Abstract
With the outbreak of coronavirus disease (COVID-19) caused by novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the world has been facing an unprecedented challenge. Considering the lack of appropriate therapy for COVID-19, it is crucial to develop effective treatments instead of supportive approaches. Mesenchymal stem cells (MSCs) as multipotent stromal cells have been shown to possess treating potency through inhibiting or modulating the pathological events in COVID-19. MSCs and their exosomes participate in immunomodulation by controlling cell-mediated immunity and cytokine release. Furthermore, they repair the renin-angiotensin-aldosterone system (RAAS) malfunction, increase alveolar fluid clearance, and reduce the chance of hypercoagulation. Besides the lung, which is the primary target of SARS-CoV-2, the heart, kidney, nervous system, and gastrointestinal tract are also affected by COVID-19. Thus, the efficacy of targeting these organs via different delivery routes of MSCs and their exosomes should be evaluated to ensure safe and effective MSCs administration in COVID-19. This review focuses on the proposed therapeutic mechanisms and delivery routes of MSCs and their exosomes to the damaged organs. It also discusses the possible application of primed and genetically modified MSCs as a promising drug delivery system in COVID-19. Moreover, the recent advances in the clinical trials of MSCs and MSCs-derived exosomes as one of the promising therapeutic approaches in COVID-19 have been reviewed.
Graphical abstract

Similar content being viewed by others
Introduction
The novel coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged for the first time in Wuhan, China, at the end of December 2019 [1]. COVID-19 is rapidly spreading worldwide, with more than 53.5 million confirmed cases and 1,350,000 deaths up to the middle of November 2020 [2]. After isolation and identification of SARS-CoV-2, a growing body of efforts has been made to understand its transmission and epidemiological features and improve diagnostics tests and emergency therapeutic strategies. The majority of SARS-CoV-2 infected cases are symptom-free (80%) or display moderate flu-like symptoms, including fever, sore throat, cough, myalgia, shortness of breath, and fatigue. However, new manifestations such as gastrointestinal and CNS symptoms, anosmia, and ageusia were reported [3, 4]. Approximately 15% of infected cases display severe pneumonia, and 5% develop acute respiratory distress syndrome (ARDS), the most severe complication of COVID-19, which is characterized by diffuse alveolar-capillary damage [5, 6]. Although the certain underlying reason for the life-threatening condition in COVID-19 is still unknown, severe inflammation related to a high level of pro-inflammatory cytokines is hypothesized to be the primary cause of disease severity and death [7]. Considering the immune system's pivotal role in COVID-19 pathogenesis, targeting the immune system helps to suggest curative therapies to surmount the disease. Despite the impressive progress in the diagnosis and management of COVID-19 patients, the treatment mainly consists of supportive care, and no curative vaccine or drug has been approved. These supportive treatments for ARDS mainly consist of continuous renal replacement therapy (CRRT), invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) [8, 9]. Although studies have suggested a variety of treatments such as antimalarial drugs (chloroquine, hydroxychloroquine) [10], antiviral drugs (antiretrovirals, remdesivir) [11, 12], renin-angiotensin-aldosterone system-related drugs (ACE inhibitors) [13], and immunomodulatory agents (sarilumab, tocilizumab) [14,15,16], the efficacy of these treatments are under question. Considering the fast global spread of the virus, developing effective therapies is necessary. Within this context, we discuss the mechanisms involved in therapeutic features of MSCs and their exosomes, possible drug delivery roles of MSCs, and recent clinical trials in COVID-19.
MSCs as a potential therapy for severe cases of COVID-19
Cell-based therapies (CBT), particularly using stem cells, are promising therapeutic approaches for treating many incurable diseases. Although CBT has many advantages as a therapeutic approach, some important limitations include inadequate cell source, high immunogenicity, and the ethical issue remained unsolved. Mesenchymal stem cells (MSCs) are a group of non-hematopoietic stem cells that originate from several adult tissues such as bone marrow, adipose tissue, dental pulp, amniotic membrane, placenta, and amniotic fluid. Based on the International Society for Cellular Therapy (ISCT), MSCs of different tissues express common surface markers such as CD73, CD90, and CD105, while do not express CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR surface molecules, which are defined as a part of the recognition criteria of MSCs. These cells are plastic-adherent in standard culture conditions and have capability to differentiate into osteoblasts, adipocytes, and chondroblasts [17]. Some essential features of MSCs, including regeneration ability, anti-inflammatory effects, immune evasive characteristics, and interaction with various intra-/extra-cellular pathways, bring them up as a novel treatment for different pathologic conditions [18]. Also, MSCs proliferate simply under the appropriate condition that results in expansion to usable clinical volume. These cells are preserved in a standard condition to be ready to use source for clinical administration. Also, various clinical trials of MSCs administration did not show side effects in allogeneic transplantation. These features make MSC-based therapy an excellent stem cell source compared with other types of stem cells [19].
The therapeutic effects of MSCs are related to the simultaneous influence of direct cell-cell contact and paracrine effects. MSCs release secretome, consisting of lipids, proteins, free nucleic acids, and extracellular vehicles (EVs). Based on release pathways, biogenesis, size, content, and function, EVs are categorized into three main sub-types: microvesicles (MVs), apoptotic bodies, and exosomes [20]. Application of the secretome instead of cellular counterparts provides important advantages, including determining dosage and potency, avoiding invasive cell biopsy, providing storable and easy-accessible sources, more stability, low immunogenicity, and a higher chance of crossing the blood-brain barrier (BBB) [21]. Considering the emerging and rapid spreading of new SARS-CoV-2, the pathology of COVID-19 as a treating target and application of stem cells as a novel treatment strategy are of interest. Herein, we discuss the therapeutic role of MSCs and their exosomes in negative consequences of COVID-19, including cytokine storm, RAAS dysfunction, alveolar fluid accumulation, and hypercoagulation.
Immune system-virus interaction and immunomodulation of MSCs
SARS-CoV-2 belongs to the virus family that enters into host cells through surface angiotensin-converting enzyme 2 (ACE2) expressed in type II surfactant-secreting alveolar cells, oral mucosa, GI tract, kidney, and heart [22,23,24]. After invasion of the virus, the innate and adaptive immune responses are activated. A variety of immune cells including T-helper (CD3+ CD4+) and T-cytotoxic (CD3+ CD8+) lymphocytes, B cells (CD19+), NK cells (CD16+CD56+), monocytes, and macrophages involve in immunologic response against SARS-CoV-2 [25, 26]. These cells produce and secrete various cytokines, chemokines, and other pro-inflammatory factors to induce an immune response. It is expected that a well-coordinated inflammatory response limits the distribution of viruses and eliminates the infection, while an exaggerated and uncontrolled immune response causes tissue damage, both locally and systemically [27]. It has been shown that high amounts of neutrophils and elevated neutrophil-to-lymphocyte ratio in the lung tissue usually show more severity and unfortunate clinical outcome [28]. Evaluation of patients with severe COVID-19 revealed that significant leukopenia consists of a reduction in T-helper, T-cytotoxic, B cells, Natural killer cells, monocytes, eosinophils, and basophils [6, 29].
MSCs have shown promising immunomodulatory features in severe inflammatory conditions. MSCs suppress maturation, activation, and antigen presentation of dendritic cells (DC) and immune cell infiltration to the lung tissue. These cells can modulate T lymphocytes' proliferation, cytokine secretion, Th1/Th2 balance, regulatory T cell production, B cell proliferation, and antibody production. They also inhibit natural killer cell activation while increasing T reg cells [30]. MSC derived extracellular vesicles play an important role in T reg production and also increase M2 macrophage phenotype [31, 32].
Along with the cellular dysregulated immune response, massive release of pro-inflammatory factors, cytokines, and chemokine, called "cytokine storm" or "macrophage activation syndrome (MAS)," promotes tissue damage processes such as acute lung injury (ALI). Most patients with severe symptoms display higher serum levels of pro-inflammatory factors such as IL-6, IL-1β, IL-2, IL-8, IL-17, TNF-α, G-CSF, GM-CSF, IP10, MCP1, and MIP1α [7, 33]. This imbalanced immune system reaction causes simultaneous hyperinflammatory/immunodeficiency states that result in intense inflammation against host cells along with the ineffective function of T-cytotoxic and natural killer to induce apoptosis in virally infected cells. It seems that TNF-α, IL-6, and IL-1 play pivotal roles in COVID-19 pathogenesis. TNF-α is mainly secreted by monocyte lineage and possesses a complex mechanism of action against viral infection. Although this protein induces the antiviral effect, paradoxically, TNF-α can cause pathological complications related to the activation of different downstream signaling pathways. TNF receptors (TNFR1 and TNFR2), as two essential receptors of TNF-α, are responsible for inducing this mediator's effects, including cell growth, NF-κB activation, and cytokine genes upregulation. It has been shown that TNF- α did not play a pivotal role in the clearance of viral infections such as influenza and poxvirus, while TNF-α depletion dysregulates antiviral inflammation. Highly expressed TNF-α results in lung tissue fibrotic remodeling by inducing uncontrolled recruitment of immune cells and decreased level of antioxidant molecules in parenchymal and endothelial cells of lung tissue [34, 35]. TNF- α activates the host cells' extrinsic apoptotic pathway by activating death receptors TNFR1, Fas, and TNF-related apoptosis-inducing ligand (TRAIL) [36, 37]. Activation of these receptors can trigger apoptosis by different mechanisms such as activation of the caspase-8 pathway, producing reactive oxygen species (ROS), and activation of mitochondrial enzymes [38, 39]. It seems that triggering different apoptosis pathways by imbalanced and over-secreted TNF-α may promote severe tissue damage during the cytokine storm of COVID-19. IL-6, as another player in ARDS of COVID-19, is a soluble mediator that participates in different processes, including inflammation, immune response, liver function, and hematopoiesis. IL-6 links innate to the adaptive immune response by stimulating B cells to produce antibodies and differentiating naïve T cells. This cytokine is a potent production inducer of acute-phase proteins such as C-reactive protein (CRP), an important indicator of lung lesions and the disease severity. It has been shown that the regulated release of IL-6 is necessary for lung repair in the early inflammatory stage and controlling infection following viral damages [40,41,42]. On the other hand, IL-6 overproduction causes different harmful properties in the lungs. IL-6, in combination with transforming growth factor β (TGF-β), stimulates the differentiation of naïve cells to T-helper 17, which plays a pivotal role in neutrophil evoking, while IL-6 prevents TGF-β-induced Treg differentiation [43]. It seems that the reduction in Treg, which was observed in severe COVID-19 patients, leads to imbalanced T cell ratios and uncontrolled immune response [27]. IL-6 function is dependent on how its signal is transmitted to target cells. Two different pathways translate the effect of IL-6: 1) classic signaling, and 2) trans-signaling. In classic signaling, target cells of IL-6 express an 80 kDa IL-6 receptor (IL-6R); however, there are limited cells, including hepatocytes and some leukocytes, that can be affected via IL-6R. Classic signaling mediates the activation of anti-inflammatory effects of IL-6 on target cells. On the other hand, if the soluble IL-6 receptor/IL-6 complex associates with gp130a, expressed on all cells, they immediately initiate an inflammatory cascade, so-called “trans-signaling” [44, 45]. It is indicated that Type II surfactant-secreting alveolar cells, which are host cells for SARS-CoV-2, can be stimulated by the IL-6 receptor/IL-6 complex, which induces an inflammatory response in alveoli that increase the risk of pulmonary fibrosis [46]. It seems that IL-6 increases the chance of severe complications of COVID-19, through immune response dysregulation and causing pulmonary fibrosis. IL-1 is a family of cytokines consisting of 11 members believed to have pro-inflammatory and antagonistic functions. Production of IL-1 is triggered by virus-mediated activation of some pathways such as double-stranded-RNA-dependent protein kinase (PKR), NF-κB, and extracellular signal-regulated kinase (ERK). IL-1β, an important member of the IL-1 family, is an inflammatory cytokine that induces pulmonary fluid accumulation in ARDS. This mediator induces rapid accumulation of neutrophils in the virus-infected lung; however, it has been shown that neutrophils do not have important effects on viral clearance [47]. The presence of IL-1β, which is elevated in severe COVID-19 patients, can lead to considerable fibrotic response in the lung [48]. There are some concerns about the incidence of pulmonary fibrotic changes in patients who have recovered from COVID-19 [49]. It seems that regulating IL-1 secretion, along with other mechanisms, may prevent the risk of pulmonary fibrosis as long-term pulmonary consequences of COVID-19. Moreover, it has been demonstrated that IL-1β plays an important role in the pathogenesis of virus complications in the central nervous system (CNS). Hyper-inflammation and elevated IL-1β in CNS exacerbate neurodegeneration through oxidopamine mediated toxicity and increased nitric oxide level [50]. It is suggested that coronaviruses can induce immune-mediated demyelination in the acute phase of infection. It seems that high amounts of IL-1β participate in virus-induced neuronal demyelination via promoting IL-17 production, which results in inhibiting apoptosis in virus-infected cells, blocking T cytotoxic cell function, and increasing cell infiltration to the CNS [51, 52]. Another study showed that IL-1β causes excitotoxic neuronal injury by inhibiting astrocyte glutamate transport, which withdraws glutamate from the synaptic cleft [53]. Considering the association between COVID-19 and demyelination, it is probable that SARS-CoV-2 induces CNS damage through IL-1β.
