Published online Jun 6, 2021. doi: 10.12998/wjcc.v9.i16.4090
Peer-review started: January 22, 2021
First decision: March 25, 2021
Revised: March 28, 2021
Accepted: April 6, 2021
Article in press: April 6, 2021
Published online: June 6, 2021
There are no effective antiviral therapies for coronavirus disease 2019 (COVID-19) at present. Although most patients with COVID-19 have a mild or moderate course of disease, up to 5%-10% of patients may have a serious and potentially life-threatening condition, indicating an urgent need for effective therapeutic drugs. The therapeutic effect of thymosin on COVID-19 has not been previously studied. In this paper, for the first time we report a case of thymosin treatment of COVID-19.
A 51-year-old man with imported COVID-19 was admitted with definite symptoms of chest tightness, chest pain, and fatigue. The polymerase chain reaction results for severe acute respiratory syndrome coronavirus 2 were negative. The antibody test was positive, confirming the diagnosis of COVID-19. As many orally administered drugs were not well tolerated due to gastrointestinal symptoms, an emergency use of thymosin, a polypeptide consisting of 28 amino acids, was administered by injection. Finally, after the implementation of the treatment program, symptoms and lung imaging improved significantly.
In this case report, it is confirmed that thymosin may help alleviate the severity of COVID-19 symptoms.
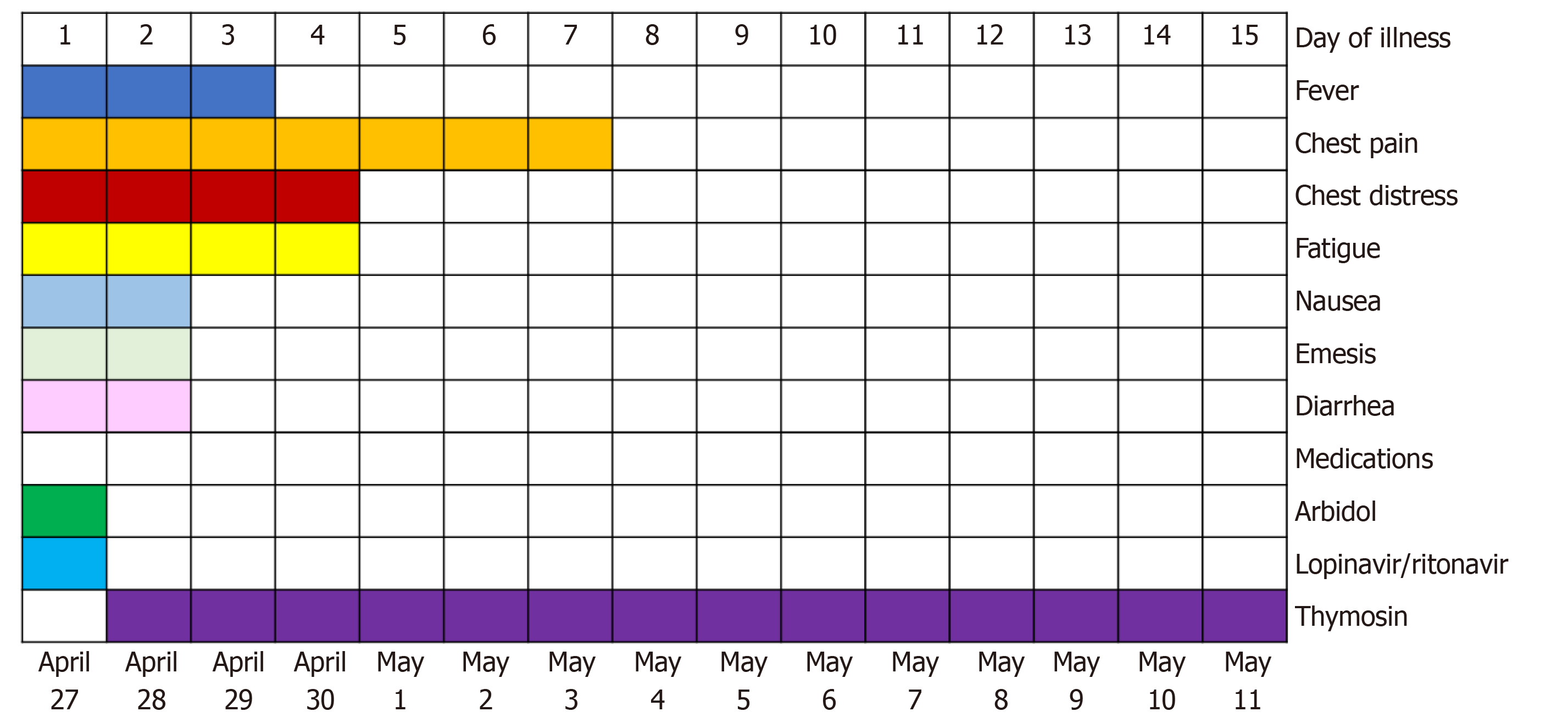
Core Tip: A 51-year-old Chinese man returned from Russia to Yueqing Hospital Affiliated to Wenzhou Medical University on April 27, 2020. He had 2-wk symptoms of chest pain, chest tightness, fatigue, and conscious fever. Oxygen inhalation was given and thymosin was injected twice a week, at 1.6 mg per injection. The patient was successfully treated and discharged after 15 d in the hospital.
