proteolytic spike protein (S protein) mediated membrane fusion activation (transition), SARS-CoV-2 engages the transmembrane protease serine 2 (TMPRSS2) or lysosomal cathepsin L (CTSL) of target cells following a clathrin and an angiotensin converting enzyme-2 (ACE2) receptor-mediated endocytosis [32–37]. Like in SARS-CoV and other coronaviruses, S protein transition is enabled through two proteolytic cleavage steps following ACE2 engagement, where the first step is localized to the S1–S2 boundary and the second is localized to the S2′ site in the S2 subunit. For SARS-CoV, both sites are cleaved by proteases in the target cell. However, in the case of SARS-CoV-2, the S1–S2 boundary is cleaved by furin in the virus producer cell, whereas the S2′ site cleavage still requires target-cell proteases TMPRSS2 or CTSL [33].
ACE2 is a highly expressed membrane receptor and widely distributed in immunocompetent and non-immunocompetent cells [lung (airwave epithelial cells), heart, liver, testis, kidney, brain, intestine (pancreas and colon), and several other tissues]; and in addition, a soluble form of ACE2 circulating in blood vessels (circulating plasma ACE2) is existing [10, 38, 39]. The main entry route is supposed to be the upper airways, where both ACE2 and TMPRSS2 expressing cells (e.g., pneumocytes, nasal goblet secretory cells, and nasal ciliated cells) are present and ACE2 is more expressed than in the lower airways [33]. To date, the preferred entry route in case of concurrent expression of ACE2 and TMPRSS2 remains unknown and is the subject of controversy [35, 40, 41], also in view of the possible therapeutic intervention.
Sepsis and severe SARS-CoV-2 infection/COVID-19
Commonalities of sepsis and severe SARS-CoV-2 infection/COVID-19
COVID-19 and sepsis show various similarities in immunopathogenesis, pathophysiology, and clinical manifestations [10, 17, 42]. Similar to sepsis, a life-threatening organ dysfunction resulting from a dysregulated host response to infection (e.g., bacteria, viruses, and fungi) [1, 2], severe SARS-CoV-2 infection/COVID-19 meets the current diagnostic criteria of sepsis (Sepsis-3) [1] and pre-disposes the infected host to sepsis and septic shock [17, 42]. However, the pathogenesis of disease-dependent coagulopathy differs somewhat between the two entities [10, 17].
The initial studies published on COVID-19 observed a correlation of serum levels of pro-inflammatory cytokines [e.g., interleukin (IL)-6, IL-1b, IL-2, IL-8, IL-17, tumor necrosis factor (TNF)-α, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage-colony stimulating factor (GM-CSF)] with the severity of COVID-19 suggesting a relevance of cytokines in disease progress (Table 1) [6, 43]. Therefore, it was natural to compare the alteration in serum levels in COVID-19 vs. other cytokine releasing syndromes, to identify resemblances and to classify the emerging disease. The comparisons yielded inconsistent results. One meta-analysis revealed lower cytokine level in COVID-19 compared to other cytokine releasing syndromes [e.g., ARDS, sepsis, chimeric antigen receptor (CAR) T cell-induced cytokine release syndrome (CRS)] [4]. Further studies indicated no apparent difference in plasma cytokine profile in severe COVID-19, sepsis, and ARDS [44] or demonstrated lower cytokine levels in patients with SARS-CoV-2 sepsis compared to bacterial sepsis. Thereby immunoglobulins (Igs) IgA and IgG were higher [45] and complement activation (e.g., C3, C4, C5a, sC5b-9, C5aR1, factor D, and factor B) was increased [3, 45, 46]. Blood (lymphocyte and monocytes counts) and inflammatory biomarkers [C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH)] hardly differ between SARS-CoV-2 sepsis and bacterial sepsis patients, except for neutrophil count and procalcitonin (PCT) in bacterial sepsis patients and D-dimer in critical COVID-19 patients [4, 45].
Impact of small molecules on inflammatory messengers and growth factors in sepsis and severe SARS-CoV-2 infection/COVID-19
| Mediator | Sepsis | COVID-19 | Lysosomotropic small molecules | |
|---|---|---|---|---|
| TNFα | ↑ | ↑/w/o | Target | ↓ |
| IL-1β | ↑ | ↑/w/o | Target | ↓ |
| IL-1RA | ↑ | ↑ | n.d. | Unknown |
| IL-2 | ↑ | w/o | - | o |
| IL-6 | ↑ | ↑ | Target | ↓↓ |
| IL-7 | ↑ | ↑ | Target | ↓ |
| IL-8 | ↑ | ↓/↑ | n.d. | Unknown |
| IL-10 | ↑ | ↑ | Target° | ↑° |
| IL-15 | ↑ | ↑ | - | o |
| IL-17A | w/o | ↑/w/o | - | o |
| IFN-γ | ↓/↑ | ↑ | n.d. | Unknown |
| TGF-β | ↓/w/o | ↑ | - | o |
| MCP-1/CCL2 | ↑ | ↑ | Target | ↓** |
| CCL4 | ↑/w/o | w/o | Target | ↓↓ |
| CCL5 | ↑ | ↑/w/o | - | o |
| CCL22 | - | ↑ | - | o |
| PTX3 | ↑ | ↑ | Target | ↓↓ |
| CXCL2 | - | - | Target | ↓↓ |
| CXCL3 | - | - | Target | ↓↓ |
| CXCL10 | ↑ | ↑ | Target | ↓↓ |
| C3/C3aR | ↓ | ↑/w/o | Target C3aR | o/↓+ |
| C4 | ↓ | w/o | n.d. | Unknown |
| C5b-C9 | ↑ | ↑ | n.d. | Unknown |
| C5a/C5aR | ↑ | ↑ | Target C5aR | o/↓+ |
| GM-CSF | ↓/↑ | ↓/↑ | n.d. | Unknown |
| ICAM-1 | ↑ | ↑ | Target | ↓ |
| VCAM1 | ↑ | ↑ | Target | ↓# |
| PTGS2 | - | - | Target | ↓# |
Messenger/growth factor release depends on disease severity in sepsis [47–62] and severe SARS-CoV-2 infection/COVID-19 [3–9, 63–65]: increased (↑), decreased (↓), without (relevant) changes (w/o), (yet) unknown (-). Targets of lysosomotropic small molecules, inhibitors of endolysosomal acidification, and disruptors of lysosomal proton gradients are indicated as: target (Target), no target (-), or not determined (n.d.). Effects on gene expression [messenger RNA (mRNA)] in LPS stimulated human monomac 6 cells are indicated as: increased (↑), decreased (↓), no effect (o). Reference compound for small lysosomotropic molecules: NB 06 [22], plus literature data of fluvoxamine [66]#, amitriptyline [67]°, and ambroxol [68]**. + determined as C5 mRNA; IFN-γ: interferon-γ; TGF-β: transforming growth factor-β; MCP-1: monocyte chemoattractant protein-1; CCL2: C-C motif chemokine ligand 2; CXCL2: CXC motif chemokine ligand 2; ICAM-1: intercellular adhesion molecule-1; VCAM1: vascular cell adhesion molecule 1; PTGS2: prostaglandin-endoperoxide synthase 2
Long pentraxin 3 (PTX3) plasma concentrations, typically increased in infections of fungal, bacterial, and viral origin; severe inflammatory response syndrome; sepsis; and cardiovascular diseases, correlate with disease severity and mortality [9, 51, 69, 70]. In COVID-19 patients, PTX3 was likewise increased in plasma and emerged as a strong independent predictor of 28-d mortality [9] and in severe COVID-19 circulating PTX3 not differing from levels of patients with other pulmonary sepsis [71] indicating similarity.