Considering the pivotal role of the cytokine storm in the mortality of COVID-19 patients and long-lasting pulmonary function impairment, there is an urgent need for effective treatments for facing the virus-induced cytokine storm. Several studies suggested that MSCs have significant inhibitory effects on the cells and major cytokines participating in the cytokine storm. Various paracrine mediators exert immunosuppressive features of MSCs, including IL-10, TGF-β, indoleamine-2,3-dioxygenase (IDO), TNFα-stimulated gene-6 (TSG6), and prostaglandin E2 (PGE-2). MSCs can switch between inflammatory and anti-inflammatory phenotypes in different conditions [54]. Recently it is suggested that immunomodulatory features of MSCs are affected by their microenvironment; for instance, inflammation increases MSCs' immunomodulatory effects [55, 56]. MSCs display immunosuppressive characteristics only during exposure to sufficiently high levels of pro-inflammatory cytokines and nitric oxide, which in COVID-19 occurs as the cytokine storm. The co-presence of IFN-γ and another cytokines, including TNFα, IL-1α, or IL-1β in the inflammation microenvironment is essential for immunomodulatory effects of MSCs [57]. These mediators exist in the lung and other affected organs during COVID-19 pathogenesis, help MSCs express their immunosuppressive effects. MSCs can influence TNF-α dependent pathways through different mechanisms. Highly expressed TNF-α and IFN-γ in the inflammation site are well known to induce MSCs to secrete PGE-2, Cyclooxygenase 2 (COX-2), and IDO. PGE-2 stimulates alveolar macrophages to produce IL-10, which reduces MHC II and costimulatory molecules' expression on macrophages and reduces neutrophils migration to the lung tissue by inducing a reduction in rolling, adhesion, and trans-epithelial migration of these immune cells [58, 59]. Moreover, PGE-2, IDO, and COX-2 inhibit T cell and B cell proliferation [60,61,62,63]. High amounts of IFN-γ also induce MSCs to upregulate inducible nitric oxide synthase (iNOS), which suppresses the immune system overactivity and delays hypersensitivity responses [64]. MSC-derived exosomes also participate in the modulation of the TNF-α level. MSCs transfer exosomes to the macrophages that inhibit TNF-α production by affecting alveolar macrophages in ARDS preclinical models. It seems that mir-451, as an exosome content, suppresses TNF-α and the expression of macrophage migration inhibitory factor (MIF) in macrophages [65, 66]. MSC injection in ALI/ARDS preclinical models resulted in increased lung tissue recovery. They raised the level of anti-inflammatory molecules such as IL-10 while decreased inflammatory cytokines, including TNF-α, MIP-2, IL-1β, TGF-β, vascular endothelial growth factor (VEGF), IL-6, IFN-γ, MPO, RANTES, and NOS levels [67,68,69,70,71,72,73,74]. Although MSCs secrete some cytokines, the effect of such cytokines depends on the type of transmitted signal. For instance, MSCs release IL-6, which has a dual effect on the target cells, mediated by classical anti-inflammatory signaling or pro-inflammatory trans-signaling [75]. IL-1 receptor antagonist (IL-1Ra), which prevents toxic immune response, is secreted from MSCs and competes with IL-1β for the IL-1 receptors. As a result, MSC-secreted IL-1Ra suppresses immune cell infiltration into the lung. Under the effect of IL-1Ra, MSCs secrete VEGF, which increases type II surfactant-secreting alveolar cell regeneration and prevents endothelial cell apoptosis [76]. MSC therapy modulates immune response and decreases lung tissue fibrosis in ALI/ARDS in vivo models [71, 77,78,79]
Considering the immunomodulatory effects of MSCs, they prevent and eliminate cytokine storm in COVID-19 and decrease life-threatening complications. Besides, MSCs are also well known to reduce immune cell infiltration to the site of inflammation. MSCs produce and secrete insulin-like growth factor I (IGF-1) which inhibits immune cell infiltration to the alveolus. Keratinocyte growth factor (KGF) secretion by MSCs inhibits the influx of neutrophils to the lung tissue [80, 81]. Angiopoietin-1 (Ang-1) is another regulatory molecule secreted by MSCs that supresses endothelium-leukocyte interaction by changing endothelial cell adhesion molecules, and preventing immune cell adhesion and rolling [82]. MSCs also secrete hepatocyte growth factor (HGF), which stabilizes the endothelium and repairs injured endothelial cells in lung tissue by inhibiting Rho GTPase [83]. MSC-derived MVs decrease neutrophil influx to the injured lung tissue. Moreover, these exosomes diminish the recruitment of Ly6Chi monocytes as stimulator lung tissue fibrosis [65].
MSCs also interact with the cells in a direct contact manner. Many studies have shown that the transfer of healthy mitochondria to damaged cells can improve inflammatory and destructive processes. These studies have suggested using MSCs as a viable option for transferring healthy mitochondria to damaged cells. Mitochondrial transfer from MSCs significantly reduced inflammatory cytokines, improved mitochondrial dysfunction of alveolar cells, and reduced asthma-induced inflammation in the animal model [84]. One study found that MSC-derived mitochondria were involved in protecting against ALI. Mitochondrial transfer from MSCs to pneumocytes occurs over 24 hours through tunneling the gap junctions between two cells which regulates cell bioenergy and increases ATP concentration in the recipient cells [85]. Moreover, mitochondrial transfer from MSCs to T cells induces their differentiation into Treg and limits the inflammatory response. The mitochondrial transmission reduces inflammatory cells as well as tissue damage in the animal model of host transplant disease (GVHD) [86].
Effect of MSCs in RAAS Regulation
The RAAS is a pivotal regulator of blood pressure and Na+/K+ balance. This regulatory system consists of several factors, such as angiotensinogen, renin, different types of angiotensin, angiotensin-converting enzymes (ACE), and AT1 receptor. Some parts of RAAS exist in the lung, which convert angiotensin I to angiotensin II by ACE 1. Angiotensin II affects the AT1 receptor or is converted to other angiotensin types by ACE 2. As mentioned above, cell entry of SARS-CoV-2 depends on the interaction of the viral spike (S) proteins to cellular receptors ACE2, with the assistance of the cellular serine protease TMPRSS2. Binding of ACE 2 and (S) proteins activate clathrin-dependent endocytosis of SARS-CoV-2 and ACE2. Receptor-virus interaction also triggers metalloprotease 17 (ADAM17), which results in the inhibition and shedding of ACE2, which increases the local accumulation of angiotensin II [24, 87]. It is suggested that angiotensin II has a dual effect on COVID-19 pathogenesis; as a restrictive infection mechanism, angiotensin II inhibits ACE 2 by competing SARS-CoV-2 or downregulating ACE2 thorough AT1 receptor [88]. As a progressive factor, angiotensin II binds AT1 receptor and acts as a lung damage-promoting factor by inducing vasoconstriction, inflammation, fibrosis, and apoptosis of alveolar epithelial cells [89, 90]. However, clinical studies suggest that administration of ACE inhibitors, which block ACE 1 and consequently reduce angiotensin II, did not affect the risk of COVID-19 that weaken the protective effects of angiotensin II [91]. It is obvious that regulated amounts of ACE 2 convert angiotensin II to angiotensin 1 to 7, which protects lung tissue against fibrosis, apoptosis, oxidative stress, and inflammation [92, 93].
MSCs can interfere with RAAS and change the expression pattern of angiotensins. Intratracheal administration of MSCs increases alveolarization and diminishes angiotensin II, AT1 receptor, and ACE1 in the injured lung [94]. As a paracrine effect, MSCs–derived MVs can alter the RAAS balance, from ACE1- angiotensin II- AT1 receptor axis to ACE2- angiotensin (1–7). Inhibition of ACE1- angiotensin II- AT1 receptor axis can reduce angiotensin II/AT1 mediated complications of COVID-19, especially its cardiovascular problems [95].
Effects of MSCs in Alveolar Fluid Clearance
Hyaluronic acid (HA), also called hyaluronan, is a lung matrix component necessary for the lung's physiological function. Studies suggest that inflammation increases HA, IL-1, and TNF production, which are elevated in COVID-19, promote HA production by stimulating HA-synthase-2 (HAS2) in type II surfactant-secreting alveolar cells and fibroblasts [96]. High amounts of HA play two crucial roles in COVID-19 pathogenesis. First, HA supports the leukocyte infiltration, which is linked with edema formation [97]. Second, HA accumulation in alveoli prevents gas diffusion through the alveolar wall because HA can absorb a high amount of water up to 1000 times its molecular weight [96]. Clinical evidence from the lungs of COVID-19 patients indicates the existence of white patches (ground glass), which present as the accumulation of fluid in the alveoli [98]. Along with imaging findings, the pathological evaluation of autopsies confirms that alveoli are filled with liquid jelly termed exudate [99]. Some clinical studies suggest hyaluronic acid and type III procollagen as early indicators of poor prognosis for COVID-19 patients [100].
MSCs inhibit fluid accumulation in the lung through suppression of leukocyte infiltration to the lung tissue. MSCs also release KGF and Ang-1, which facilitate endothelial repair in a paracrine manner. Both KGF and Ang-1 reduce alveolar epithelium permeability in lung tissue and inhibit uncontrolled proteins and alveolar fluid transport and fluid accumulation in the lung [74, 80, 101,102,103,104]. KGF reduces BAL protein level in acute lung edema models, and MSC derived Ang-1 has been shown to decrease permeability to albumin [101, 105]. Similar effects have been reported for HGF and EVs secreted from MSCs as a paracrine effect [106, 107]. MSCs transfer Ang-1 mRNA to the lung's injured endothelial cells through their secreted microvesicles [108]. Ang-1 secreted from MSCs inhibits F actin fibers' transformation into stress fibers and suppresses cytoskeleton fibers' central organization in alveolar type 2 cells and endothelial cells [101, 108]. The central organization of actin and myosin fibers results in increased protein permeability. MSC derived KGF upregulates alpha epithelial Na channels (aENaC) gene expression and Na+/K+-ATPase activity, which results in improved alveolar fluid transport in the lungs [109]. aENaC facilitates Na reabsorption from the apical surface of alveolar epithelial cells and inhibits fluid accumulation in alveoli [101, 108, 110]. Furthermore, MSC-derived MVs increase the alveolar fluid clearance during acute lung injury in a dose-dependent manner. Both MSCs and their microvesicles increase the expression of eNOS as a NO producer enzyme in endothelial cells of lung tissue. A higher amount of NO results in vasodilation and decreases pulmonary arterial pressure and pulmonary vascular resistance [111]. It seems that MSCs improve the outcome of ARDS by increasing alveolar fluid clearance and lung tissue compliance.