- Citation: Zheng QN, Xu MY, Gan FM, Ye SS, Zhao H. Thymosin as a possible therapeutic drug for COVID-19: A case report. World J Clin Cases 2021; 9(16): 4090-4094
- URL: https://www.wjgnet.com/2307-8960/full/v9/i16/4090.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i16.4090
Since the outbreak in China in December 2019, coronavirus disease 2019 (COVID-19) has spread worldwide[1]. The numbers of infections and deaths have increased significantly[2]. Although there are many reports on the treatment of COVID-19, it is clear that currently no specific drugs are available to treat COVID-19 infection[3]. Thus, new treatment strategies for COVID-19 are urgently needed to be developed. Research confirms that COVID-19 is a human-to-human transmission caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[4]. Similar to other virally infectious diseases, the immune function plays a decisive role in the conse
A 51-year-old male patient had 2-wk symptoms of chest pain, chest tightness, fatigue, and conscious fever.
On April 13, 2020, the patient developed chest pain, chest tightness, fatigue, and conscious fever, and was not treated in Russia. On April 27, 2020, he was admitted to Yueqing Hospital Affiliated to Wenzhou Medical University for treatment.
The patient had no history of hypertension, diabetes, or other systemic diseases.
The patient had no history of smoking or underlying lung disease or any other risk factors. His family members were healthy.
Physical examination showed that a body temperature of 37.9 °C, blood pressure of 132/87 mmHg, pulse of 94 beats per minute, respiration of 20 breaths per minute, and SPO2 of 94%. Moist rales were heard in the right lung.
The results showed a white blood cell count of 4.6 × 109 cells/L (reference range: 4.0-10.0 × 109 cells/L). Reverse transcription polymerase chain reaction (RT-PCR) for SARS-CoV-2 was negative. The IgM antibodies for common pathogens such as influenza A, influenza B, adenovirus, respiratory syncytial virus, parainfluenza, Mycoplasma pneumoniae, and Chlamydia pneumoniae were all negative. SARS-CoV-2-specific IgM and IgG antibodies (the results of the kit produced by Xiamen Innodx Biotech Co., Ltd., China) were immediately detected and the results were all positive.