To date, the precise immunopathogenesis of COVID-19 still remains unclear, as is whether the cytokine storm is the predominant driver of disease severity and organ dysfunction in COVID-19. Moreover, there is an ongoing discussion about the role of hyperinflammation following the cytokine storm and the role of immunosuppression by defected cellular host response [72, 73].
Sepsis, COVID-19, and the complement system
The serum-operating complement system is a complex defense mechanism against invading pathogens and an essential part of innate immune response that comprises of multiple signaling pathways that can be activated by various triggers [74–76]: firstly, the classic pathway after contact with IgG- and IgM-containing immune complexes, by PTXs, including CRP, serum amyloid P component (SAP), and PTX3; secondly, the alternative pathway, typically triggered by LPS-derived from gram-negative bacteria; thirdly, the lectin pathway that involves the interaction of mannose-binding lectin (MBL), a serum protein, with mannose residues on bacterial surfaces, and with ficolins; and finally, however, not commonly classified as a complement-activation pathway by proteases from neutrophils and macrophages that can cleave C5 [77, 78]. Anaphylatoxins C3a and C5a formed in the complement cascades bind to their corresponding receptors C3aR, C5aR1, and C5aR2, leading to downstream production of inflammatory mediators [74, 78]. In turn, C5a/C3a and C5aR/C3aR stimulate the activation of CD4+ and CD8+ T cells and bridge the innate and adaptive immune responses [74, 79] or C3d the B cell response [80, 81].
Complement activation and generation resulting in high levels of complement peptides C3a and C5a during sepsis are considered to be one of the hallmarks of sepsis [2], similar to those identified in severe and critical COVID-19 patients [11, 46]. In COVID-19 the classical pathway is activated in all patients, while hyperactivation of the lectin and alternative pathways is associated with disease severity [13] and patients requiring ICU admission C5a levels were significantly higher compared with those without [3].
This results in the traditional therapeutic approaches using antibodies against C3 (AMY101, Compstatin), C5 (Eculizumab, Ravulizumab), C5a (BDB-001, Vilobelimab), C5aR1 [Avdoralimab (IPH5401)]; antagonists C5aR1 [Avacopan (CCX168), PMX53]; and enzyme inhibitors of C3 cleavage (APL-2 and AMY-101) or C5 cleavage (Zilucoplan) [75, 78, 82–84].
Complement system, endolysosomal trafficking, endosomes, and lysosomes
The complement system is in common perceived as a mainly serum-operating defense mechanism of innate immunity. However, there is evidence suggesting immune cell-derived and intrinsically operating complement activation fragments are key in driving and modulating adaptive T cell immunity [76, 79]. In T cells upon receptor triggering CTSL/CTSL1, a papain-like lysosomal cysteine protease and even occurring extracellular [85, 86], cleaves C3 into active C3a and C3b fragments and mediates the C3 convertase-independent rapid local production of these fragments. Interestingly, resting T cells contain substantial intracellular endosomal and lysosomal pools of CTSL and C3 proposing that C3 is continuously cleaved to C3a in lysosomes. The resulting C3a is targeting the inward-facing C3aR receptor in the lumen of the lysosome and suggesting that sustaining basal mechanistic target of rapamycin (mTOR) activation is required for homeostatic T cell survival [76, 87].
The TLRs were the first to be innate immune receptors discovered and include (with ligands), among others, TLR3 [double-stranded RNA (dsRNA)], TLR7/TLR8 (ssRNA), and TLR9 (CpG DNA) that intracellular located to the inner endosomal membrane and the plasma membrane receptor TLR4 (LPS) [2, 78, 88, 89]. In the presence of the plasma membrane bound LPS co-receptor CD14, TLR4 is capable of dynamin and clathrin dependent endocytosis occurring within 15 min of LPS stimulation [90, 91]. With translocating to endosomes and phagosomes the TLR4/CD14/LPS receptor complex enters the endosomal entry route [78, 88, 90], with subsequent maturation of early endosomes (EE) via late endosomes (LE) to endolysosomes (EL), and finally lysosomes.
In S-protein-mediated endosomal entry SARS-CoV-2 particles engage cell surface ACE2 as receptor for clathrin mediated endocytosis in airway cells [33, 34, 37, 92, 93]. Similar to the TLR4/LPS receptor complex, the SARS-CoV-2/ACE2 complex enters endosomal entry route and after maturation and acidification to EL or mature lysosomes, CTSL with an optimum pH of 5.0–5.5 [94] performs the cleavage of S2′ site. S2′ site cleavage exposes the fusion peptide (FP) and dissociation of S1 from S2 induces dramatic conformational changes in the S2 subunit, inducing the membrane fusion of SARS-CoV-2 with host cells [33]. Yet, and with SARS-CoV, the cleavage at the S1/S2 cleavage site was considered to be the fusion triggering step [95]; however, according to the latest hypothesis with SARS-CoV-2 [33], this cleavage is supposed to occur during virus maturation in an infected cell.
The acidic environment for CTSL within the lysosome and the proton gradient across the lysosomal membrane are established by two proton pumps, the vacuolar ATPase (V-ATPase) and the lysosomal RedOx-chain [86, 96, 97], and activation of the CTSL-dependent lysosomal C3 cleavage and fusion of SARS-CoV-2 particles with host airway cells. That implies that in addition to the direct inhibition of CTSL by experimental cysteine protease inhibitor E64d [92, 98, 99] maturation and acidification of the endosome as well as the maintenance of the acidic pH are promising targets. However, to date only experimental inhibitors such as bafilomycin A1 are reported and available [100, 101]. A way out is given by active compounds that can decrease or abolish the lysosomal transmembrane proton gradient and raise the endolysosomal pH from 4.5–5 to 6–6.5, e.g., lysosomotropic approved drugs [102].
Current disease management of sepsis and COVID-19
Biologics and small molecules
Currently, immunomodulators, antivirals, and biologics (e.g., anti-SARS-CoV-2 antibodies) are the therapeutic pillars of COVID-19 treatment strategies (Table 2). Depending on the severity of the disease (pneumonia, ARDS) and associated organ dysfunction; however, they are substantially different from the therapy of typical sepsis patients [16, 103, 104]. In-depth knowledge about these drugs and immunomodulatory effectors in oncology and rheumatology suggested their use to COVID-19 [105, 106].