Effects of MSCs in Coagulation
Three essential factors predispose vessels to thrombosis; endothelial damage or dysfunction, blood flow changes (stasis, turbulence), and hypercoagulability, known as Virchow's triad. Several studies reported that thrombotic events are frequent among patients with COVID-19 [112,113,114]. It is vital to consider Virchow's triad to understand the possible mechanisms of thrombosis formation. As the first factor of this triad, ACE2 is highly expressed in endothelial cells of arteries and veins in some tissues [115]. Thus, the presence of ACE2 makes endothelial cells susceptible to SARS-CoV-2 infection and injuries. Moreover, lymphocytic endotheliitis and apoptotic bodies were observed in autopsy specimens, which show vascular endothelial inflammation in COVID-19 patients [116]. As the next factor, vascular wall thickening, occlusion, and lumen stenosis cause pulmonary hypertension and abnormal blood flow in the advanced stage of COVID-19 [117]. Virus-induced inflammation triggers hypercoagulation as the last factor of Virchow's triad. IL-6 can increase tissue factor (TF) expression in mononuclear cells that initiates the extrinsic coagulation pathway. Also, both IL-6 and TNF-α suppress endogenous anticoagulant pathways that results in a hypercoagulable state. Systemic infection may result in excessive amounts of von Willebrand factor, which mediates platelet aggregation and exposes vessels to clot formation. The excess von Willebrand amount results from the infection-induced deficiency of ADAMTS13, which reduces coagulation by cleaving the von Willebrand factor [118, 119]. A combination of these mechanisms increases the risk of thrombosis and disseminated intravascular coagulation (DIC) that appear in paraclinical data as elevated D-dimer, fibrin degradation products (FDP), and more prolonged PT and PTT values [120].
MSCs can reduce complications of thrombosis and DIC mainly through immune system regulation, recanalization, and neovascularization. MSCs decrease organ deterioration and DIC by regulating the release of pro-inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, and IL-6. The mentioned cytokines are involved in apoptosis of vascular endothelial cells, vessel injury, overexpression of TF, and DIC development. Besides, MSCs inhibit fibrin microthrombi formation via suppressing T-cytotoxic lymphocytes, NK cells, and B cells [121, 122]. MSCs induce recanalization and angiogenesis through recruiting macrophages. They alter M1 macrophages, which encourage inflammation, to M2 macrophages, which promote tissue repair. Macrophages participate in thrombosis lysis and endocytosis and also vessel recanalization. MSCs induce vascular-like structures through the infiltration of macrophages in thrombosis sites. The macrophages inside thrombosis induce 1) proliferation and migration of endothelial cells, 2) lysis of extracellular matrix, 3) secreting angiogenesis factors such as VEGF and basic fibroblast growth factor (bFGF) [123]. It seems that administration of MSCs may reduce coagulation complications in COVID-19 by controlling Haemostasis factors.
Effects of MSCs in the Regeneration of Lung Tissue
MSCs play a positive role in the regeneration of damaged or destroyed tissues. Up to now, MSCs have been used to regenerate different organs such as bone, cartilage, nervous system, cornea, trachea, and skin [124,125,126,127,128,129]. MSCs induce reconstruction mainly through two mechanisms; differentiation to specific cell types and paracrine signals that promote target progenitor cells. Hypoxia can act as a promoter for the differentiation of MSCs to type II surfactant-secreting alveolar cells as the main target cell of SARS-CoV-2. This effect is mediated by microRNA-145 (miR-145), which targets TGF-β receptor II (TGFβRII) and consequently inhibits TGF-β signaling [130]. It is indicated that under appropriate conditions, MSCs express Clara cell secretory protein (CCSP), pro-surfactant protein C (pro-SPC), thyroid transcription factor-1 (TTF-1), and cystic fibrosis transmembrane conductance regulator (CFTR) proteins, which are pivotal functional factors of alveolar cells [130]. Moreover, MSCs recruit domestic cells by producing paracrine signals to enhance proliferation and regeneration in damaged tissues. MSCs release KGF and HGF in the damaged lung, which both induce proliferation and inhibit apoptosis in type II surfactant-secreting alveolar cells [131]. Another study showed that the secretome of MSCs contains several essential factors such as fibronectin, lumican, periostin, and insulin-like growth factor-binding protein 7 (IGFBP-7), which play a vital role in the stimulation of regeneration in lung tissues by affecting type II surfactant-secreting alveolar cells [132]. Along with regenerative effects, MSCs can protect pulmonary cells by inhibiting apoptosis pathways. It is indicated that MSCs prevent apoptosis in the lung fibrosis model of rats by reducing caspase-3 protein expression and increasing the Bcl-2/Bax ratio [133]. The other study showed that MSCs reduce apoptosis by inhibiting PI3K/Akt and Caspase-3 in lung fibroblasts [134]. These studies suggest that both MSC and its secretome can participate in lung regeneration and protection against ALI as a serious consequence of COVID-19. The underlying mechanisms of SARS-CoV-2 pathogenesis and MSCs’ role in eliminating COVID-19 complications are shown in Fig. 1.
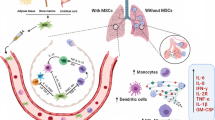
COVID-19 pathogenesis and MSCs role in the elimination of SARS-CoV-2 related complications (1) SARS-CoV-2 enters alveolar type II cells through binding ACE2. As a result, angiotensin II accumulates in the alveoli and binds to ATR1, which acts as a lung damage promoting factor and induces vasoconstriction, inflammation, fibrosis, and apoptosis of alveolar epithelial cells. (2.1) M1 alveolar macrophages produce various pro-inflammatory cytokines, including TNF-α, IL-6, IL-8, and IL-1. TNF-α depletion during the severe phase of COVID-19 activates the extrinsic apoptosis pathway in the alveolar cells and results in massive lung tissue fibrosis. (2.2) TNF-α, IL-6, and IL-8 secretion by M1 alveolar macrophages attract both MSCs to the site of inflammation. (2.3) IL-1β and TNF-α increase hyaluronic acid production by type II surfactant-secreting alveolar cells and fibroblasts. (3.1) MSCs produce IL-1ra, which competes with IL-1, secreted from M1 macrophages, for the IL-1 receptor and suppresses IL-1 effects. (3.2) MSCs decrease TNF-α production of macrophages by their secreted exosomes such as mir-451 containing exosomes. (4.1) IL-1, TNF-α, IL-6, and IL-8 production by alveolar macrophages increase immune cell accumulation in the alveoli. (4.2, 3) M1 macrophages secrete IL-6 and TGF-β, increasing naïve T cell differentiation to T helper 17 cells and decreasing naïve T cell differentiation to regulatory T cells. (5) MSCs reduce immune cell infiltration to the alveoli through the secretion of IL-10, KGF, and IGF-1. (6.1) MSCs increase M2 phenotype macrophages and, as a result, decrease inflammatory cytokines production by M1 macrophages. MSCs increase the infiltration of macrophages to the site of thrombosis. (6.2) M2 macrophages promote tissue repair through several mechanisms, such as vessel recanalization. They secrete VEGF and bFGF, which support the proliferation of endothelial cells. (7) PGE-2, IDO, and COX-2 are secreted by MSC that inhibit the proliferation of infiltrated B cells and T cells. (8) MSC-derived EVs increase T cell differentiation to Regulatory T cells. T reg cells inhibit over-activation of immune responses. (9) Hypoxia, which is a consequence of severe chronic inflammation, promotes MSCs differentiation to type II alveolar cells. (10) MSCs secrete KGF, HGF, pro-SPC, TTF-1, and CFTR, which reduce the accumulation of angiotensin II. As a result, they decrease the apoptosis of alveolar cells while they increase their proliferation. (11) MSCs transfer their mitochondria to the alveolar cells and improve their function. (12) MSCs facilitate injured endothelium repair through secretion of HGF, KGF, and angiopoietin 1. (13) MSC-derived microvessels decrease pulmonary arterial pressure and pulmonary vascular resistance that results in diminished fluid accumulation in the alveoli.
Delivery Routes of MSCs
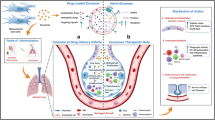
MSCs can detect several chemoattractant molecules, pro-inflammatory cytokines, and cell-cell adhesion molecules expressed in the site of inflammation. High levels of IFN-γ, TNF-α, IL-6, IL-8, and TGF-β in the site of inflammation in the COVID-19 patients act as chemotactic molecules, resulting in MSCs migration to the damaged tissues [134]. In order to increase the efficacy of MSCs and their secretome therapy, several parameters should be optimized, such as cell dose, secretome concentration, and delivery method [135]. The efficacy of MSCs’ migration to the site of inflammation depends on the delivery route of MSCs. Considering the type of disease, there are various delivery methods for MSCs. One of the potentially effective delivery routes for MSCs in treating respiratory disorders such as COVID-19 is intravenous (IV), which results in a significant number of MSCs accumulating in the lungs. MSCs modulate immune system reactions against themselves by suppressing B cell proliferation and inhibit immunoglobulin production. They also express a lower level of surface antigenic molecules such as major histocompatibility complex (MHC) class I and II or costimulatory molecules including CD80, CD86, or CD40, resulting in lower stimulation of the immune system against administered MSCs [136]. This immune-modulatory function makes MSCs an appropriate candidate for passage through circulation when they are injected systematically and also makes them able to track damaged organs without immune system disruption. Besides, IV injection of MSCs results in MSC entrapment in the lung microvasculature due to their large sizes that cause higher efficacy in lung disease. Another route of delivery is the administration of MSCs via intratracheal liquid bolus administration, which increases the chance of MSC migration to the targeted regions of lung tissue [137]. Underlying mechanisms of intratracheal delivery of MSCs are suggested to be similar to intratracheal surfactant replacement therapy and based on the deposition of the cells on the surfaces and traveling through the airways. The efficacy of intratracheal delivery has been approved by several studies [138, 139]. However, there is a lack of enough information on improving intratracheal cell delivery [140]. Intranasal delivery is another potential route of MSC delivery to the lungs, which is feasible, noninvasive, and effective. In this method of delivery, MSCs’ viability is near 100%. The volume of cells given to the patient and multiple-dose administration are important in intranasal cell delivery, which improves its efficacy [141].
Condition media (CM) of MSCs, which contains secretome, has shown promising therapeutic effects in both in vitro and in vivo models of several inflammatory diseases of the lung [142]. In vitro and in vivo experimental models of ALI and ARDS have shown that MSCs secrete several anti-inflammatories, anti-apoptosis, anti-fibrosis molecules and consequently improve lung tissue regeneration. Additionally, cell-free therapies with MSC components have a lower risk of immune system stimulation. These features make them appropriate candidates for treating severe conditions following infection with various microorganisms and viruses such as SARS-CoV-2. As shown in several clinical studies, MSC-derived exosomes have demonstrated higher safety, which attributes to their natural properties such as their small size and natural presence in different organs. Also, the delivery route of exosomes via aerosol inhalation seems safer than the IV route of MSC therapies. Furthermore, from the economic point, the exosomes have a much lower final cost of production and more accessible storage and shipping, which makes their scale-up more plausible for general public use [143, 144]. However, there are some reports on the efficacy of MSC condition medium to be lower than MSC injection in ALI [145]. This difference in the amount of clinical evidence may consist of limited follow-up and a lower number of exosomes used in clinical studies than stem cell application [146]. It has been only in the past decade, which exosomes found a way to be in the scientific discussion for potential therapeutic use. The MSCs have generated reliable results in several studies of respiratory disorders such as ARDS and pulmonary fibrosis, which resemble the respiratory problems caused by COVID-19. MSCs’ entrapment in lung tissue may be an important reason for successfully applying these cells in pulmonary disease. Accumulation of MSCs in the pulmonary tissues prolongs these cells' presence; thus, it provides long-acting therapeutic agents that would remove possible chronic complications of COVID-19.
MSCs as a Drug Delivery System
Recently, genetically modified or pretreated MSCs with various drugs have been used as a vehicle of drug delivery for various molecules such as chemotherapeutic drugs, cytokines, RNA, and DNA [147]. Manipulated MSCs gradually release their content by exocytosis or within EVs in the microenvironment of damaged tissues. Application of primed MSCs decreases the systemic collateral toxicity of drugs, provides a sustained release system that increases the duration of drug effect in the tissue, and acts in a targeted manner. Drug–loaded MSCs are administered in two different ways; direct cell-cell contact and condition media (CM). Considering the tropism features of MSCs, they can accumulate in inflammation sites, directly communicate with local cells, and release drugs in the inflammation microenvironments. CM of drug-loaded MSCs contains important biological factors, including EVs. Moreover, MSCs can release loaded drugs by recruiting EVs that transport drugs between MSCs and target cells [147,148,149,150]. Several research groups have used MSCs to provide appropriate drug carrier systems. MSCs were administered successfully in lung delivery of IFN-β and IFN-α, which are among the foremost candidates to treat COVID-19 in various ongoing clinical trials (available on clinicaltrials.gov) [148,149,150,151]. VEGF plays an important role in brain inflammation during COVID-19 pathogenesis [152]. Engineered MSCs can secret soluble Fms related receptor tyrosine kinase 1 (Flt-1), a receptor of VEGF, that competitively inhibits the factor from binding to the organ receptors [153]. It seems that the idea of drug-loaded and engineered MSC application is hopeful and encouraging as a future therapy to fight the COVID-19 pandemic. The special features of MSCs, such as immunomodulation, RAAS controlling, alveolar fluid reduction, and coagulation suppression along with loading appropriate drugs, bring them up as a beneficial treatment intervention of COVID-19.
Clinical Trials
Based on the immunomodulatory characteristics and regenerative effects of MSCs in preclinical studies and their successful outcomes in the treatment of various respiratory disorders such as ARDS and H5N1 viral infections in the past, several clinical studies have been launched to assess the safety and efficacy of MSCs from different cell sources for COVID-19 [154, 155]. Up to December 6, 2020, our search by the keywords “COVID-19”, “exosome” and “mesenchymal stem cell” revealed that there are 66 registered clinical trials of MSCs application in clinicaltrial.gov, of which 38 are active and recruiting patients, and six have completed their trials. The proportion of MSCs administration of different sources is shown as umbilical cord (36%), adipose tissue (18%), bone marrow (13%), Wharton's jelly (10%), dental pulp (3%), olfactory mucosa (2%), induced pluripotent stem cell (IPSCs) (2%), placenta (1%), and unmentioned origin or off-the-shelf MSC products (15%) (available on clinicaltrials.gov) [156]. The list of administered MSCs and their sources are mentioned in the Table 1 of supplementary information. It is indicated that different sources of MSCs share common features that bring them up as an appropriate therapeutic option. For instance, MSCs from adipose tissue, amniotic fluid, bone marrow, and umbilical cord minimally express ACE2 and TMPRSS2, indicating that MSCs from these sources are resistant to SARS-CoV-2 infection. Also, bone marrow-derived MSCs do not alter their ACE2 or TMPRSS2 expression under inflammatory conditions that are important in increasing the efficacy of MSC application in cytokine storm during the sever phase of COVID-19 [157, 158]. However, selecting the most appropriate source of MSCs for clinical administration requires evaluating future results of ongoing clinical trials and better investigating molecular and biological features of MSCs. Besides, eight ongoing clinical trials of exosome administration are available, of which one study is successfully terminated. Out of these eight studies, only one study has used nonstem cell-derived EVs (generated by immune cells), and the rest used stem cell-derived EVs (Table 1).
These trials' main inclusion and exclusion criteria are the same signs of disease exacerbation used by healthcare systems worldwide. This type of intervention is mostly used in hospitalized patients in the ICU (Intensive Care Unit) because it needs close observation of patients to monitor probable side effects of CBT. Besides, CBT strategies only make sense for severe and critical patients because the mechanism of action of such therapies is based on immunomodulation. In addition, the probability of Cytokine Release Syndrome (CRS) is significantly higher in ICU admitted patients [159]. It is also important to consider costs, availability, and condition of cell isolation, which are limiting factors in CBTs.
When the efficacy of CBT is proven in a preclinical model, and before initiating a clinical trial, it is necessary to ensure that Cellular and Tissue-Based Products (HCT/Ps) are produced and preserved under clinical-grade standards. Good Manufacturing Practice (GMP), which is obligatory by the Food and Drug Administration (FDA), Health Canada, and European Medicines Agency (EMA), is defined as applying standard operating procedures for manufacturing and controlling therapeutic cell production quality [160]. Because of the substantial need to HCT/Ps during the COVID-19 pandemic, it is essential to consider GMP guidelines in cell isolation and processing. However, some factors influence GMP's quality in the pandemic situation, including time limitation, lack of appropriate equipment, and financial problems. One of the most important aspects of GMP in the COVID-19 pandemic is the viral contamination that can originate from several sources, including HCT/Ps, biological raw materials, manufacturing environment, and clinicians who administer HCT/Ps to patients. In order to reduce the chance of virus transmission, it is suggested to test the final products by employing validated tests for SARS-CoV-2, screen tissue donors for contamination, screen operators who are in close contact with HCT/Ps or patients, and obey guidelines of GMP rigorously. However, our understanding of GMP quality in CBT clinical trials of COVID-19 is insufficient, and further studies are essential to improve HCT/Ps preparing processes and clinical practice during the pandemic situation.
The optimum dosing and required number of injections of MSCs are also not precise, and therefore some trials are using flexible dosing to find out the appropriate measures at the end of the trial. The overall number of cells injected is somewhere between 10 million/per kg to 50 million/per kg for each patient, and the number of injections is between one and five times of injection. These trials' primary outcome is the change in lung CT, blood biochemical examination, lymphocyte subsets, and inflammatory factors shift, mortality outcome, and ICU and hospital stay duration. These outcomes will be assessed for up to one year in some trials to observe MSC treatment's long-term consequences.
There have been four published studies of the registered clinical trials showing promising results. The first study is a case report of a 65 y/o critically ill COVID-19 patient with severe pneumonia and respiratory failure requiring mechanical ventilation whose disease had progressed despite intensive therapy, with markers showing evidence of multiple organ damage [161]. Considering the severe organ injury caused by an inflammatory response and side effects, the glucocorticoid and antiviral therapy were withdrawn. This patient was treated with allogeneic human umbilical cord MSC (hUC-MSC) alongside the conventional therapy, which she had not responded to, using three intravenous infusions of 5 × 107 hUC-MSCs, three days apart. During the therapy, antibiotics were given to prevent infection, and thymosin α1 was also given to amplify the immunomodulation effects of the MCSs.
She was transferred out of the ICU as she recovered, with most of her laboratory results returning to normal. Six days after the third infusion, her CT scan changes in the lungs significantly improved. All measured parameters, including T cell counts, returned to normal levels. No short-term side effects were observed for this patient.
The second clinical trial was a pilot study to assess whether MSC intravenous infusions of allogeneic MSCs from the umbilical cord source could improve clinical outcomes for patients with COVID-19 pneumonia [162]. Seven confirmed COVID-19 patients were enrolled, including one critical type, four severe types, and two mild types. Before transplantation, all had shortness of breath, high body temperature, and low oxygen saturation. Treatment was a single intravenous injection of GMP grade MSCs, 1 × 106 cells per kilogram of patient weight. There were no adverse reactions to follow-up over 14 days, and within two days, all patients had significantly improved respiratory function. Overall, peripheral lymphocytes increased with a shift to regulatory phenotypes, and inflammatory cytokines decreased significantly, while anti-inflammatory factors such as IL-10 increased after treatment.
The third study assessed the efficacy of UC-MSCs for COVID-19 treatment at Taikangtongji Hospital in Wuhan, China. The study included 31 patients; 30 were either in the severe or critical condition of the disease. The UC-MSC treatment showed the restoration of oxygenation and downregulation of inflammatory factors and mitigated cytokine storms in patients hospitalized with severe COVID-19 without any noticeable side effects. In the end, 27 of those patients were discharged from the hospital [163].
As the fourth clinical study, a parallel assigned controlled, non-randomized, phase 1 clinical trial was performed in China to evaluate the safety and efficacy of UC-MSCs administration in moderate to severe COVID-19 patients. They enrolled 18 patients divided into two groups, and three cycles of intravenous infusion of hUC-MSCs were administered in three-day intervals. Assessment of clinical manifestations and laboratory data suggest that application of MSCs had no infusion-associated adverse effect. It also reduced mortality and improved long-term treatment outcomes [164].
Some immunocompromised conditions may co-exist with COVID-19, such as malignancies, diabetes mellitus, organ transplantation, and acquired immunodeficiency syndrome (AIDS) [165]. Besides, some immunosuppressive drugs like glucocorticoids, azathioprine, and calcineurin inhibitors influence immune responses [166]. Since immunocompromised conditions is one of the excluding criteria of MSC-based therapy in COVID-19, MSC-based therapy's exact role in immunocompromised patients with COVID-19 is unclear. However, clinical administration of MSCs in some immunocompromised conditions like AIDS and type 2 diabetes (T2DM) were evaluated. MSCs may be useful in these immunocompromising diseases by controlling the dysregulated immune system or boosting endogenous repair by altering the microenvironment. In a phase 1/phase 2 clinical trial, patients with T2DM received cord blood-derived multipotent stem cells. The results revealed that the CBT reverses immune dysfunctions via balancing Th1/Th2/Th3 cytokine production [167]. In another clinical study, it is shown that all patients who received MSC transfusions tolerate the intervention all over the clinical trial. Transplanted MSCs elevated circulating naive and central memory CD4 T-cell and returned IFN-γ and IL-2 production in the immune nonresponder patients. The immune enhancements were correspondingly associated with systemic immunomodulation and inflammation [168]. These results show that MSCs can exert immunomodulation even in immunocompromised patients. Besides, MSCs participate in several regenerative mechanisms when they are administered in COVID-19 patients. For example, MSCs promote cell regeneration, improve cell bioenergy, control coagulation disorders, and regulate the RAAS axis, which are helpful in immunocompromised COVID-19 patients. However, more evaluation is essential for shedding light on MSCs administration outcomes and possible advantages and disadvantages in the immune system disorders and COVID-19.
Limitations of MSC-based Therapy in COVID-19:
Although MSCs provide several advantages in combating COVID-19, there are still some doubts and limitations that should be evaluated in the future studies. The first issue in applying MSCs in COVID-19 is the eligibility of the patients for CBT. There is insufficient information on use of MSC-based therapy in patients with a history of chronic diseases or excluding conditions such as malignancies, immune system disorders, allergies, pregnancy, and lactating mothers [18, 169]. As the second problem, the majority of clinical trials using MSCs or their exosomes in COVID-19 are in phase I/II which led to insufficient outcomes. In addition, the main problem with published studies, in addition to their small sample size, is the absence of control groups, which makes the outcome of these studies challenging. Clinical administration of MSCs requires strong evidence of safety and efficacy, which is achieved by evaluating probable side effects and unwanted long-lasting results. Finally, the absence of standard therapeutic protocols for various aspects of MSC-based therapy, including isolation methods, selecting an appropriate source, storage conditions, route of delivery, administration methods, dosage, and appropriate phase of the disease are important limitations of MSCs application in COVID-19. Nevertheless, MSC-based therapy in the COVID-19 pandemic requires more analysis and reconsideration in the expectations of surmounting their hurdles in future studies.
Conclusion
MSCs and their secreted molecules can modulate immune system reactions in severe conditions followed by infection and other pulmonary severe situations, accompanied by over activation of the immune system. Their secretome can also inhibit lung tissue fibrosis and accumulation of alveolar fluid in alveoli in ARDS clinical and preclinical models. There are only a few reports of using MSCs in the treatment of COVID-19, and further studies would be required on the mechanisms involving the therapeutic effects of MSCs in severe COVID-19.
References
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., & China Novel Coronavirus Investigating and Research Team. (2020). A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England journal of medicine, 382(8), 727–733. https://doi.org/10.1056/NEJMoa2001017.
WHO Coronavirus Disease (COVID-19) Dashboard. (n.d.). Retrieved November 16, 2020, from https://covid19.who.int/
Baig, A. M., Khaleeq, A., Ali, U., & Syeda, H. (2020). Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS chemical neuroscience, 11(7), 995–998. https://doi.org/10.1021/acschemneuro.0c00122.
Vaira, L. A., Salzano, G., Deiana, G., & De Riu, G. (2020). Anosmia and Ageusia: Common Findings in COVID-19 Patients. The Laryngoscope. https://doi.org/10.1002/lary.28692
Xu, Z., Shi, L., Wang, Y., Zhang, J., Huang, L., Zhang, C., et al. (2020). Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respiratory medicine, 8(4), 420–422. https://doi.org/10.1016/S2213-2600(20)30076-X.
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., Manson, J. J., & HLH Across Speciality Collaboration, UK. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet, 395(10229), 1033–1034. https://doi.org/10.1016/S0140-6736(20)30628-0.
Han, F., Sun, R., Ni, Y., Hu, X., Chen, X., Jiang, L., et al. (2015). Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. The American journal of the medical sciences, 349(3), 199–205. https://doi.org/10.1097/MAJ.0000000000000379.
Henry, B, M. (2020). COVID-19, ECMO, and lymphopenia: a word of caution. The Lancet. Respiratory medicine. https://doi.org/10.1016/S2213-2600(20)30119-3
Yao, X., Ye, F., Zhang, M., Cui, C., Huang, B., Niu, P., et al. (2020). In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 71(15), 732–739. https://doi.org/10.1093/cid/ciaa237.
Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell research, 30(3), 269–271. https://doi.org/10.1038/s41422-020-0282-0.
Chan, K. S., Lai, S. T., Chu, C. M., Tsui, E., Tam, C. Y., Wong, M. M. L., et al. (2003). Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong medical journal = Xianggang yi xue za zhi / Hong Kong Academy of Medicine, 9(6), 399–406 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14660806.
Gurwitz, D. (2020). Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug development research, 81(5), 537–540. https://doi.org/10.1002/ddr.21656.
Antwi-Amoabeng, D., Kanji, Z., Ford, B., Beutler, B, D., Riddle, M, S., & Siddiqui, F. (2020). Clinical outcomes in COVID-19 patients treated with tocilizumab: An individual patient data systematic review. Journal of medical virology. https://doi.org/10.1002/jmv.26038
Benucci, M., Giannasi, G., Cecchini, P., Gobbi, F, L., Damiani, A., Grossi, V., … Manfredi, M. (2020). COVID-19 pneumonia treated with Sarilumab: A clinical series of eight patients. Journal of medical virology. https://doi.org/10.1002/jmv.26062
Xu, X., Han, M., Li, T., Sun, W., Wang, D., Fu, B., et al. (2020). Effective treatment of severe COVID-19 patients with tocilizumab. Proceedings of the National Academy of Sciences of the United States of America, 117(20), 10970–10975. https://doi.org/10.1073/pnas.2005615117.
Dominici, M., Le Blanc, K., Mueller, I., Slaper-Cortenbach, I., Marini, F., Krause, D., et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 8(4), 315–317. https://doi.org/10.1080/14653240600855905.
Babajani, A., Soltani, P., Jamshidi, E., Farjoo, M, H., & Niknejad, H. (2020). Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Frontiers in Bioengineering and Biotechnology. https://doi.org/10.3389/fbioe.2020.00748
Golchin, A., Seyedjafari, E., & Ardeshirylajimi, A. (2020). Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem cell reviews and reports, 16(3), 427–433. https://doi.org/10.1007/s12015-020-09973-w.
Doyle, L, M., & Wang, M, Z. (2019). Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells , 8(7). https://doi.org/10.3390/cells8070727
Yin, K., Wang, S., & Zhao, R. C. (2019). Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomarker research, 7, 8. https://doi.org/10.1186/s40364-019-0159-x.
Oudit, G. Y., Kassiri, Z., Jiang, C., Liu, P. P., Poutanen, S. M., Penninger, J. M., & Butany, J. (2009). SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. European journal of clinical investigation, 39(7), 618–625. https://doi.org/10.1111/j.1365-2362.2009.02153.x.
Xu, H., Zhong, L., Deng, J., Peng, J., Dan, H., Zeng, X., et al. (2020). High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. International journal of oral science, 12(1), 8. https://doi.org/10.1038/s41368-020-0074-x.
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 181(2), 271–280.e8. https://doi.org/10.1016/j.cell.2020.02.052.
Merad, M., & Martin, J. C. (2020). Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nature reviews. Immunology, 20(6), 355–362. https://doi.org/10.1038/s41577-020-0331-4.
Wang, F., Nie, J., Wang, H., Zhao, Q., Xiong, Y., Deng, L., et al. (2020). Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. The Journal of infectious diseases, 221(11), 1762–1769. https://doi.org/10.1093/infdis/jiaa150.
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., et al. (2020). Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 71(15), 762–768. https://doi.org/10.1093/cid/ciaa248.
Zhang, B., Zhou, X., Zhu, C., Song, Y., Feng, F., Qiu, Y., et al. (2020). Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Frontiers in molecular biosciences, 7, 157. https://doi.org/10.3389/fmolb.2020.00157.
Li Y, X., Wu W., Yang T., Zhou W., Fu Y, M., Feng Q, M., & Ye J, M. (2020). [Characteristics of peripheral blood leukocyte differential counts in patients with COVID-19]. Zhonghua nei ke za zhi [Chinese journal of internal medicine], 59(0), E003. 3760. https://doi.org/10.3760/cma.j.cn112138-20200221-00114
Gao, F., Chiu, S. M., Motan, D. A. L., Zhang, Z., Chen, L., Ji, H.-L., et al. (2016). Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell death & disease, 7, e2062. https://doi.org/10.1038/cddis.2015.327.
Bernardo, M. E., & Fibbe, W. E. (2013). Mesenchymal stromal cells: sensors and switchers of inflammation. Cell stem cell, 13(4), 392–402. https://doi.org/10.1016/j.stem.2013.09.006.
Prockop, D. J. (2013). Concise review: two negative feedback loops place mesenchymal stem/stromal cells at the center of early regulators of inflammation. Stem cells, 31(10), 2042–2046. https://doi.org/10.1002/stem.1400.
Henderson, L. A., Canna, S. W., Schulert, G. S., Volpi, S., Lee, P. Y., Kernan, K. F., et al. (2020). On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis & rheumatology (Hoboken, N.J.), 72(7), 1059–1063. https://doi.org/10.1002/art.41285.
Tuazon Kels, M. J., Ng, E., Al Rumaih, Z., Pandey, P., Ruuls, S. R., Korner, H., et al. (2020). TNF deficiency dysregulates inflammatory cytokine production, leading to lung pathology and death during respiratory poxvirus infection. Proceedings of the National Academy of Sciences of the United States of America, 117(27), 15935–15946. https://doi.org/10.1073/pnas.2004615117.
Damjanovic, D., Divangahi, M., Kugathasan, K., Small, C.-L., Zganiacz, A., Brown, E. G., et al. (2011). Negative regulation of lung inflammation and immunopathology by TNF-α during acute influenza infection. The American journal of pathology, 179(6), 2963–2976. https://doi.org/10.1016/j.ajpath.2011.09.003.
Galluzzi, L., Vitale, I., Abrams, J. M., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., et al. (2012). Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell death and differentiation, 19(1), 107–120. https://doi.org/10.1038/cdd.2011.96.
Parameswaran, N., & Patial, S. (2010). Tumor necrosis factor-α signaling in macrophages. Critical reviews in eukaryotic gene expression, 20(2), 87–103. https://doi.org/10.1615/critreveukargeneexpr.v20.i2.10.
Kim, J. J., Lee, S. B., Park, J. K., & Yoo, Y. D. (2010). TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell death and differentiation, 17(9), 1420–1434. https://doi.org/10.1038/cdd.2010.19.
Wang, L., Du, F., & Wang, X. (2008). TNF-alpha induces two distinct caspase-8 activation pathways. Cell, 133(4), 693–703. https://doi.org/10.1016/j.cell.2008.03.036.
Wang, L. (2020). C-reactive protein levels in the early stage of COVID-19. Medecine et maladies infectieuses, 50(4), 332–334. https://doi.org/10.1016/j.medmal.2020.03.007.
Tanaka, T., Narazaki, M., & Kishimoto, T. (2014). IL-6 in inflammation, immunity, and disease. Cold Spring Harbor perspectives in biology, 6(10), a016295. https://doi.org/10.1101/cshperspect.a016295.
Kobayashi, T., Tanaka, K., Fujita, T., Umezawa, H., Amano, H., Yoshioka, K., et al. (2015). Bidirectional role of IL-6 signal in pathogenesis of lung fibrosis. Respiratory research, 16, 99. https://doi.org/10.1186/s12931-015-0261-z.
Bettelli, E., Carrier, Y., Gao, W., Korn, T., Strom, T. B., Oukka, M., et al. (2006). Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature, 441(7090), 235–238. https://doi.org/10.1038/nature04753.
Reeh, H., Rudolph, N., Billing, U., Christen, H., Streif, S., Bullinger, E., et al. (2019). Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: fusing experimental insights and dynamic modelling. Cell communication and signaling: CCS, 17(1), 46. https://doi.org/10.1186/s12964-019-0356-0.
Rose-John, S. (2012). IL-6 trans-signaling via the soluble IL-6 receptor: importance for the pro-inflammatory activities of IL-6. International journal of biological sciences, 8(9), 1237–1247. https://doi.org/10.7150/ijbs.4989.
Le, T.-T. T., Karmouty-Quintana, H., Melicoff, E., Le, T.-T. T., Weng, T., Chen, N.-Y., et al. (2014). Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. Journal of immunology, 193(7), 3755–3768. https://doi.org/10.4049/jimmunol.1302470.
Schmitz, N., Kurrer, M., Bachmann, M. F., & Kopf, M. (2005). Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. Journal of virology, 79(10), 6441–6448. https://doi.org/10.1128/JVI.79.10.6441-6448.2005.
Gasse, P., Mary, C., Guenon, I., Noulin, N., Charron, S., Schnyder-Candrian, S., et al. (2007). IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. The Journal of clinical investigation, 117(12), 3786–3799. https://doi.org/10.1172/JCI32285.
Spagnolo, P., Balestro, E., Aliberti, S., Cocconcelli, E., Biondini, D., Casa, G. D., et al. (2020). Pulmonary fibrosis secondary to COVID-19: a call to arms? The Lancet. Respiratory medicine, 8(8), 750–752. https://doi.org/10.1016/S2213-2600(20)30222-8.
Pott Godoy, M. C., Tarelli, R., Ferrari, C. C., Sarchi, M. I., & Pitossi, F. J. (2008). Central and systemic IL-1 exacerbates neurodegeneration and motor symptoms in a model of Parkinson’s disease. Brain: a journal of neurology, 131(Pt 7), 1880–1894. https://doi.org/10.1093/brain/awn101.
Kim, B. S., Jin, Y.-H., Meng, L., Hou, W., Kang, H. S., Park, H. S., & Koh, C.-S. (2012). IL-1 signal affects both protection and pathogenesis of virus-induced chronic CNS demyelinating disease. Journal of neuroinflammation, 9, 217. https://doi.org/10.1186/1742-2094-9-217.
Wu, G. F., & Perlman, S. (1999). Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. Journal of virology, 73(10), 8771–8780. https://doi.org/10.1128/JVI.73.10.8771-8780.1999.
Prow, N. A., & Irani, D. N. (2008). The inflammatory cytokine, interleukin-1 beta, mediates loss of astroglial glutamate transport and drives excitotoxic motor neuron injury in the spinal cord during acute viral encephalomyelitis. Journal of neurochemistry, 105(4), 1276–1286. https://doi.org/10.1111/j.1471-4159.2008.05230.x.
Waterman, R. S., Tomchuck, S. L., Henkle, S. L., & Betancourt, A. M. (2010). A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PloS one, 5(4), e10088. https://doi.org/10.1371/journal.pone.0010088.
Crop, M. J., Baan, C. C., Korevaar, S. S., Ijzermans, J. N. M., Pescatori, M., Stubbs, A. P., et al. (2010). Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clinical and experimental immunology, 162(3), 474–486. https://doi.org/10.1111/j.1365-2249.2010.04256.x.
Raicevic, G., Najar, M., Najimi, M., El Taghdouini, A., van Grunsven, L. A., Sokal, E., & Toungouz, M. (2015). Influence of inflammation on the immunological profile of adult-derived human liver mesenchymal stromal cells and stellate cells. Cytotherapy, 17(2), 174–185. https://doi.org/10.1016/j.jcyt.2014.10.001.
Li, W., Ren, G., Huang, Y., Su, J., Han, Y., Li, J., et al. (2012). Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell death and differentiation, 19(9), 1505–1513. https://doi.org/10.1038/cdd.2012.26.
Ajuebor, M. N., Das, A. M., Virág, L., Flower, R. J., Szabó, C., & Perretti, M. (1999). Role of resident peritoneal macrophages and mast cells in chemokine production and neutrophil migration in acute inflammation: evidence for an inhibitory loop involving endogenous IL-10. Journal of immunology, 162(3), 1685–1691 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9973430.
Németh, K., Leelahavanichkul, A., Yuen, P. S. T., Mayer, B., Parmelee, A., Doi, K., et al. (2009). Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature medicine, 15(1), 42–49. https://doi.org/10.1038/nm.1905.
Wang, Y., Chen, X., Cao, W., & Shi, Y. (2014). Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nature immunology, 15(11), 1009–1016. https://doi.org/10.1038/ni.3002.
Ge, W., Jiang, J., Arp, J., Liu, W., Garcia, B., & Wang, H. (2010). Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation, 90(12), 1312–1320. https://doi.org/10.1097/TP.0b013e3181fed001.
Jarvinen, L., Badri, L., Wettlaufer, S., Ohtsuka, T., Standiford, T. J., Toews, G. B., et al. (2008). Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. Journal of immunology, 181(6), 4389–4396. https://doi.org/10.4049/jimmunol.181.6.4389.
Mazzoni, A., Bronte, V., Visintin, A., Spitzer, J. H., Apolloni, E., Serafini, P., et al. (2002). Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. Journal of immunology, 168(2), 689–695. https://doi.org/10.4049/jimmunol.168.2.689.
Nemeth, K., Keane-Myers, A., Brown, J. M., Metcalfe, D. D., Gorham, J. D., Bundoc, V. G., et al. (2010). Bone marrow stromal cells use TGF-beta to suppress allergic responses in a mouse model of ragweed-induced asthma. Proceedings of the National Academy of Sciences of the United States of America, 107(12), 5652–5657. https://doi.org/10.1073/pnas.0910720107.
Zhu, Y.-G., Feng, X.-M., Abbott, J., Fang, X.-H., Hao, Q., Monsel, A., et al. (2014). Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem cells, 32(1), 116–125. https://doi.org/10.1002/stem.1504.
Park, M.-C., Kwon, O. C., Lee, S.-W., Song, J. J., & Park, Y.-B. (2020). MiR-451 suppresses inflammatory responses in ankylosing spondylitis by targeting macrophage migration inhibitory factor. Clinical and experimental rheumatology, 38(2), 275–281 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/31287414.
Güldner, A., Maron-Gutierrez, T., Abreu, S. C., Xisto, D. G., Senegaglia, A. C., da Silva Barcelos, P. R., et al. (2015). Expanded endothelial progenitor cells mitigate lung injury in septic mice. Stem cell research & therapy, 6, 230. https://doi.org/10.1186/s13287-015-0226-7.
Curley, G. F., Ansari, B., Hayes, M., Devaney, J., Masterson, C., Ryan, A., et al. (2013). Effects of intratracheal mesenchymal stromal cell therapy during recovery and resolution after ventilator-induced lung injury. Anesthesiology, 118(4), 924–932. https://doi.org/10.1097/ALN.0b013e318287ba08.
Gupta, N., Krasnodembskaya, A., Kapetanaki, M., Mouded, M., Tan, X., Serikov, V., & Matthay, M. A. (2012). Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax, 67(6), 533–539. https://doi.org/10.1136/thoraxjnl-2011-201176.
Liang, Z.-X., Sun, J.-P., Wang, P., Tian, Q., Yang, Z., & Chen, L.-A. (2011). Bone marrow-derived mesenchymal stem cells protect rats from endotoxin-induced acute lung injury. Chinese medical journal, 124(17), 2715–2722 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22040430.
Kim, E. S., Chang, Y. S., Choi, S. J., Kim, J. K., Yoo, H. S., Ahn, S. Y., et al. (2011). Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Respiratory research, 12, 108. https://doi.org/10.1186/1465-9921-12-108.
Sun, J., Han, Z.-B., Liao, W., Yang, S. G., Yang, Z., Yu, J., et al. (2011). Intrapulmonary delivery of human umbilical cord mesenchymal stem cells attenuates acute lung injury by expanding CD4+CD25+ Forkhead Boxp3 (FOXP3)+ regulatory T cells and balancing anti- and pro-inflammatory factors. Cellular physiology and biochemistry: international journal of experimental cellular physiology, biochemistry, and pharmacology, 27(5), 587–596. https://doi.org/10.1159/000329980.
Lee, S.-H., Jang, A.-S., Kim, Y.-E., Cha, J.-Y., Kim, T.-H., Jung, S., et al. (2010). Modulation of cytokine and nitric oxide by mesenchymal stem cell transfer in lung injury/fibrosis. Respiratory research, 11, 16. https://doi.org/10.1186/1465-9921-11-16.
Gupta, N., Su, X., Popov, B., Lee, J, W., Serikov, V., & Matthay, M, A. (2007). Intrapulmonary Delivery of Bone Marrow-Derived Mesenchymal Stem Cells Improves Survival and Attenuates Endotoxin-Induced Acute Lung Injury in Mice. The Journal of Immunology. https://doi.org/10.4049/jimmunol.179.3.1855
Scheller, J., Chalaris, A., Schmidt-Arras, D., & Rose-John, S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochimica et biophysica acta, 1813(5), 878–888. https://doi.org/10.1016/j.bbamcr.2011.01.034.
Harrell, C. R., Markovic, B. S., Fellabaum, C., Arsenijevic, N., Djonov, V., & Volarevic, V. (2020). The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. BioFactors, 46(2), 263–275. https://doi.org/10.1002/biof.1587.
Kim, Y.-H., Cho, K.-A., Park, M., Kim, H. S., Park, J.-W., Woo, S.-Y., & Ryu, K.-H. (2019). Conditioned Medium from Tonsil-Derived Mesenchymal Stem Cells Relieves CCl-Induced Liver Fibrosis in Mice. Tissue engineering and regenerative medicine, 16(1), 51–58. https://doi.org/10.1007/s13770-018-0160-8.
Tai, W.-L., Dong, Z.-X., Zhang, D.-D., & Wang, D.-H. (2012). Therapeutic effect of intravenous bone marrow-derived mesenchymal stem cell transplantation on early-stage LPS-induced acute lung injury in mice. Nan fang yi ke da xue xue bao = Journal of Southern Medical University, 32(3), 283–290 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22445968.
Chang, Y. S., Choi, S. J., Sung, D. K., Kim, S. Y., Oh, W., Yang, Y. S., & Park, W. S. (2011). Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell transplantation, 20(11–12), 1843–1854. https://doi.org/10.3727/096368911X565038.
Lee, J, W., Fang, X., Gupta, N., Serikov, V., & Matthay, M, A. (2009). Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences. https://doi.org/10.1073/pnas.0907996106
Monsel, A., Zhu, Y, G., Gudapati, V., Lim, H., & Lee, J, W. (2016). Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opinion on Biological Therapy. https://doi.org/10.1517/14712598.2016.1170804
Gamble, J, R., Drew, J., Trezise, L., Underwood, A., Parsons, M., Kasminkas, L., … Vadas, M, A. (2000). Angiopoietin-1 Is an Antipermeability and Anti-Inflammatory Agent In Vitro and Targets Cell Junctions. Circulation Research. https://doi.org/10.1161/01.res.87.7.603
Birukova, A. A., Alekseeva, E., Mikaelyan, A., & Birukov, K. G. (2007). HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB journal: official publication of the Federation of American Societies for Experimental Biology, 21(11), 2776–2786. https://doi.org/10.1096/fj.06-7660com.
Yao, Y., Fan, X.-L., Jiang, D., Zhang, Y., Li, X., Xu, Z.-B., et al. (2018). Connexin 43-mediated mitochondrial transfer of iPSC-MSCs alleviates asthma inflammation. Stem cell reports, 11(5), 1120–1135. https://doi.org/10.1016/j.stemcr.2018.09.012.
Paliwal, S., Chaudhuri, R., Agrawal, A., & Mohanty, S. (2018). Regenerative abilities of mesenchymal stem cells through mitochondrial transfer. Journal of biomedical science, 25(1). https://doi.org/10.1186/s12929-018-0429-1.
Court, A. C., Le-Gatt, A., Luz-Crawford, P., Parra, E., Aliaga-Tobar, V., Bátiz, L. F., et al. (2020). Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO reports, 21(2), e48052. https://doi.org/10.15252/embr.201948052.
Seif, F., Aazami, H., Khoshmirsafa, M., Kamali, M., Mohsenzadegan, M., Pornour, M., & Mansouri, D. (2020). JAK Inhibition as a New Treatment Strategy for Patients with COVID-19. International archives of allergy and immunology, 181(6), 467–475. https://doi.org/10.1159/000508247.
Busse, L. W., Chow, J. H., McCurdy, M. T., & Khanna, A. K. (2020). COVID-19 and the RAAS-a potential role for angiotensin II? Critical care / the Society of Critical Care Medicine, 24(1), 136. https://doi.org/10.1186/s13054-020-02862-1.
Kuba, K., Imai, Y., Rao, S., Jiang, C., & Penninger, J. M. (2006). Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. Journal of molecular medicine, 84(10), 814–820. https://doi.org/10.1007/s00109-006-0094-9.
Kuba, K., Imai, Y., & Penninger, J. M. (2006). Angiotensin-converting enzyme 2 in lung diseases. Current opinion in pharmacology, 6(3), 271–276. https://doi.org/10.1016/j.coph.2006.03.001.
Mancia, G., Rea, F., Ludergnani, M., Apolone, G., & Corrao, G. (2020). Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. The New England journal of medicine, 382(25), 2431–2440. https://doi.org/10.1056/NEJMoa2006923.
Papinska, A. M., Soto, M., Meeks, C. J., & Rodgers, K. E. (2016). Long-term administration of angiotensin (1-7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacological research: the official journal of the Italian Pharmacological Society, 107, 372–380. https://doi.org/10.1016/j.phrs.2016.02.026.
Meng, Y., Yu, C.-H., Li, W., Li, T., Luo, W., Huang, S., et al. (2014). Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. American journal of respiratory cell and molecular biology, 50(4), 723–736. https://doi.org/10.1165/rcmb.2012-0451OC.
Chen, C.-M., & Chou, H.-C. (2018). Human mesenchymal stem cells attenuate hyperoxia-induced lung injury through inhibition of the renin-angiotensin system in newborn rats. American journal of translational research, 10(8), 2628–2635 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30210699.
Liu, Z., Liu, J., Xiao, M., Wang, J., Yao, F., Zeng, W., et al. (2018). Mesenchymal stem cell-derived microvesicles alleviate pulmonary arterial hypertension by regulating renin-angiotensin system. Journal of the American Society of Hypertension: JASH, 12(6), 470–478. https://doi.org/10.1016/j.jash.2018.02.006.
Shi, Y., Wang, Y., Shao, C., Huang, J., Gan, J., Huang, X., et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell death and differentiation, 27(5), 1451–1454. https://doi.org/10.1038/s41418-020-0530-3.
Johnson, P., Arif, A. A., Lee-Sayer, S. S. M., & Dong, Y. (2018). Hyaluronan and Its Interactions With Immune Cells in the Healthy and Inflamed Lung. Frontiers in immunology, 9, 2787. https://doi.org/10.3389/fimmu.2018.02787.
Meng, H., Xiong, R., He, R., Lin, W., Hao, B., Zhang, L., et al. (2020). CT imaging and clinical course of asymptomatic cases with COVID-19 pneumonia at admission in Wuhan, China. The Journal of infection, 81(1), e33–e39. https://doi.org/10.1016/j.jinf.2020.04.004.
Carsana, L., Sonzogni, A., Nasr, A., Rossi, R, S., Pellegrinelli, A., Zerbi, P., … Nebuloni, M. (2020). Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. The Lancet infectious diseases. https://doi.org/10.1016/S1473-3099(20)30434-5
Ding, M., Zhang, Q., Li, Q., Wu, T., & Huang, Y.-Z. (2020). Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respiratory medicine, 167, 105981. https://doi.org/10.1016/j.rmed.2020.105981.
Fang, X., Neyrinck, A. P., Matthay, M. A., & Lee, J. W. (2010). Allogeneic human mesenchymal stem cells restore epithelial protein permeability in cultured human alveolar type II cells by secretion of angiopoietin-1. The Journal of biological chemistry, 285(34), 26211–26222. https://doi.org/10.1074/jbc.M110.119917.
Murakami, M., Nguyen, L. T., Zhuang, Z. W., Moodie, K. L., Carmeliet, P., Stan, R. V., & Simons, M. (2008). The FGF system has a key role in regulating vascular integrity. The Journal of clinical investigation, 118(10), 3355–3366. https://doi.org/10.1172/JCI35298.
Mei, S. H. J., McCarter, S. D., Deng, Y., Parker, C. H., Liles, W. C., & Stewart, D. J. (2007). Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS medicine, 4(9), e269. https://doi.org/10.1371/journal.pmed.0040269.
McCarter, S. D., Mei, S. H. J., Lai, P. F. H., Zhang, Q. W., Parker, C. H., Suen, R. S., et al. (2007). Cell-based angiopoietin-1 gene therapy for acute lung injury. American journal of respiratory and critical care medicine, 175(10), 1014–1026. https://doi.org/10.1164/rccm.200609-1370OC.
Lee, J. W., Fang, X., Krasnodembskaya, A., Howard, J. P., & Matthay, M. A. (2011). Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem cells, 29(6), 913–919. https://doi.org/10.1002/stem.643.
Potter, D. R., Miyazawa, B. Y., Gibb, S. L., Deng, X., Togaratti, P. P., Croze, R. H., et al. (2018). Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. The journal of trauma and acute care surgery, 84(2), 245–256. https://doi.org/10.1097/TA.0000000000001744.
Wang, H., Zheng, R., Chen, Q., Shao, J., Yu, J., & Hu, S. (2017). Mesenchymal stem cells microvesicles stabilize endothelial barrier function partly mediated by hepatocyte growth factor (HGF). Stem cell research & therapy, 8(1), 211. https://doi.org/10.1186/s13287-017-0662-7.
Hu, S., Park, J., Liu, A., Lee, J., Zhang, X., Hao, Q., & Lee, J.-W. (2018). Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem cells translational medicine, 7(8), 615–624. https://doi.org/10.1002/sctm.17-0278.
Guery, B. P., Mason, C. M., Dobard, E. P., Beaucaire, G., Summer, W. R., & Nelson, S. (1997). Keratinocyte growth factor increases transalveolar sodium reabsorption in normal and injured rat lungs. American journal of respiratory and critical care medicine, 155(5), 1777–1784. https://doi.org/10.1164/ajrccm.155.5.9154891.
Wang, Y., Folkesson, H. G., Jayr, C., Ware, L. B., & Matthay, M. A. (1999). Alveolar epithelial fluid transport can be simultaneously upregulated by both KGF and beta-agonist therapy. Journal of applied physiology, 87(5), 1852–1860. https://doi.org/10.1152/jappl.1999.87.5.1852.
Gennai, S., Monsel, A., Hao, Q., Park, J., Matthay, M. A., & Lee, J. W. (2015). Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 15(9), 2404–2412. https://doi.org/10.1111/ajt.13271.
Helms, J., Tacquard, C., Severac, F., Leonard-Lorant, I., Ohana, M., Delabranche, X., et al. (2020). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive care medicine, 46(6), 1089–1098. https://doi.org/10.1007/s00134-020-06062-x.
Klok, F. A., Kruip, M. J. H. A., van der Meer, N. J. M., Arbous, M. S., Gommers, D. A. M. P. J., Kant, K. M., et al. (2020). Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis research, 191, 145–147. https://doi.org/10.1016/j.thromres.2020.04.013.
Klok, F. A., Kruip, M. J. H. A., van der Meer, N. J. M., Arbous, M. S., Gommers, D., Kant, K. M., et al. (2020). Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thrombosis research, 191, 148–150. https://doi.org/10.1016/j.thromres.2020.04.041.
Hamming, I., Timens, W., Bulthuis, M. L. C., Lely, A. T., Navis, G. J., & van Goor, H. (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. The Journal of pathology, 203(2), 631–637. https://doi.org/10.1002/path.1570.
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A. S., et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. The Lancet, 395(10234), 1417–1418. https://doi.org/10.1016/S0140-6736(20)30937-5.
Luo, W., Yu, H., Gou, J., Li, X., Sun, Y., Li, J., & Liu, L. (2020). Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints, 2020, 2020020407. Retrieved from https://www.researchgate.net/profile/Weiren_Luo/publication/339939319_Clinical_Pathology_of_Critical_Patient_with_Novel_Coronavirus_Pneumonia_COVID-19_First_Case_of_the_Whole_Lung_Biopsy/links/5e888de14585150839befe5d/Clinical-Pathology-of-Critical-Patient-with-Novel-Coronavirus-Pneumonia-COVID-19-First-Case-of-the-Whole-Lung-Biopsy.pdf
Levi, M., Thachil, J., Iba, T., & Levy, J. H. (2020). Coagulation abnormalities and thrombosis in patients with COVID-19. The Lancet. Haematology, 7(6), e438–e440. https://doi.org/10.1016/S2352-3026(20)30145-9.
Peyvandi, F., Garagiola, I., & Baronciani, L. (2011). Role of von Willebrand factor in the haemostasis. Blood transfusion = Trasfusione del sangue, 9(Suppl 2), s3–s8. https://doi.org/10.2450/2011.002S.
Liao, D., Zhou, F., Luo, L., Xu, M., Wang, H., Xia, J., … Hu, Y. (2020). Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID-19: a retrospective cohort study. The Lancet. Haematology. https://doi.org/10.1016/S2352-3026(20)30217-9
Wang, B., Wu, S., Wang, T., Ma, Z., & Liu, K. (2017). Bone Marrow-Derived Mesenchymal Stem Cells-Mediated Protection Against Organ Dysfunction in Disseminated Intravascular Coagulation Is Associated With Peripheral Immune Responses. Journal of cellular biochemistry, 118(10), 3184–3192. https://doi.org/10.1002/jcb.25964.
Wang, B., Wu, S.-M., Wang, T., Liu, K., Zhang, G., Zhang, X.-Q., et al. (2012). Pre-treatment with bone marrow-derived mesenchymal stem cells inhibits systemic intravascular coagulation and attenuates organ dysfunction in lipopolysaccharide-induced disseminated intravascular coagulation rat model. Chinese medical journal, 125(10), 1753–1759 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22800895.
Zhang, J., Kong, X., Jin, X., Gao, P., Wang, M., & Yang, L. (2019). Bone marrow stromal cells transplantation promotes the resolution and recanalization of deep vein thrombosis in rabbits through regulating macrophage infiltration and angiogenesis. Journal of cellular biochemistry. https://doi.org/10.1002/jcb.28447
Pelizzo, G., Avanzini, M. A., Mantelli, M., Croce, S., Maltese, A., Vestri, E., et al. (2018). Granulation tissue-derived mesenchymal stromal cells: a potential application for burn wound healing in pediatric patients. Journal of stem cells & regenerative medicine, 14(1), 53–58 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/30018473.
de Windt, T. S., Vonk, L. A., Slaper-Cortenbach, I. C. M., van den Broek, M. P. H., Nizak, R., van Rijen, M. H. P., et al. (2017). Allogeneic Mesenchymal Stem Cells Stimulate Cartilage Regeneration and Are Safe for Single-Stage Cartilage Repair in Humans upon Mixture with Recycled Autologous Chondrons. Stem cells, 35(1), 256–264. https://doi.org/10.1002/stem.2475.
Rohaina, C. M., Then, K. Y., Ng, A. M. H., Wan Abdul Halim, W. H., Zahidin, A. Z. M., Saim, A., & Idrus, R. B. H. (2014). Reconstruction of limbal stem cell deficient corneal surface with induced human bone marrow mesenchymal stem cells on amniotic membrane. Translational research: the journal of laboratory and clinical medicine, 163(3), 200–210. https://doi.org/10.1016/j.trsl.2013.11.004.
Macchiarini, P., Jungebluth, P., Go, T., Asnaghi, M. A., Rees, L. E., Cogan, T. A., et al. (2008). Clinical transplantation of a tissue-engineered airway. The Lancet, 372(9655), 2023–2030. https://doi.org/10.1016/S0140-6736(08)61598-6.
Fan, L., Yu, Z., Li, J., Dang, X., & Wang, K. (2014). Schwann-like cells seeded in acellular nerve grafts improve nerve regeneration. BMC musculoskeletal disorders, 15, 165. https://doi.org/10.1186/1471-2474-15-165.
Westhauser, F., Senger, A.-S., Reible, B., & Moghaddam, A. (2017). In Vivo Models for the Evaluation of the Osteogenic Potency of Bone Substitutes Seeded with Mesenchymal Stem Cells of Human Origin: A Concise Review. Tissue engineering. Part C, Methods, 23(12), 881–888. https://doi.org/10.1089/ten.TEC.2017.0164.
Li, Y., Shi, X., Yang, L., Mou, Y., Li, Y., Dang, R., & Li, C. (2017). Hypoxia promotes the skewed differentiation of umbilical cord mesenchymal stem cells toward type II alveolar epithelial cells by regulating microRNA-145. Gene, 630, 68–75. https://doi.org/10.1016/j.gene.2017.08.006.
Li, X., Wang, Y., An, G., Liang, D., Zhu, Z., Lian, X., et al. (2017). Bone marrow mesenchymal stem cells attenuate silica-induced pulmonary fibrosis via paracrine mechanisms. Toxicology letters, 270, 96–107. https://doi.org/10.1016/j.toxlet.2017.02.016.
Akram, K. M., Samad, S., Spiteri, M. A., & Forsyth, N. R. (2013). Mesenchymal stem cells promote alveolar epithelial cell wound repair in vitro through distinct migratory and paracrine mechanisms. Respiratory research, 14, 9. https://doi.org/10.1186/1465-9921-14-9.
Chen, S., Cui, G., Peng, C., Lavin, M. F., Sun, X., Zhang, E., et al. (2018). Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem cell research & therapy, 9(1), 110. https://doi.org/10.1186/s13287-018-0846-9.
Kim, S.-Y., Lee, J.-H., Kim, H. J., Park, M. K., Huh, J. W., Ro, J. Y., et al. (2012). Mesenchymal stem cell-conditioned media recovers lung fibroblasts from cigarette smoke-induced damage. American journal of physiology. Lung cellular and molecular physiology, 302(9), L891–L908. https://doi.org/10.1152/ajplung.00288.2011.
Weiss, D. J. (2014). Concise review: current status of stem cells and regenerative medicine in lung biology and diseases. Stem cells, 32(1), 16–25. https://doi.org/10.1002/stem.1506.
Guo, M., Sun, Z., Sun, Q.-Y., Han, Q., Yu, C.-L., Wang, D.-H., et al. (2009). A modified haploidentical nonmyeloablative transplantation without T cell depletion for high-risk acute leukemia: successful engraftment and mild GVHD. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation, 15(8), 930–937. https://doi.org/10.1016/j.bbmt.2009.04.006.
Abreu, S. C., Antunes, M. A., Maron-Gutierrez, T., Cruz, F. F., Ornellas, D. S., Silva, A. L., et al. (2013). Bone marrow mononuclear cell therapy in experimental allergic asthma: intratracheal versus intravenous administration. Respiratory physiology & neurobiology, 185(3), 615–624. https://doi.org/10.1016/j.resp.2012.11.005.
Filoche, M., Tai, C.-F., & Grotberg, J. B. (2015). Three-dimensional model of surfactant replacement therapy. Proceedings of the National Academy of Sciences of the United States of America, 112(30), 9287–9292. https://doi.org/10.1073/pnas.1504025112.
Halpern, D., Fujioka, H., Takayama, S., & Grotberg, J. B. (2008). Liquid and surfactant delivery into pulmonary airways. Respiratory physiology & neurobiology, 163(1–3), 222–231. https://doi.org/10.1016/j.resp.2008.05.012.
Kim, J., Guenthart, B., O’Neill, J. D., Dorrello, N. V., Bacchetta, M., & Vunjak-Novakovic, G. (2017). Controlled delivery and minimally invasive imaging of stem cells in the lung. Scientific reports, 7(1), 13082. https://doi.org/10.1038/s41598-017-13280-9.
Moreira, A., Winter, C., Joy, J., Winter, L., Jones, M., Noronha, M., et al. (2020). Intranasal delivery of human umbilical cord Wharton’s jelly mesenchymal stromal cells restores lung alveolarization and vascularization in experimental bronchopulmonary dysplasia. Stem cells translational medicine, 9(2), 221–234. https://doi.org/10.1002/sctm.18-0273.
Fung, M. E., & Thébaud, B. (2014). Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatric research, 75(1–1), 2–7. https://doi.org/10.1038/pr.2013.176.
Zhu, X., Badawi, M., Pomeroy, S., Sutaria, D. S., Xie, Z., Baek, A., et al. (2017). Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. Journal of extracellular vesicles, 6(1), 1324730. https://doi.org/10.1080/20013078.2017.1324730.
Bari, E., Ferrarotti, I., Saracino, L., Perteghella, S., Torre, M. L., & Corsico, A. G. (2020). Mesenchymal Stromal Cell Secretome for Severe COVID-19 Infections: Premises for the Therapeutic Use. Cells. https://doi.org/10.3390/cells9040924.
Hayes, M., Curley, G, F., Masterson, C., Devaney, J., O’Toole, D., & Laffey, J, G. (2015). Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Medicine Experimental. https://doi.org/10.1186/s40635-015-0065-y
Johnson, C. L., Soeder, Y., & Dahlke, M. H. (2017). Concise Review: Mesenchymal Stromal Cell-Based Approaches for the Treatment of Acute Respiratory Distress and Sepsis Syndromes. Stem cells translational medicine, 6(4), 1141–1151. https://doi.org/10.1002/sctm.16-0415.
Park, J, S., Suryaprakash, S., Lao, Y, H., & Leong, K, W. (2015). Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods. https://doi.org/10.1016/j.ymeth.2015.03.002
Ren, C., Kumar, S., Chanda, D., Chen, J., Mountz, J. D., & Ponnazhagan, S. (2008). Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem cells, 26(9), 2332–2338. https://doi.org/10.1634/stemcells.2008-0084.
Chen, X., Wang, K., Chen, S., & Chen, Y. (2019). Effects of mesenchymal stem cells harboring the Interferon-β gene on A549 lung cancer in nude mice. Pathology, research and practice, 215(3), 586–593. https://doi.org/10.1016/j.prp.2019.01.013.
Jalkanen, J., Hollmén, M., & Jalkanen, S. (2020). Interferon beta-1a for COVID-19: critical importance of the administration route. Critical care (London, England), 24(1), 335. https://doi.org/10.1186/s13054-020-03048-5.
Nile, S. H., Nile, A., Qiu, J., Li, L., Jia, X., & Kai, G. (2020). COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine & growth factor reviews, 53, 66–70. https://doi.org/10.1016/j.cytogfr.2020.05.002.
Yin, X.-X., Zheng, X.-R., Peng, W., Wu, M.-L., & Mao, X.-Y. (2020). Vascular endothelial growth factor (VEGF) as a vital target for brain inflammation during the COVID-19 outbreak. ACS chemical neuroscience, 11(12), 1704–1705. https://doi.org/10.1021/acschemneuro.0c00294.
Li, G., Miao, F., Zhu, J., & Chen, Y. (2017). Anti-angiogenesis gene therapy for hepatocellular carcinoma via systemic injection of mesenchymal stem cells engineered to secrete soluble Flt-1. Molecular medicine reports, 16(5), 5799–5806. https://doi.org/10.3892/mmr.2017.7310.
Matthay, M. A. (2015). Therapeutic Potential of Mesenchymal Stromal Cells for Acute Respiratory Distress Syndrome. Annals of the American Thoracic Society, 12(Suppl 1), S54. https://doi.org/10.1513/AnnalsATS.201406-254MG.
Loy, H., Kuok, D. I. T., Hui, K. P. Y., Choi, M. H. L., Yuen, W., Nicholls, J. M., et al. (2019). Therapeutic Implications of Human Umbilical Cord Mesenchymal Stromal Cells in Attenuating Influenza A(H5N1) Virus–Associated Acute Lung Injury. The Journal of infectious diseases, 219(2), 186. https://doi.org/10.1093/infdis/jiy478.
Search of: mesenchymal stem cell. (n.d.). Retrieved August 27, 2020, from https://clinicaltrials.gov/ct2/results?cond=Covid19&term=mesenchymal+stem+cell&cntry=&state=&city=&dist=
Schäfer, R., Spohn, G., Bechtel, M., Bojkova, D., Baer, P, C., Kuçi, S., … Cinatl, J. (2020). Human mesenchymal stromal cells are resistant to SARS-CoV-2 infection under steady-state, inflammatory conditions and in the presence of SARS-CoV-2-infected cells. Stem cell reports. https://doi.org/10.1016/j.stemcr.2020.09.003
Cao, Y., Wu, H., Zhai, W., Wang, Y., Li, M., Li, M., et al. (2020). A safety consideration of mesenchymal stem cell therapy on COVID-19. Stem cell research, 49(102066), 102066. https://doi.org/10.1016/j.scr.2020.102066.
Khoury, M., Cuenca, J., Cruz, F. F., Figueroa, F. E., Rocco, P. R. M., & Weiss, D. J. (2020). Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology, 55(6), 2000858. https://doi.org/10.1183/13993003.00858-2020.
Bedford, P., Jy, J., Collins, L., & Keizer, S. (2018). Considering cell therapy product “good manufacturing practice” status. Frontiers in medicine, 5, 118. https://doi.org/10.3389/fmed.2018.00118.
Liang, B., Chen, J., Li, T., Wu, H., Yang, W., Li, Y., et al. (2020). Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine, 99(31), e21429. https://doi.org/10.1097/MD.0000000000021429.
Leng, Z., Zhu, R., Hou, W., Feng, Y., Yang, Y., Han, Q., et al. (2020). Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and disease, 11(2), 216–228. https://doi.org/10.14336/AD.2020.0228.
Guo, Z., Chen, Y., Luo, X., He, X., Zhang, Y., & Wang, J. (2020). Administration of umbilical cord mesenchymal stem cells in patients with severe COVID-19 pneumonia. Critical care, 24(1), 1–3. https://doi.org/10.1186/s13054-020-03142-8.
Meng, F., Xu, R., Wang, S., Xu, Z., Zhang, C., Li, Y., et al. (2020). Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal transduction and targeted therapy, 5(1), 172. https://doi.org/10.1038/s41392-020-00286-5.
Fung, M., & Babik, J, M. (2020). COVID-19 in immunocompromised hosts: What we know so far. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. https://doi.org/10.1093/cid/ciaa863
Hartono, C., Muthukumar, T., & Suthanthiran, M. (2013). Immunosuppressive drug therapy. Cold Spring Harbor perspectives in medicine, 3(9), a015487. https://doi.org/10.1101/cshperspect.a015487.
Zhao, Y., Jiang, Z., Zhao, T., Ye, M., Hu, C., Zhou, H., et al. (2013). Targeting insulin resistance in type 2 diabetes via immune modulation of cord blood-derived multipotent stem cells (CB-SCs) in stem cell educator therapy: phase I/II clinical trial. BMC medicine, 11(1), 160. https://doi.org/10.1186/1741-7015-11-160.
Zhang, Z., Fu, J., Xu, X., Wang, S., Xu, R., Zhao, M., et al. (2013). Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS (London, England), 27(8), 1283–1293. https://doi.org/10.1097/QAD.0b013e32835fab77.
Gao, Y., Cai, G.-Y., Fang, W., Li, H.-Y., Wang, S.-Y., Chen, L., et al. (2020). Machine learning based early warning system enables accurate mortality risk prediction for COVID-19. Nature communications, 11(1), 5033. https://doi.org/10.1038/s41467-020-18684-2.
Acknowledgements
The authors would also like to express their most sincere words of appreciation to Dr. Amirhossein Asgari and Dr. Mehran Rashidi for their valuable contribution.
Funding
This work was supported by the Pharmacology Department of School of Medicine, Shahid Beheshti University of Medical Sciences. In addition, it was supported by the National Institutes for Medical Research Development (NIMAD), Theran, Iran (963951).
Author information
Authors and Affiliations
Contributions
AB, EJ, and HN contributed to the conception. AB, PS, and EJ reviewed the articles and wrote the original draft. HN, AB and EJ contributed to the manuscript revision and editing. All the authors read and approved the submitted version.
Corresponding author
Ethics declarations
Consent to Publish
The Authors grant the Publisher the sole and exclusive license of the full copyright in the Contribution, which license the Publisher hereby accepts. Consequently, the Publisher shall have the exclusive right throughout the world to publish and sell the Contribution in all languages.
Competing Interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elham Jamshidi, Amirhesam Babajani and Pegah Soltani share the first authorship
Supplementary Information
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Jamshidi, E., Babajani, A., Soltani, P. et al. Proposed Mechanisms of Targeting COVID-19 by Delivering Mesenchymal Stem Cells and Their Exosomes to Damaged Organs. Stem Cell Rev and Rep 17, 176–192 (2021). https://doi.org/10.1007/s12015-020-10109-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10109-3