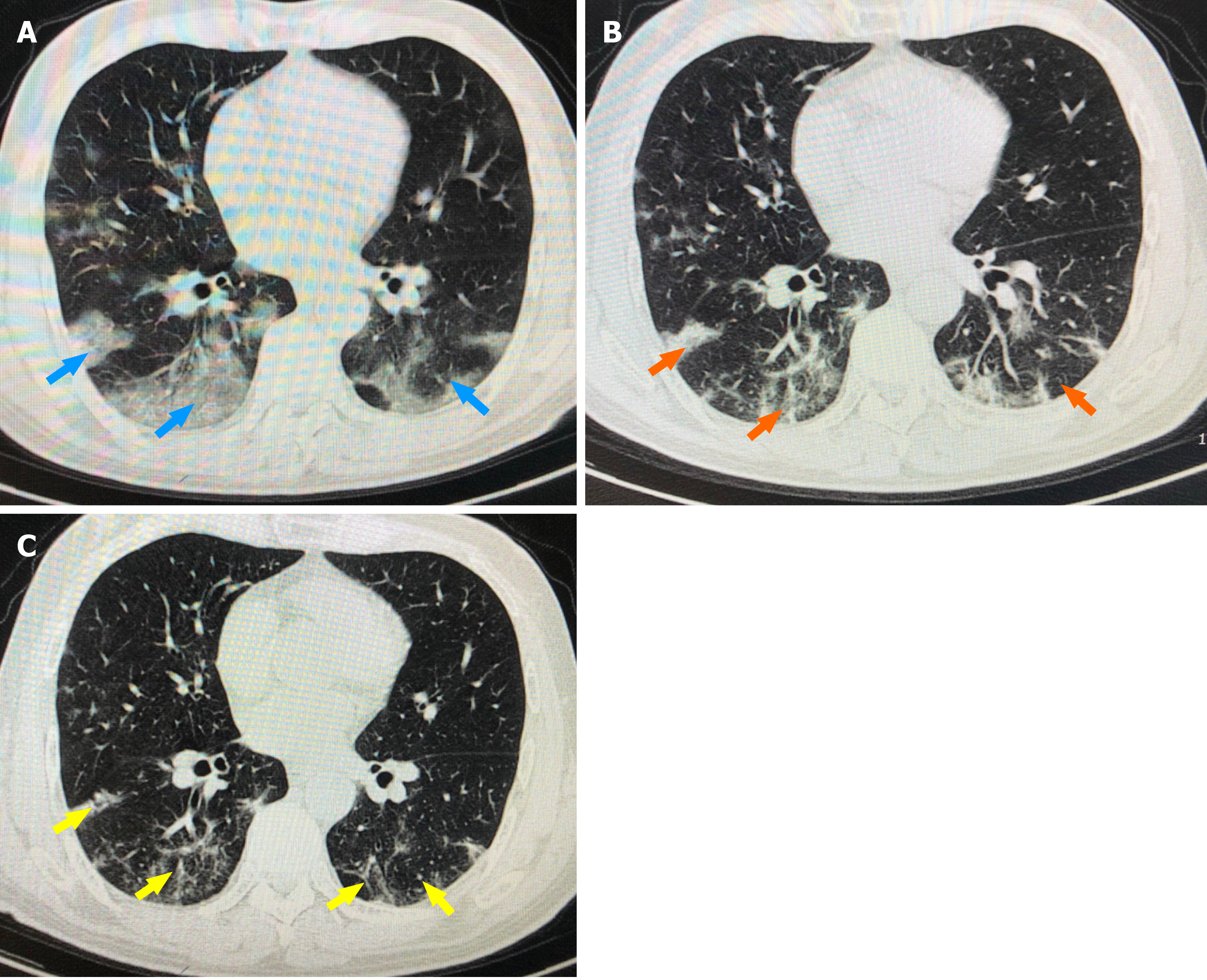
On April 28, chest computed tomography (CT) showed multiple patchy shadows and ground glass shadows in both lungs (Figure 1A). On May 1, chest CT showed obvious absorption of both lungs lesions (Figure 1B). On May 11, reexamination of chest CT showed that the lesions in both lungs were absorbed (Figure 1C).
The final diagnosis was COVID-19.
The patient was then sent to the air isolation ward for clinical observation and immediately given an oxygen therapy. The patient was also treated with arbidol and lopinavir-ritonavir, but due to obvious nausea, vomiting, and diarrhea, the drug treatments were halted. On April 28, thymosin was subcutaneously injected twice a week, at 1.6 mg per injection.
At the follow-up one month after discharge, the patient showed no discomfort, and the chest CT lesions were completely absorbed.