Recommendation of immune-modulating therapeutics according to NIH COVID-19 treatment guidelines as of November 2021 [16] and current status of clinical development according to ClinicalTrials.gov as of December 2021
| Target | Drug | Clinical relevance and level of development |
|---|---|---|
| NFκB inhibition | Recommendation: hospitalized patients require supplemental oxygen, high-flow device or non-invasive ventilation, invasive ventilation, ECMO | |
| IL-1Ra | Clinical trials (phase II/III)Insufficient evidence | |
| IL-1 | Recommendation against, except clinical trials | |
| JAK 1/2 kinaseJAK 1/3 kinase | Recommendation: hospitalized patients require high-flow device or non-invasive ventilation(Not in combination with anti-IL6R/anti-IL-6) | |
| GM-CSF | Clinical trials (phase II)Clinical trials (phase II/III)Insufficient evidence | |
| IL-6 | Recommendation: hospitalized patients require high-flow device or non-invasive ventilation, invasive ventilation, ECMO(Not in combination with JAK inhibition) | |
| IL-4, IL-13 | Clinical trials (phase II) | |
| IL-17 | Clinical trials (phase III) | |
| IL-2 | Clinical trials (phase III) | |
| ICAM-1 | - | - |
| IL-10 | - | - |
| IL-15 | Clinical trials (phase I) | |
| IL-7 | Clinical trials (phase II) | |
| TNF-α | Clinical trials (phase III)Clinical trials (phase II/III) | |
| Complement C3 | Clinical trials (phase I/II)Clinical trials (phase II) | |
| Complement C5 | Clinical trials (phase III)Clinical trials (phase II) | |
| Complement C5a | Clinical trials (phase III)Clinical trials (phase II/III) | |
| VCAM1 | - | - |
Mode of action is classified in: corticoids; antagonists; antibodies; small molecule; agonists. NFκB: nuclear factor kappa B; ECMO: extracorporeal membrane oxygenation; -: no targeting drug, no drug in development, no drug with clinical relevance
As of December 2021, more than 900 drug-related interventional clinical trials targeting treatment COVID-19 are registered at ClinicalTrials.gov. During the early pandemic period, the strength of the cytokine storm driven by COVID-19 infection was considered to figure prominently in predicting patient outcomes [6, 43]. Large-scale clinical trials with IL-6 (siltuximab) and IL-6R (sarilumab, tocilizumab) antibodies have yielded conflicting results and failed to demonstrate a significant benefit in COVID-19, except for IL-6R antibodies in severe COVID-19 (hospitalized patients who require supplemental oxygen, high-flow oxygen, non-invasive ventilation, or invasive mechanical ventilation) [16]. The non-response to blockade of IL-6/IL-6R argues against IL-6 as the assumed predominant driver of COVID-19 CRS/cytokine storm, disease severity, and organ dysfunction.
A number of studies focused on inhibiting the host humoral immune response yielded promising results, suggesting current immunomodulatory drugs (e.g., corticosteroids, biologics, and small molecules) as constituents of a successful therapeutic strategy for moderate and severe COVID-19 [16, 104]. In addition, biologics and small molecules provide effective tools to interrupt the relevant signaling pathways of cytokines, cytokine receptors, JAKs, and complement proteins [105, 107–109]. Some other small molecules and well-known drugs or their metabolites, most of them lysosomotropic, exhibit modulatory effects on sepsis and COVID-19 relevant signaling pathways and raise hope for the successful treatment of both diseases (Table 1, Figure 1) [20–22, 41, 110, 111].
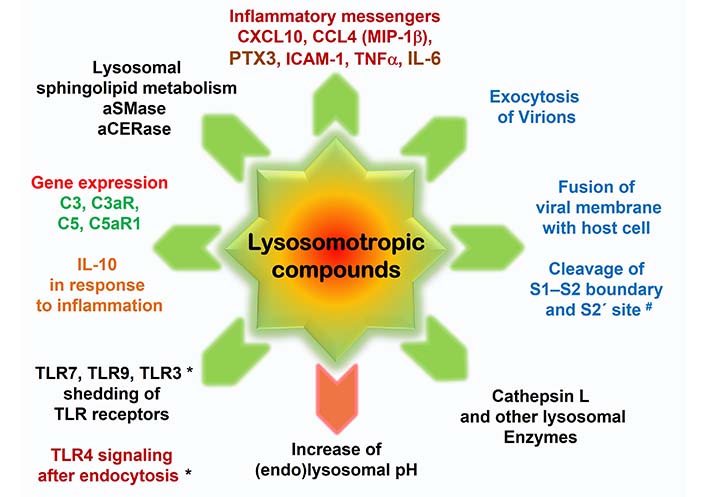
Targets of lysosomotropic drugs and metabolites in sepsis, SARS-CoV-2 infection, and severe SARS-CoV-2 infection/COVID-19. Lysosomotropic compounds aim various targets in host cells: lysosomal pH, enzymes, and metabolism (black); in response to LPS-induced inflammation/severe viral infection (dark red); in SARS-CoV-2 infection of host cells and viral replication (blue); and the expression of C3, C3aR, C5, and in particular of C5aR1, a highly probable promoter of sepsis and COVID-19 related vasculitides (green) [11, 112]. Effects are indicated as decreased (green arrow) and increased (red arrow). * TLR3 shedding and decreasing effects on TLR4 signaling after endocytosis are yet not confirmed, however, highly probable. MIP-1β: macrophage inflammatory protein-1β
In contrast to the humoral immune response, therapeutic option to intervene the hyperactivation of inflammatory response in sepsis failed in large multicenter clinical trials [113]. However, the use of hydrocortisone is the only immune-modulating drug recommended in septic shock, given the ongoing need requirement of vasoactive drugs [103].
The promising results in vitro as well as in TLR4-null mice targeting of the TLR4 signaling pathway and the production of pro-inflammatory cytokines failed to meet the high expectations in clinical trials. For example, resatorvid (TAK-242), a small molecule TLR4 antagonist, does not suppress cytokine levels in patients with sepsis and shock or respiratory failure/ARDS [114–117]. Other promising targets such as high mobility group box 1 (HMGB1) in sepsis still lack clinical trials [118].
Shortcoming of single targeting with biologics in signaling pathways
Antibodies are highly specific and highly selective tools for capturing peptide messengers, blocking the corresponding receptors or neutralizing the viral pathogen, so the natural choice was to apply antibodies for treating sepsis and SARS-CoV-2 infection/COVID-19. Contrary to expectations, however, the results of clinical trial demonstrate that the benefit is unincisive or very poor, except for the antibodies addressing the S protein receptor-binding domain (RBD) of SARS-CoV- (bamlanivimab plus etesevimab/casirivimab plus imdevimab) [16] or targeting an evolutionarily conserved epitope outside the rapidly evolving receptor binding motif to neutralize SARS-CoV-2, its variants (e.g., Omicron), and multiple other sarbecoviruses, including SARS-CoV-1 (sotrovimab) [119, 120].
In sepsis, inhibition of single downstream pro-inflammatory cytokines, such as TNF-α, IL-1b, and IL-6, have failed in clinical trials, not to be unexpected given the substantial amounts of mediators involved in the pathogenesis [78]. To improve outcome in SARS-CoV-2 infection/COVID-19, antibody cocktails consisting of anti-IL-6, IL-1 receptor blocker, IL-1 type 1 receptor, and TNF-α are suggested [121], irrespective of the risk of serious adverse effects (e.g., bacterial pneumonia) due to more pronounced interference with the immune defense.
Quest for multi target small molecules and drugs
Hydroxychloroquine is a well-known disease-modifying anti-rheumatic drug (DMARD) indirectly reducing the production of anti-inflammatory cytokines (e.g., IL-1, IL-6, TNF-α, and IFN-γ) by mononuclear cells and of TNF, IFN-α, IL-6, and CCL4 (MIP-1β) in plasmacytoid dendritic cells (pDCs), natural killer cell co-cultures stimulated with RNA-containing immune complexes, and other antigen-presenting cells [122]. Despite the successful in vitro inhibition of SARS-CoV-2 infection of cells in vitro [123, 124], hydroxychloroquine has failed to demonstrate benefits in clinical trials, leading to a dissuasion from the application [16]. Terminal elimination half-life of 41 ± 11 days, poor and/or delayed pulmonary accumulation (steady state on day 10) [125], severe adverse effects (e.g., dysrhythmias, prolonging the QTc interval in and beyond the therapeutic window, serious drug-drug interactions with various other drugs) [16, 126] rendered hydroxychloroquine unsuitable for widespread use in prophylaxis and therapy. However, the concept of multi-targeting pro-inflammatory cytokines, similar to the suggested antibody cocktail outlined above, can alternatively be implemented with small molecules that have DMARD characteristics and a suitable drug profile. In both sepsis and SARS-CoV-2 infection/COVID-19, it seems to be preferable to prevent the excessive emergence and release of pro-inflammatory cytokines than to trap them after excessive release. Small molecules have the advantage of being able to act within cells at the subcellular level.
Disease management with targeting endolysosomal acidification and signaling in sepsis and severe SARS-CoV-2 infection/COVID-19
Lysosomotropism of drugs is multi-targeting
Lysosomotropism is a characteristic of small molecules accumulating in lysosomes, usually by passive diffusion across the lysosomal membrane and trapped in the lysosome lumen [127]. Typically, lysosomotropic compounds are characterized by one or more easily protonatable aliphatic nitrogen atoms localized in side chains or saturated ring systems, possessing a ClogP > 2 (lipophilicity) and a basic pKa between 6.5 and 11 [25, 128]. Trapping and enrichment results in an increase in lysosomal pH from 4.5–5 to 6–6.5 [102] and an inactivation of lysosomal enzymes through a lysosomal pH beyond their optimum pH range (pH 4.5–5.5). Various well-known approved drugs such as ambroxol, amitriptyline, chlorpromazine, desipramine, desloratadine, fluvoxamine, hydroxychloroquine, and azelastine (Figure 2) or the small molecule model compound NB 06 have been classified as lysosomotropic compounds [22, 100, 128–134]. The pivotal involvement of the lysosome in metabolism, endocytosis, and exocytosis renders it an interesting therapeutic target. Raising the pH in (late) endosomes and lysosomes results in non-specific partial or complete inhibition of numerous lysosomal enzymes addressing SARS-CoV-2 infection, replication, and shedding; as well as TLR receptors and host response to infection/complement system (Table 1, Figure 1).
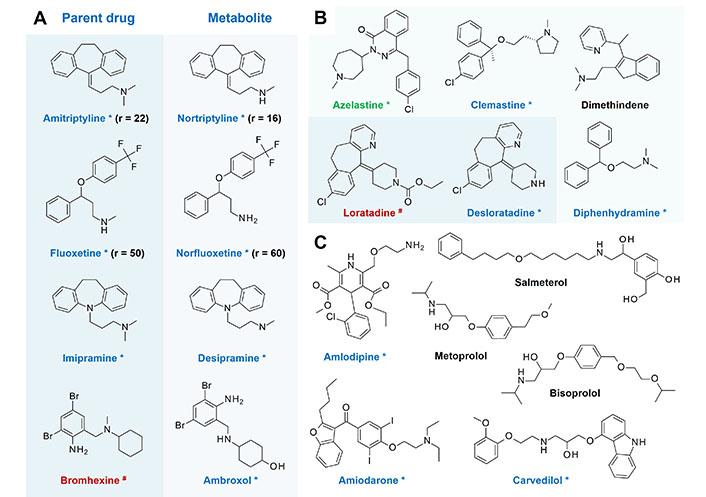
Lysosomotropic drugs, lysosomotropic metabolites as candidates for (systemic) prophylaxis of sepsis, viral infection, and transition to severe SARS-CoV-2 infection/COVID-19. (A) Pairs of confirmed lysosomotropic and non lysosomotropic drugs and their lysosomotropic N-desmethyl metabolites [22, 100, 128–133] and their ratio (r) of pulmonary tissue/plasma concentration, if available. Accumulation in pulmonary tissue is given at a ratio r > 1 [135, 136]; (B) H1-antihistamines and candidates for (systemic) prophylaxis of viral infection and transition to severe SARS-CoV-2 infection/COVID-19. Azelastine (*, green) is a nasal and ophthalmic H1-antihistamine over-the-counter (OTC) drug with confirmed lysosomotropism and anti-SARS-CoV(-2) efficacy [134]. Bromhexine, ambroxol, loratadine, desloratadine, azelastine, clemastine, dimethindene, and diphenhydramine are likewise OTC drugs and are easily accessible; (C) various commonly used cardiovascular drugs and the bronchodilator salmeterol. Salmeterol (ClogP 3.61) is administered by inhalation and decreases levels of LPS triggered pro-inflammatory cytokines TNF-α, IL-6, and IL-1β [137, 138] suggesting present lysosomotropism is indicated as confirmed (*, blue), highly probable (black), but yet not confirmed, and no lysosomotropism (#, red)
Inflammatory messengers
NB 06, in a setting addressing the effects on gene expression of lysosomotropic compounds in LPS-induced inflammation in monocytic cells, modulates the gene expression of various prominent inflammatory messengers including CXCL10, CXCL3, CXCL2, CCL20, CCL4 (MIP-1β), PTX3, ICAM-1, TNF-α, and IL-6 [22]. These findings are consistent with the reported inhibitory effects of lysosomotropic hydroxychloroquine on antigen processing and major histocompatibility complex (MHC) class II presentation; interference with TLR signaling (TLR9 and TLR7); and inhibition of TNF-α, IFN-α, IL-6, and CCL4 production [122, 131]. Furthermore, the results support the hypothesis that lysosomotropic drugs are useful in (systemic) infections involving bacterial endotoxins, such as LPS by targeting the TLR4 receptor pathway in sepsis and LPS induced lung injury [2, 23, 68].
Ceramide metabolism and related exocytosis
Apoptosis of mammalian cells is characterized by an increase in C16-ceramide [139] in response to cellular stress and independent of ceramide synthases (CerS) and the ceramide de novo synthesis at the endoplasmic reticulum (ER) [140]. In the presence of lysosomotropic compounds non-selective lysosomal ceramide degradation of acid ceramidase (aCERase) shifts to the reverse ceramide synthase activity of aCERase (revaCERase) and selective synthesis of pro apoptotic C16-ceramide and C18-ceramide without ATP consumption proceeding from palmitic acid or stearic acid and sphingosine [22, 141]. C18-ceramide triggered exocytosis [142] and forming of syncytia, e.g., in SARS-CoV(-2) infection, can be blocked by chlorpromazine [34, 92, 143, 144].
Endosomal entry route, shedding of TLR receptors, complement C3, and lysosomal enzymes
In SARS-CoV-2 infection, lysosomotropic drugs prevent maturation and acidification of endosomes laden with viral particles, thereby preventing the CTSL mediated S1–S2 boundary and S2′ site cleavage in target cells. Contagions with other coronaviruses (e.g., alpha-coronaviruses HCoV-NL63 and HCoV-229E, beta-coronaviruses HCoV-OC43 and HCoV-HKU1) causing mild upper respiratory tract infections [33] or other viral CTSL-dependent target cell infections could be managed as well.
At the level of TLR receptors, lysosomotropic drugs inhibit endolysosomal proteases, presumably cathepsins, which are required for the ectodomain shedding of receptors TLR7 (ssRNA) and TLR9 (CpG) to obtain the functional form of both receptors [145]. Exclusively the processed forms of TLR7 and TLR9 present in the phagosome are capable of recognizing their ligand and signaling downstream. Full-length TLR9 is capable of recognizing its ligand CpG, and, however, fails to recruit MyD88 in response to CpG stimulation [145]. Given that the V-ATPase inhibitor Bafilomycin A1 blocks endolysosomal acidification as well as signaling by TLR9, TLR7, and TLR3 [145], it is obvious that the functional form of dsRNA detecting TLR3 is likewise depending on ectodomain shedding in the EL. However, this regulatory mechanism is lacking in the cell surface receptor TLR4 [145]. After translocation to the EL the TLR4/CD14/LPS receptor complex is capable of downstream signaling via a toll/IL-1 receptor-domain-containing adapter-inducing IFN-β (TRIF)-related adaptor molecule (TRAM)–TRIF-dependent pathway [88, 91].
Contrary to expectations, lysosomes and in particular CTSL are part of the innate, not adaptable immune system which preserves host integrity and is essential in homeostasis [74, 76, 78]. This specificity for the hydrolysis of C3 to C3a but not of C5 to C5a/C5b or C4 [76] may indicate that lysosomal CTSL is part of the alternative pathway and responsible for the initial spontaneous hydrolysis of C3. Lysosomal CTSL-dependent conversion of C3 to C3a suggests that lysosomotropic drugs interfere with lysosomal generation of C3a and diminish C3a appearance at the cell surface after T cell stimulation. This may be of particular importance as severe COVID-19 is associated with hyperactivation of the alternative complement pathway [13].
Given the limited decrease of C3a at the cell surface of T cells after stimulation of about 20% for CD3-activation and 50% for CD3+/CD46 activation using a specific CTSL inhibitor [76], a complete inhibition of C3a generation by lysosomotropic compounds is very unlikely. Intracellular C3a generation, necessary for cell viability and homeostatic T cell survival [76], remains unaffected. In severe diseases with complement derailment such as sepsis and COVID-19 [2, 11], it may be sufficient to interfere with the lysosomal, stimulus related portion of C3a, probably linked to the endolysosomal receptors TLR3, TLR7, TLR8, and TLR9 and the translocated receptor TLR4.
Expression of complement receptor C5aR
C5a is considered to be one of the most active inflammatory peptides produced and prominently increased during sepsis, avian influenza (H5N1 and H1N1 viral infection), and severe and critical COVID-19 [2, 3, 11, 46]. In addition to C5a, the corresponding C5aR receptor is likewise overexpressed during sepsis and severe SARS-CoV-2 infection/COVID-19 [11, 46, 112]. This overexpression is most likely linked to IL-6, which is well-known to induce transcriptional upregulation of C5aR. In consequence, early massive appearance of IL-6 in the plasma is resulting in transcriptional upregulation and overexpression of C5aR in the vasculature and in various organs and finally in organ dysfunction in severe cases [77]. Consequently, the C5a-C5aR axis figures prominently in severe sepsis and COVID-19 associated organ failure [2, 46, 77, 112].
The triggering effect of IL-6 on C5aR expression suggests that the modulatory effect on IL-6 observed with lysosomotropic NB 06 will also be evident with C5aR. Indeed, like IL-6, NB 06 inhibits significantly the transcriptional upregulation of C5aR1 during LPS-induced inflammation in monocytic cells [C5aR1: 6 h; LPS (1 ng/mL): 2,215; LPS + NB 06 (10 μmol/L): 1,100; NB 06: –1,549]. Without LPS stimulation, underexpression of C5aR1 was present at the end. Similar to C5aR1, LPS-induced transcriptional upregulation of lysosomal C3aR was blocked and, without LPS, underexpression was observed [C3aR: 6h; LPS (1 ng/mL): 2,062; LPS + NB 06 (10 μmol/L): 1,039; NB 06: –1,520]. The expression of the precursors C3 and C5, however, remained unchanged the entire period [22]. Whether these results on C3aR, and in particular C5aR1, are significant in the disease progress of sepsis and severe SARS-CoV-2 infection/COVID-19 requires further evaluation.
Overexpression and release of anti-inflammatory cytokine IL-10
IL-10 or human cytokine synthesis inhibitory factor (CSIF) is a prominent anti-inflammatory cytokine expressed by macrophages and myeloid dendritic cells (DCs) in response to microbial products such as LPS. Induction of IL-10 expression often occurs together with pro-inflammatory cytokines to limit the immune response to pathogens including oxidative burst and thereby preventing damage to the host [146]. In particular, IL-10 compromises mostly the gene expression of pro-inflammatory mediators such as IL-1β, TNF-α, IL-6, GM-CSF, CCL5, and MIP-1α/CCL3 [147, 148] and disturbs class II MHC presentation by inhibition of the transport of the class II molecules to the cell membrane [148]. Some of these mediators are likewise controlled by small lysosomotropic molecules such as NB 06 and similar to NB 06, IL-10 checks inducible enzymes such as the inducible form of cyclooxygenase (COX), COX-2/PTGS2 [149].
Given the normalizing properties of IL-10 on host response to sepsis, various efforts have been made to manage the immune response and reduce mortality in sepsis by systemic application of an adenovirus expressing IL-10. In the murine sepsis model, adenovirus systemic expressing IL-10 has failed; however, it targeted local expression of IL-10 in the thymus and achieved the desired beneficial effects in the outcome of septic mice [147, 150]. Since both desipramine and amitriptyline have been tested in differing murine sepsis models and mortality of septic mice decreased, it was natural to ask whether IL-10 was determined [67, 151]. And indeed, in the cecal ligation and puncture (CLP) murine sepsis model, amitriptyline increased IL-10 levels in peritoneal lavage and pulmonary injury during sepsis [67]. These findings suggest that lysosomotropic drugs promote the expression and/or release of anti-inflammatory IL-10 and serve as an alternative to targeted local expression of IL-10.
Managing PTX3 levels in plasma
The long pentraxin PTX3 is an acute phase protein produced at the sites of infection and (systemic) inflammation by a variety of cell types including macrophages, monocytes, DCs, smooth muscle cells, vascular endothelial and cardiac muscle cells, and in particular respiratory epithelial cells [9, 70, 152, 153]. Like the short pentraxins CRP and SAP, PTX3 is linked to the humoral arm of innate immunity, in particular to recognition molecules and activators of the classical and the lectin pathway of the complement [78, 154, 155]. In a murine model, intratracheal instillation of LPS triggered rapidly increasing, dose-dependent expression of PTX3 in pulmonary tissue provoking acute lung injury, and finally ARDS [70]. Besides LPS, pro-inflammatory cytokines (IL-1β, TNF-α), TLR agonists, microbes, and microbial components, trigger PTX3 expression; however, IL-6 and IFN-γ failed to trigger PTX3 expression [70, 154]. Considerably increased plasma concentrations of PTX3 are present in infections of fungal, bacterial, and viral origin; sepsis and septic shock; cardiovascular diseases; and as more recently ascertained, likewise in severe SARS-CoV-2 infection/COVID-19, not differing from pulmonary sepsis [9, 51, 60, 62, 71]. PTX3 plasma concentrations correlate with disease severity and mortality in sepsis and severe SARS-CoV-2 infection/COVID-19 [9, 51, 60, 62] and when expressed by the heart and vasculature in response to primary inflammatory stimuli, PTX3 is thought to be involved in numerous inflammatory diseases such as atherosclerosis and vasculitis [153, 156]. In sepsis and severe SARS-CoV-2 infection/COVID-19, pulmonary vasculitis, endothelial dysfunction, inhibiting the vasorelaxation induced by acetylcholine, and determining morphological changes in endothelial cells by PTX3 appears to be of particular importance [9, 71, 154]. Furthermore, in severe sepsis and septic shock, early high PTX3 is assumed to predict subsequent new organ failures, while a smaller decrease in circulating PTX3 over time is associated with an increased risk of death [62].
The impact of lysosomotropism of hydroxychloroquine or NB 06 has already been linked to vascular diseases such as atherosclerosis [131, 157], however, never with regard to PTX3 as a marker of vascular diseases, acute lung injury, sepsis, or SARS-CoV-2 infection/COVID-19 [9, 152, 153, 156]. Rather coincidentally, a screening experiment on the effect of NB 06 on LPS-stimulated Mono-Mac-6 cells at the level of gene expression suggested that lysosomotropic compounds such as NB 06 markedly and time-dependently decrease the expression of PTX3 [22]. Given that PTX3 is a marker of sepsis and SARS-CoV-2 infection/COVID-19 severity, the efficacy of desipramine and amitriptyline in the murine sepsis model, as well as fluvoxamine in clinical trials may be attributable to this experimental finding [20, 26, 67, 110] and encourages further evaluation of lysosomotropic compounds in this context.
Endolysosomal acidification and transmembrane proton gradient as targets
Apparently, the underlying effect for the observed effects of lysosomotropic compounds in cells and SARS-CoV-2 infection is the increase of the lysosomal pH. The lysosomal pH or more precisely the lysosomal transmembrane gradient depends on various factors. First, the lysosomal transmembrane pH gradient is established by two proton pumps, the V-ATPase using cytosolic ATP as its energy source and the lysosomal RedOx-chain using cytosolic NADH [86, 96] to transfer protons from the cytosol into the lumen of the lysosome. Therefore, both proton pumps are dependent on energy-providing processes such as the oxidative branch of the pentose phosphate pathway or fatty acid oxidation to provide sufficient amounts of ATP or NADH [158, 159]. Second, from the presence of compounds that disrupt or collapse the transmembrane gradient. Lysosomotropism, i.e., the accumulation in the lysosome, is probably only one way to lower the lysosomal proton transmembrane gradient and increase the pH in the lysosome. Compounds such as metformin, which exhibit similar effects on pro-inflammatory messengers [e.g., IL-1β, TNF-α, IL-6, MCP-1 (CCL2), and CXCL10 in mouse lungs] as lysosomotropic compounds fall short of the molecular characteristics of lysosomotropism and do not accumulate in the lysosome [160, 161]. Currently, it is impossible to predict whether other drugs possessing the lysosomotropism-mimicking of metformin can be identified.
Impact of lysosomotropism in sepsis models and SARS-CoV-2 infection/COVID-19 observational studies and clinical trials
Some of the lysosomotropic drugs and metabolites in Figure 2, in particular desipramine, fluoxetine, amitriptyline, and ambroxol, have already been tested for their efficacy in LPS-induced septic shock or polymicrobial sepsis/peritoneal contamination and infection (PCI) induced sepsis in animal models. In the murine PCI model, desipramine significantly reduced sepsis-typical hallmarks such as sepsis-induced cardiomyopathy, cardiac dysfunction, endothelial stress response, hepatic biotransformation capacity, activation of hepatic stellate cell in the acute-phase, and development of hepatic sepsis sequelae (e.g., post sepsis liver fibrosis) [19, 26, 151, 162].
Likewise, amitriptyline tested in the CLP model decreased mortality, mitigates neutrophil/monocyte accumulation in pulmonary tissue, and pulmonary injury during sepsis associated with increasing IL-10 levels in peritoneal lavage and decreasing MCP-1/CCL2 [67]. Preventive treatment with desipramine and fluoxetine significantly reduced TNF-α production and mortality in the murine LPS-induced septic shock model [163]. Ambroxol, with recently confirmed lysosomotropism, decreases infiltration of inflammatory cells into lung tissue, IL-1β, TNF-α, and MCP-1/CCL2 expression after LPS-induced inflammation and lung injuries following inhalation [68, 132]. The inhaled bronchodilator salmeterol (ClogP 3.61) decreases levels of LPS triggered pro-inflammatory cytokines TNF-α, IL-6, and IL-1β [137, 138], however, still lacking experimental lysosomotropism confirmation. Although not considered a typical lysosomotropic drug due to its somewhat low lipophilicity (ClogP –0.81), in the LPS-induced septic shock model ciprofloxacin shows similar effects on mortality, TNF-α, IL-1β, and CXCL2 [164].
Chloroquine and hydroxychloroquine emerged as the first lysosomotropic drugs to be trialed in the setting of SARS-CoV-2 infections/COVID-19, unfortunately without the desired benefit [16]. Random observations in psychiatric patients in France with a lower prevalence (about 4%) of symptomatic and severe forms of SARS-CoV-2 infections/COVID-19 compared to healthcare professionals (about 14%) when taking chlorpromazine or decreased mortality when using hydroxyzine pioneered subsequent and wider observational study on lysosomotropic drugs [30, 165, 166]. There, for example, escitalopram, sertraline, amiodarone, and amitriptyline reduced the risk of intubation or death in severe forms of SARS-CoV-2 infections/COVID-19 [21]. At the same time, a placebo-controlled, randomized, adaptive platform clinical trial of the lysosomotropic selective serotonin reuptake inhibitor (SSRI) fluvoxamine was initiated with the goal of evaluating its benefit in patients at known risk for a severe progression of SARS-CoV-2 infection. Treatment with fluvoxamine (100 mg twice daily for 10 days) among high-risk outpatients with early diagnosed COVID-19 reduced the risk of emergency care and hospitalization [20, 130].
Another strategy of drug repurposing is the nasal application of azelastine to prevent viral infections and to reduce viral load of SARS-CoV-2 in the nasopharynx. Azelastine is lysosomotropic and holds a marketing authorization as a nasal spray for allergic rhinitis [167, 168]. Therefore, it is natural to test azelastine placebo-controlled for its antiviral and viral load-lowering characteristics. After the preliminary evaluation, 0.1% azelastine nasal spray treatment decreased viral load, in particular in patients with initial high viral load and turning negative much faster than in the placebo group [134]. In all these successfully applied small molecules with various indications, lysosomotropism is a common feature [22, 129, 130, 169] that was not necessarily a consideration in the selection process.
Rational drug repurposing: the quest for lysosomotropism and the perfect drug profile
To identify new, readily available drugs with existing marketing authorization, a rational repurposing strategy is needed to become independent of random observations. Various lysosomotropic drugs and metabolites (e.g., in Figure 2) demonstrated anti-SARS-CoV(-2) efficacy, and some of them in severe forms of SARS-CoV-2 infections/COVID-19 and murine models of sepsis [19, 20, 25, 98, 110, 163, 167, 170, 171]. Drugs such as desipramine, fluoxetine, fluvoxamine or chlorpromazine are, however, not suitable for the systemic prophylaxis in healthy individuals due to their intrinsic pharmacology as anti-depressants. Eligibility criteria for drugs could include (Figure 3): lysosomotropism of drugs or their metabolites is present within the therapeutic margin, no or acceptable undesirable systemic pharmacological effects at lysosomotropic drug concentrations, and favorable systemic drug profile (clinical pharmacology considerations). In the case of viral infection prophylaxis of the respiratory tract, attention must be paid to the accumulation of the drug or its active metabolites in the nasopharynx and the lung. However, local application is a fallback option for otherwise suitable drugs [41, 111]. Clinical pharmacology considerations for small molecule in treatments for severe forms of SARS-CoV-2 infections/COVID-19 and sepsis can be performed using the liberation, absorption, distribution, metabolism, and excretion (LADME) model [172]. Antihistamines such as azelastine, clemastine, dimethindene, diphenhydramine, and loratadine (active metabolite desloratadine) or the expectorants bromhexine/ambroxol could be such readily available drugs with broad therapeutic window and safe drug profile. The generic adverse effects of lysosomotropic prescription drugs (e.g., QTc prolongation, Torsade de Pointes, ventricular arrhythmia, bundle branch heart block, and cardiac deaths, in particular from overdosing) are very rare (loratadine/desloratadine) [173] or absent (ambroxol) [174].
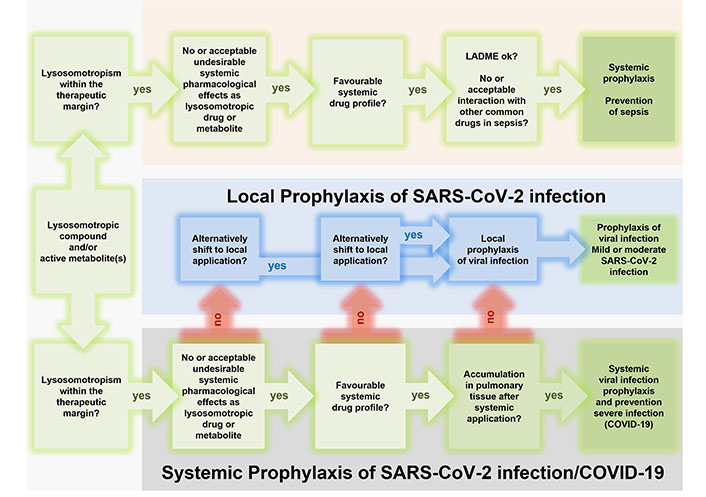
Rational repurposing of lysosomotropic drugs and metabolites for systemic prophylaxis of transition to sepsis and prophylaxis of (systemic) SARS-CoV-2 infection and prevention of transition to severe SARS-CoV-2 infection/COVID-19. Candidates (active compound and/or one or more of its metabolites) should meet the following criteria: weak protonatable nitrogen bases with lipophilicity (ClogP > 2) and a basic pKa between 6.5 and 11 [25, 128], lysosomotropism is present within the therapeutic margin, with or without acceptable undesirable systemic pharmacological effects as lysosomotropic drug or originating from its metabolites. Systemic prophylaxis of sepsis (top) and transition of viral infection to severe SARS-CoV-2 infection/COVID-19 (bottom) requires a favorable systemic drug profile; however, in the case of an unfavorable drug profile, in prophylaxis of viral infection local application can serve as a fallback (middle). Systemic prophylaxis of viral infection requires an accumulation in pulmonary tissue after systemic application of the drug
Multi-targeting of lysosomotropic drugs vs. antibodies and antibody cocktails
Antibodies are undoubtedly a highly specific tool to inhibit pro-inflammatory mediators such as IL-6, and, however, are lacking success in clinical sepsis trials and severe SARS-CoV-2 infection/COVID-19 [2, 16, 78]. IL-6 inhibitors (e.g., sarilumab, tocilizumab) are only recommended in hospitalized patients, and COVID-19 patients who require supplemental oxygen, high-flow oxygen, non-invasive ventilation, or invasive mechanical ventilation [16]. In an effort to inhibit more pro-inflammatory mediators simultaneously, antibody cocktails consisting of anti-IL-6, IL-1 receptor blocker, IL-1 type 1 receptor, and TNF-α are suggested [121] despite the risk of serious adverse effects due to massive interventions in the immune system (e.g., bacterial upper respiratory tract infection, pneumonia, and viral infections). Even the JAK-1 inhibitors tofacitinib and baricitinib have also failed to meet expectations and are only recommended for COVID-19 under defined conditions [16]. Although lysosomotropic compounds likewise interfere with prominent mediators of the immune defense, adverse effects of IL-6 and TNF-α antibodies are not reported. In contrast to antibodies, the resynthesis of IL-6 and TNF-α and thus the available amount of the mediators is reduced, but not completely inhibited, still allowing a moderate immune response. To reiterate the benefits of lysosomotropic drugs and metabolites, here are a handful of examples of their benefit in sepsis and SARS-CoV-2 infection/COVID-19.
Managing the onset of sepsis and severe SARS-CoV-2 infections/COVID-19
The key to the success of lysosomotropism of drugs and metabolites in the early clinical trials and observational studies in SARS-CoV-2 infections and COVID-19 [20, 21, 110] most likely relates to the various targets (Table 1, Figure 1) in the pathogenesis of COVID-19 and sepsis. Initially, lysosomotropic drugs target the onset of sepsis that is associated with the early appearance of IL-6 in the plasma, resulting in transcriptional upregulation of C5aR in various organs and present in sepsis and severe SARS-CoV-2 infection/COVID-19 [46, 77, 175]. Given that IL-6 plays a central role as mediator of toxicity in the CRS/cytokine storm, that is associated with severe cases of SARS-CoV-2 infection/COVID-19 [176, 177], controlling IL-6 is likewise a measure to avoid effectively an IL-6-driven CRS/cytokine storm. In influenza A virus [A(H1N1)pdm09]-induced pulmonary infections, lysosomotropic drugs are presumed to reduce both peaks of IL-6 plasma level on day 2 and day 5 after infection, respectively, thus preventing increased expression of C5aR [178]. At the onset of SARS-CoV-2 infections and COVID-19 lysosomotropic drugs are multi-targeting on core processes of the viral infection, addressing the formation of multinucleate syncytia and alteration of tissue structure, ceramide metabolism, and the release of virions.
The most significant advantage of lysosomotropic compounds is, however, that they address targets such as the pH of the lysosome, allowing a variety of signaling pathways and enzymatic reactions to be controlled (Figure 1, Table 1). Although lacking the specificity of antibodies, the broadness of the modulating effects with predictable side effects, in particular the intrinsic pharmacological effects, lysosomotropic compounds could become a valuable tool to better manage severe diseases such as sepsis or SARS-CoV-2 infection/COVID-19.
Managing the immune response and the complement C3a–C3aR/C5a–C5aR1 axis
The dysregulated or over activated complement system in trauma, sepsis and severe SARS-CoV-2 infection/COVID-19 is a challenging therapeutic target [2, 11, 13, 46, 75, 112, 175]. As a result of improved rodent survival by blocking C5 or C5aR1 [77, 179], development of various antibodies, antagonists, and convertase enzyme inhibitors began (Table 2); however, none of these have been granted marketing authorization to date [75, 78, 82–84]. Interestingly, we discovered that the lysosomotropic NB 06 downregulates the complement receptors C3aR and especially C5aR1 [22] considered to be of relevance for the disease progress, severity, and outcome. This finding suggests that C5aR1 overexpression in sepsis and in severe SARS-CoV-2 infection/COVID-19, the C5aR1-mediated vasculature and endarteritis in various organs, and the organ dysfunction in severe cases [77] can be controlled with lysosomotropic drugs. In addition to the expression of the C3a receptor C3aR, the conversion of C3 to C3a is likewise targeted in the lysosomal C3a/C3aR axis by lysosomotropic compounds. The increase of lysosomal pH triggered in presence of lysosomotropic compounds reduces the pH-dependent lysosomal CTSL-dependent conversion of C3 to C3a and C3b and decreases the free amount of C3b for downstream processes such as formation of C5 convertase [75, 76, 78]. Given that the hyperactivation of the lectin and in particular the alternative pathways are associated with disease severity in COVID-19 patients [13], it is likely that multi-targeting with lysosomotropic drugs is superior to the other concepts such as antibodies.
Shedding, acidification of EL, cross-talk, and TLR-receptors
Acidification of EL that can be impeded with lysosomotropic compounds, is required for endolysosomal protease-depended ectodomain shedding of TLR7 and TLR9 to obtain their functional form [145] and certainly for translocated TLR4/CD14/LPS receptor complex to be capable of downstream signaling via a TRAM–TRIF-dependent pathway [88, 91].
Strikingly, TLR4 is a co-stimulus of both lysosomal C3a/C3aR and C5a/C5aR1. With TLR-4 co-stimulation, C3a can induce in human monocytes and monocyte-derived macrophages signaling by producing pro-inflammatory mediators, such as IL-1β, TNF-α, IL-6, and prostaglandin E2 (PGE2) [180]. More pronounced, as demonstrated in the murine model, is the synergistic effect of C5a/C5aR with TLR4 in eliciting a stronger inflammatory response in endothelial cells. This endothelial-cell derived production of CC-chemokines, IL-6, and the IL-8 family CXC-chemokines for priming adherent neutrophils via their CXC-chemokine receptors as well as C5a-induced expression of C5aR in endothelial cells can be controlled with lysosomotropic drugs [74, 77, 181]. Thus, C5a cannot exert its effect as one of the most active inflammatory peptides produced during sepsis and severe SARS-CoV-2 infection/COVID-19 [2, 46, 77, 112].
Conclusions
So far, lysosomotropism of drugs has hardly been considered as a therapeutic option, since it cannot be defined by effects on receptors or enzymes; however, it is a pharmacological effect targeting a cell organelle, a characteristic of the compound and requires testing on cells to be confirmed [22, 128, 129]. Given the lysosome and its pH as target, any pH-dependent processes such as enzymatic activity, receptor shedding, and antigen presentation within the lysosome are affected resulting in multi-targeting of lysosomotropic drugs, often in contrast to the common philosophy of single targeting, such as antibodies.
Lysosomotropic compounds in general target the expression of pro-inflammatory mediators, in particular IL-6 and PTX3, the expression of the complement receptors C3aR and C5aR1, the release of the anti-inflammatory cytokine IL-10, shedding of the TLR receptors TLR7 and TLR9, and alteration of lysosomal sphingolipid metabolism, notably by preventing the formation of stress-induced pro-apoptotic C16 ceramide (Figure 1).
In both sepsis and SARS-CoV-2 infection/COVID-19, the decreasing expression of the C5aR1 receptor and in particular PTX3 is of major interest, considering PTX3 as a marker for disease progression. COVID-19 requires upstream infection with SARS-CoV-2. There, in host cell infection, lysosomotropic drugs can interfere with the CTSL-dependent cleavage of the viral S1–S2 boundary and S2′ site during host cell infection by raising the intra-lysosomal pH and thus preventing fusion viral with the host cell and cutting the S protein. It is obvious, that even if infection has already been established, modulation of PTX3 expression is rated to positively affect disease progression and to prevent transition to COVID-19.
Given that lysosomotropism is known to exist among many approved drugs and their metabolites already or has been investigated during repurposing for the treatment of severe SARS-CoV-2 infection/COVID-19, suitable drugs can be selected based on the proposed selection flow-chart (Figure 3). Ideally, the drugs can also treat the patient’s underlying diseases simultaneously and in the near future new drugs can be developed that exhibit no pharmacological effect beyond their lysosomotropic effect. Furthermore, the mechanism of action can also be applied to any viral disease where the triggering virus requires an intact lysosome to infect the host cell, or the progression to severe forms triggered by mediators that can be modulated by lysosomotropic compounds.
Unlike IL-6 antibodies or TNF-α-antibodies, lysosomotropic drugs are less likely to impair the immune response and do not have an associated increased risk of bacterial or viral infections. Adverse effects are mostly well documented, and cutaneous adverse effects in particular can usually be managed properly [111].
Despite the emerging success in SARS-CoV-2 infection/COVID-19 and in rodent sepsis models and the commonalities now discovered with sepsis, lysosomotropism of drugs and metabolites has hardly been considered as a useful drug characteristic and treatment option in the field of sepsis. By suggesting multi-targeting lysosomotropic drugs, we would like to bring new impetus to the treatment and prophylaxis of transition to sepsis and provide food for thought beyond the well-known treatments with corticosteroids and antibodies.
Abbreviations
| ACE2: | angiotensin converting enzyme-2 |
| ARDS: | acute respiratory distress syndrome |
| CCL2: | C-C motif chemokine ligand 2 |
| CLP: | cecal ligation and puncture |
| COVID-19: | coronavirus disease 2019 |
| CRP: | C-reactive protein |
| CRS: | cytokine release syndrome |
| CTSL: | cathepsin L |
| CXCL2: | CXC motif chemokine ligand 2 |
| EL: | endolysosomes |
| GM-CSF: | granulocyte macrophage-colony stimulating factor |
| ICAM-1: | intercellular adhesion molecule-1 |
| IFN-γ: | interferon-γ |
| Igs: | immunoglobulins |
| IL: | interleukin |
| JAK: | Janus kinase |
| LPS: | lipopolysaccharide |
| MCP-1: | monocyte chemoattractant protein-1 |
| MIP-1β: | macrophage inflammatory protein-1β |
| PTGS2: | prostaglandin-endoperoxide synthase 2 |
| PTX3: | pentraxin 3 |
| SARS-CoV-2: | severe acute respiratory syndrome coronavirus 2 |
| S protein: | spike protein |
| ssRNA: | single-stranded RNA |
| TLR4: | toll-like receptor 4 |
| TMPRSS2: | transmembrane protease serine 2 |
| TNF: | tumor necrosis factor |
| TRIF: | toll/interleukin 1 receptor-domain-containing adapter-inducing interferon-β |
| V-ATPase: | vacuolar ATPase |
| VCAM1: | vascular cell adhesion molecule 1 |
Declarations
Acknowledgments
We thank Edith Walther for her tremendous technical support in performing elaborate cell experiments and sample preparation in the initial stages of conceptual work. Tremendous support by Petra and Peter Bauer, Davina Miglietta, Lars Kaiser, the staff at Riesling Apotheke (Ellerstadt, Germany), Europa Apotheke, (Bensheim, Germany), and Apotheke im Markt (Heidelberg, Germany) is gratefully acknowledged.
Author contributions
MB conceived the work; MB, OS, RC, and HPD wrote the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Availability of data and materials
Not applicable.
Funding
Funding from the Institute of Precision Medicine and the Institute for Applied Research (IAF, Furtwangen University, Schwenningen, Germany) is gratefully acknowledged. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Copyright
© The Author(s) 2022.