In this case, although the RT-PCR results were negative for SARS-CoV-2, the specific IgM and IgG antibodies for SARS-CoV-2 were all positive, indicating a clear diagnosis of COVID-19[7]. The patient was initially treated with arbidol and lopinavir-ritonavir, but because of severe nausea, vomiting, and diarrhea, the treatment was switched to thymosin injection, which was the only medicine. To date, the pathogenesis of COVID-19 is not clearly understood and there are no effective drug treatments available for the disease. Although various therapeutic drugs have been reported, their biological effects are non-specific. Thymosin, as an immune enhancer, has a therapeutic potential for the treatment of infections and immunocompromised diseases such as cancer[6,8,9]. Thymosin is a hormone with 28 amino acids naturally secreted by the thymus. It has a variety of immunomodulatory activities. It can activate Toll-like receptors in dendritic cells and other immune cells, thus enhancing the function of T-helper 1 cells, natural killer cell activity, and antibody response to T-cell-dependent antigen[10,11]. Thymosin has been used in China for the treatment of viral hepatitis for more than two decades[12]. The reason may be that thymosin can promote T lymphocyte responses and function involved in the host antiviral defenses. We report here an effective case of thymosin injection for the treatment of COVID-19 when other available antiviral drugs could not be tolerated. However, further studies are still needed to explain the exact efficacy and mechanism of thymosin on COVID-19. We summarize the patient's course of therapy schedule (Figure 2). The symptoms of chest tightness, chest pain, and fatigue gradually improved under the action of thymosin alone. Thymosin therapy may also increase the survival rate of sepsis and reduce the bacterial load in a sepsis model as reported by King and Tuthill[13]. Although there are no large-scale studies, considering the encouraging result and the proven safety for human use, we believe that thymosin may be a feasible therapeutic drug against COVID-19 and warrants further investigation. Clinical trials can verify the role of thymosin in alleviating the symptoms of COVID-19 and shortening the course of treatment in a large cohort.
Relieving symptoms, safeness, and effectiveness make thymosin a potentially ideal choice for the treatment of COVID-19. Although we had only one case, as described here, the significant effect in alleviating symptoms of COVID-19 demonstrates the potential therapeutic effect of this naturally derived compound.
Manuscript source: Unsolicited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aktas S, Shayestehpour M S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus Disease 2019 (COVID-19): A Perspective from China. Radiology. 2020;296:E15-E25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1106] [Cited by in F6Publishing: 918] [Article Influence: 229.5] [Reference Citation Analysis (1)] |
| 2. | Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7635] [Cited by in F6Publishing: 5811] [Article Influence: 1452.8] [Reference Citation Analysis (0)] |
| 3. | Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;323:1824-1836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 978] [Cited by in F6Publishing: 1242] [Article Influence: 310.5] [Reference Citation Analysis (0)] |
| 4. | Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81:104260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 405] [Cited by in F6Publishing: 391] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 5. | Márquez EJ, Trowbridge J, Kuchel GA, Banchereau J, Ucar D. The lethal sex gap: COVID-19. Immun Ageing. 2020;17:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Ciancio A, Rizzetto M. Thymalfasin in the treatment of hepatitis B and C. Ann N Y Acad Sci. 2010;1194:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | National Health Commission of the People’s Republic of China. Diagnosis and treatment protocol for COVID-19 (Trial Version 7). [cited 20 March 2020]. Available from: http://en.nhc.gov.cn/2020-03/29/c_78469.htm. [Cited in This Article: ] |
| 8. | Xue XC, Yan Z, Li WN, Li M, Qin X, Zhang C, Hao Q, Wang ZL, Zhao N, Zhang W, Zhang YQ. Construction, expression, and characterization of thymosin alpha 1 tandem repeats in Escherichia coli. Biomed Res Int. 2013;2013:720285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Morton B, Pennington SH, Gordon SB. Immunomodulatory adjuvant therapy in severe community-acquired pneumonia. Expert Rev Respir Med. 2014;8:587-596. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Romani L, Bistoni F, Gaziano R, Bozza S, Montagnoli C, Perruccio K, Pitzurra L, Bellocchio S, Velardi A, Rasi G, Di Francesco P, Garaci E. Thymosin alpha 1 activates dendritic cells for antifungal Th1 resistance through toll-like receptor signaling. Blood. 2004;103:4232-4239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 157] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Romani L, Bistoni F, Perruccio K, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Bistoni G, Rasi G, Velardi A, Fallarino F, Garaci E, Puccetti P. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265-2274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Gramenzi A, Cursaro C, Andreone P, Bernardi M. Thymalfasin: clinical pharmacology and antiviral applications. BioDrugs. 1998;9:477-486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | King RS, Tuthill C. Evaluation of thymosin α 1 in nonclinical models of the immune-suppressing indications melanoma and sepsis. Expert Opin Biol Ther. 2015;15 Suppl 1:S41-S49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |