SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs
Abstract
:1. Introduction
2. Virus Characteristics and Pathogenic Mechanisms
3. Diagnostics and Screening
- (1)
- RNA detection test through nucleic acid amplification by RT-PCR procedure.
- (2)
- Antigen tests based on the detection of a specific surface protein viral antigen.
- (3)
- Antibody tests based on the detection of specific antibodies against SARS-CoV-2.
- The molecular test detects the presence of the genome of SARS-CoV-2 with the material contained in the swabs, while the antigenic one detects traces of its antigen (the Spike glycoprotein).
- The antigenic test is performed on a swab, for example oropharyngeal or nasal mucosa, by detecting the presence of the Spike glycoproteins (S) specific to SARS-CoV-2. However, this could result in false negatives, and does not mean that the virus is inactive. Therefore, it is apt to repeat the test a few days later [58].
3.1. Molecular Tests
- In the reaction, an enzymatic stabilizer is used, which allows to premix everything and therefore the operator does not need help preparing the mix for the PCR;
- The primer selected aims at the short fragment and this may allow to reduce the time for the PCR stage to less than 1 h;
- The cycle of amplification of RNA target of several Coronaviruses occurs in an amplification process, “One-Step”, through MPL1 and MPL2. In this single process, both false negatives and positives are highly identified [10].
3.1.1. Test Sampling
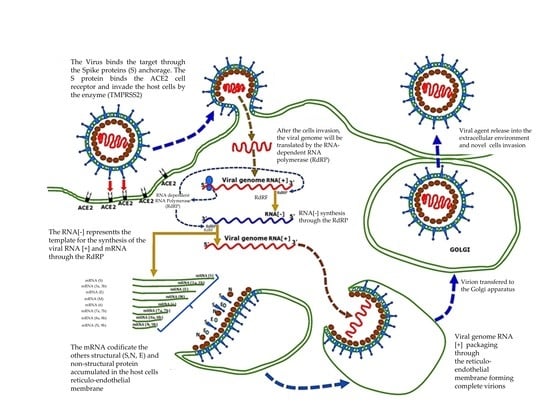
3.1.2. Receptors Mechanism and Interaction
4. Vaccines
4.1. Immunity Risk Factors
4.2. Vaccines Characteristics
- (1)
- mRNA Vaccine: a sequence of RNA is synthetized in a laboratory and injected into humans. It stimulates the synthesis of the protein, prompting the immune system to produce antibodies that will then act against the virus.
- (2)
- DNA Vaccine: similar to the RNA vaccine—a fragment of DNA is synthetized, which induces cells to produce proteins against which we require an antibody response and subsequent immune response.
- (3)
- Protein Vaccine: in a laboratory, we use sequences of the RNA of the virus to synthetize proteins or fragments of proteins of the viral capsid. Injecting it into the body, together with substances that stimulate an immune response, produces antibodies that prevent respiratory symptoms [99].
- (1)
- Sponsor: Pfizer, Identification Number: BNT162b2, Development: Germany [107].
- (2)
- Sponsor: Modernatx, Inc; mRNA-1273 Identification N.: NCT04470427 Development: United States. News [108].
- (3)
- Sponsor: University of Oxford; ChAdOx1 nCoV-19 Identification N.: NCT04400838 Development: United Kingdom [109].
- (4)
- Ad26.COV2. S di Janssen Johnson & Johnson Corp.
- (5)
- Sputnik V-Gam-COVID-Vac di Gamaleya Res. Institute. Russia.
- The minimum protective neutralizing antibody titer (MPNAT); that is the minimum level of antibodies sufficient to protect the patient from infection (at 24 months).
- Number of SARS-CoV-2 positive and confirmed patients (from first vaccination up to 24 months).
- Reports of participants with local and systemic reactions to vaccination (from the first vaccine up to day 90) will be collected.
- Data will be collected from participants who experienced grade 3 and 4 adverse events and any serious adverse events (from the first vaccine up to day 90).
4.3. Pfizer Vaccine
4.4. Moderna Vaccine (COVID-19 mRNA-1273 Vaccine)
4.5. Astrazeneca (Oxford)
4.6. Janssen Single Dose Vaccine
4.7. Sputnik V (GAM-COVID-VAC)
4.8. Coronavac/Sinovac
- The number of local and systemic adverse reactions in the first 7 days after vaccination by age group (18–59 years and 60 years or older), attributed to the vaccine.
- Seroconversion rates (two weeks after the second immunization for age group 18–59 years and 60 years or older).
- Frequency of local and systemic adverse reactions up to 28 days after the second dose by age group (18–59 years and 60 years or older), attributed to the vaccine.
- Frequency of serious adverse events up to 12 months after the first dose.
- Cell-mediated immune response (from administration up to 4 weeks after the second vaccination).
- Detection and titration of antibodies against SARS-CoV-2 (from administration, two weeks, 6 months, 12 months, 18 months, and 24 months after the second vaccination).
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- COVID-19 Map. Available online: https://coronavirus.jhu.edu/map.html (accessed on 4 January 2021).
- Santacroce, L.; Bottalico, L.; Charitos, I.A. The impact of COVID-19 on Italy: A lesson for the future. Int. J. Occup. Environ. Med. 2020, 11, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, G.; De Vito, D.; Rengo, S.; Tatullo, M. COVID-19 outbreak: An overview on dentistry. Int. J. Environ. Res. Public Health 2020, 17, 2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Catucci, O.; Amatulli, F.; Cefalo, A.; Lazzaro, R.; Aityan, K.S.; Dalagni, G.; Nico, A.; de Michele, A.; et al. Clinical and diagnostic findings in COVID-19 patients: An original research from SG Moscati Hospital in Taranto Italy. J. Biol. Regul. Homeost. Agents 2021, 35, 171–183. [Google Scholar] [PubMed]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential beneficial role of probiotics on the outcome of COVID-19 patients: An evolving perspective. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 295–301. [Google Scholar] [CrossRef]
- Bellocchio, L.; Bordea, I.; Ballini, A.; Lorusso, F.; Hazballa, D.; Isacco, C.; Malcangi, G.; Inchingolo, A.; Dipalma, G.; Inchingolo, F.; et al. Environmental Issues and Neurological Manifestations Associated with COVID-19 Pandemic: New Aspects of the Disease? Int. J. Environ. Res. Public Health 2020, 17, 8049. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Bottalico, L.; Cantore, S.; Passarelli, P.C.; Inchingolo, F.; D’Addona, A.; Santacroce, L. Special features of SARS-CoV-2 in daily practice. World J. Clin. Cases 2020, 8, 3920–3933. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Scarano, A. The impact of the novel Covid-19 on the scientific production spread: A five-month bibliometric report of the worldwide research community. Acta Med. Mediterr. 2020, 36, 1–4. [Google Scholar]
- Pham, V.H.; Gargiulo Isacco, C.; Nguyen, K.C.D.; Le, S.H.; Tran, D.K.; Nguyen, Q.V.; Pham, H.T.; Aityan, S.; Pham, S.T.; Cantore, S.; et al. Rapid and sensitive diagnostic procedure for multiple detection of pandemic coronaviridae family members SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV: A translational research and cooperation between the Phan Chau Trinh University in Vietnam and university of Bari “Aldo Moro” in Italy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7173–7191. [Google Scholar] [CrossRef]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial skin temperature and discomfort when wearing protective face masks: Thermal infrared imaging evaluation and hands moving the mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef]
- Bordea, I.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.; Malcangi, G.; Pham, V.; Inchingolo, A.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) pandemic: Future challenges for dental practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://Covid19.WHO.int (accessed on 19 December 2020).
- Davenne, E.; Giot, J.B.; Huynen, P. Coronavirus and COVID-19: Focus on a galopping pandemic. Revue Med. Liege 2020, 75, 218–225. [Google Scholar]
- Vimercati, L.; De Maria, L.; Quarato, M.; Caputi, A.; Stefanizzi, P.; Gesualdo, L.; Migliore, G.; Fucilli, F.I.M.; Cavone, D.; Delfino, M.C.; et al. COVID-19 hospital outbreaks: Protecting healthcare workers to protect frail patients. An Italian observational cohort study. Int. J. Infect. Dis. 2021, 102, 532–537. [Google Scholar] [CrossRef]
- Charitos, I.A.; Del Prete, R.; Inchingolo, F.; Mosca, A.; Carretta, D.; Ballini, A.; Santacroce, L. What we have learned for the future about COVID-19 and healthcare management of it? Acta Biomed. 2020, 91, e2020126. [Google Scholar]
- Fan, Y.; Zhao, K.; Shi, Z.-L.; Zhou, P. Bat Coronaviruses in China. Viruses 2019, 11, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Santacroce, L.; Charitos, I.A.; Del Prete, R. COVID-19 in Italy: An overview from the first case to date. Electron. J. Gen. Med. 2020, 17, em235. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zhao, J.; Peng, J.; Li, X.; Deng, X.; Geng, Z.; Shen, Z.; Guo, F.; Zhang, Q.; Jin, Y.; et al. Detection of 2019-nCoV in saliva and characterization of oral symptoms in COVID-19 patients. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Yuen, K.-S.; Ye, Z.-W.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. SARS-CoV-2 and COVID-19: The most important research questions. Cell Biosci. 2020, 10, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology 2020, 296, E32–E40. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2017, 23, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.-W.; Yuan, S.; Kok, K.-H.; To, K.K.-W.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.J.; Shan, J. 2019 Novel coronavirus: Where we are and what we know. Infection 2020, 48, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Conti, P. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents 2020, 34, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and functional basis of SARS-CoV-2 entry by using human ACE. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef] [PubMed]
- Giudice, R.L.; Giudice, R.L. The Severe acute respiratory syndrome coronavirus-2 (SARS CoV-2) in dentistry. Management of biological risk in dental practice. Int. J. Environ. Res. Public Health 2020, 17, 3067. [Google Scholar] [CrossRef]
- Manea, A.; Crisan, D.; Baciut, G.; Baciut, M.; Bran, S.; Armencea, G.; Crisan, M.; Colosi, H.; Colosi, I.; Vodnar, D. The importance of atmospheric microbial contamination control in dental offices: Raised awareness caused by the SARS-CoV-2 pandemic. Appl. Sci. 2021, 11, 2359. [Google Scholar] [CrossRef]
- Korber, B.; Fischer, W.M.; Gnanakaran, S. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. BioRxiv 2020. [Google Scholar] [CrossRef]
- Wong, G.; Liu, W.; Liu, Y.; Zhou, B.; Bi, Y.; Gao, G.F. MERS, SARS, and Ebola: The role of super-spreaders in infectious disease. Cell Host Microbe 2015, 18, 398–401. [Google Scholar] [CrossRef] [Green Version]
- Wahba, L.; Jain, N.; Fire, A.Z.; Shoura, M.J.; Artiles, K.L.; McCoy, M.J.; Jeong, D.-E. Identification of a pangolin niche for a 2019-NCoV-like coronavirus via an extensive meta-metagenomic search. Bioinformatics 2020. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.F.; To, K.K.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.-R.; Cao, Q.-D.; Hong, Z.-S.; Tan, Y.-Y.; Chen, S.-D.; Jin, H.-J.; Tan, K.-S.; Wang, D.-Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med Res. 2020, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Xu, Y.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective face masks: Effect on the oxygenation and heart rate status of oral surgeons during surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.L.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of Pro-Inflammatory Cytokines (IL-1 and IL-6) and Lung Inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 1. [Google Scholar] [PubMed]
- Inchingolo, F.; Martelli, F.S.; Isacco, C.G.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.; De Vito, D.; Aityan, S.K.; et al. Chronic periodontitis and immunity, towards the implementation of a personalized medicine: A translational research on gene Single Nucleotide Polymorphisms (SNPs) linked to chronic oral dysbiosis in 96 caucasian patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Coutard, B.; Valle, C.; De Lamballerie, X.; Canard, B.; Seidah, N.; Decroly, E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antivir. Res. 2020, 176, 104742. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, P.; Wang, J.; Feng, J.; Zhou, H.; Li, X.; Zhong, W.; Hao, P. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020, 63, 457–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Liu, M.; Wang, C.; Xu, W.; Lan, Q.; Feng, S.; Qi, F.; Bao, L.; Du, L.; Liu, S.; et al. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020, 30, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-E.; Li, K.; Barlan, A.; Fehr, A.R.; Perlman, S.; McCray, J.P.B.; Gallagher, T. Proteolytic processing of Middle East respiratory syndrome coronavirus spikes expands virus tropism. Proc. Natl. Acad. Sci. USA 2016, 113, 12262–12267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J. Virol. 2011, 86, 2856–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lippi, G.; Simundic, A.-M.; Plebani, M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19). Clin. Chem. Lab. Med. 2020, 58, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Byrd, A.L.; Park, M.; Kong, H.H.; Segre, J.A. Temporal stability of the human skin microbiome. Cell 2016, 165, 854–866. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Wang, M.; Zuo, Z.; Fan, C.; Ye, F.; Cai, Z.; Wang, Y.; Cui, H.; Pan, K.; Xu, A. Diagnostic value and dynamic variance of serum antibody in coronavirus disease. Int. J. Infect. Dis. 2020, 94, 49–52. [Google Scholar] [CrossRef]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. Laboratory diagnosis of COVID-19: Current issues and challenges. J. Clin. Microbiol. 2020, 58, e00512-20. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Cruz, C.S.D.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [Green Version]
- Algaissi, A.; AlFaleh, M.A.; Hala, S.; Abujamel, T.S.; Alamri, S.S.; Almahboub, S.A.; Alluhaybi, K.A.; Hobani, H.I.; Alsulaiman, R.M.; Alharbi, R.H.; et al. SARS-CoV-2 S1 and N-based serological assays reveal rapid seroconversion and induction of specific antibody response in COVID-19 patients. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Gillot, C.; Douxfils, J.; Cadrobbi, J.; Laffineur, K.; Dogné, J.-M.; Elsen, M.; Eucher, C.; Melchionda, S.; Modaffarri, É.; Tré-Hardy, M.; et al. An original ELISA-based multiplex method for the simultaneous detection of 5 SARS-CoV-2 IgG antibodies directed against different antigens. J. Clin. Med. 2020, 9, 3752. [Google Scholar] [CrossRef]
- Chirico, F.; Sacco, A.; Bragazzi, N.L.; Magnavita, N. Can Air-Conditioning Systems Contribute to the Spread of SARS/MERS/COVID-19 Infection? Insights from a Rapid Review of the Literature. Int. J. Environ. Res. Public Health 2020, 17, 6052. [Google Scholar] [CrossRef]
- Marcone, V. Reduction of Contagion Risks by SARS-Cov-2 (COVID-19) in Air-Conditioned Work Environ-ments. Pain Phys. 2020, 23, S475–S482. [Google Scholar] [CrossRef]
- Caruso, A.A.; Del Prete, A.; Lazzarino, A.I. Hydrogen peroxide and viral infections: A literature review with research hypothesis definition in relation to the current covid-19 pandemic. Med. Hypotheses 2020, 144, 109910. [Google Scholar] [CrossRef] [PubMed]
- Chinese Researchers Develop Rapid Antibody Test Kit for Coronavirus—Xinhua | english.news.cn. Available online: http://www.xinhuanet.com/english/2020-02/21/c_138805748.htm (accessed on 4 January 2021).
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Abbott Launches COVID-19 Antibody Test | Abbott Newsroom. Available online: https://www.abbott.com/corpnewsroom/diagnostics-testing/abbott-launches-covid-19-antibody-test.html (accessed on 4 January 2021).
- Taking COVID-19 Testing to a New Level | Abbott U.S. Available online: https://www.abbott.com/binaxnow-test-navica-app.html#/ (accessed on 4 January 2021).
- Ji, T.; Liu, Z.; Wang, G.; Guo, X.; Khan, S.A.; Lai, C.; Chen, H.; Huang, S.; Xia, S.; Chen, B.; et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens. Bioelectron. 2020, 166, 112455. [Google Scholar] [CrossRef] [PubMed]
- Detect COVID-19 in as Little as 5 Minutes | Abbott Newsroom. Available online: https://www.abbott.com/corpnewsroom/diagnostics-testing/detect-covid-19-in-as-little-as-5-minutes.html (accessed on 4 January 2021).
- Sweet, L. Abbott Rapid COVID-19 Test May Yield False Negatives, FDA Warns. Available online: https://chicago.suntimes.com/coronavirus/2020/5/14/21258893/abbott-labs-covid19-tests-white-house-new-york-university (accessed on 4 January 2021).
- Basu, A.; Zinger, T.; Inglima, K.; Woo, K.-M.; Atie, O.; Yurasits, L.; See, B.; Aguero-Rosenfeld, M.E. Performance of abbott ID now COVID-19 rapid nucleic acid amplification test using nasopharyngeal swabs transported in viral transport media and dry nasal swabs in a New York city academic institution. J. Clin. Microbiol. 2020, 58. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. In vitro diagnostic assays for COVID-19: Recent advances and emerging trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Bordea, I.R.; Hanna, R.; Chiniforush, N.; Grădinaru, E.; Câmpian, R.S.; Sîrbu, A.; Amaroli, A.; Benedicenti, S. Evaluation of the outcome of various laser therapy applications in root canal disinfection: A systematic review. Photodiagnosis Photodyn. Ther. 2020, 29, 101611. [Google Scholar] [CrossRef]
- Chiniforush, N.; Pourhajibagher, M.; Parker, S.; Benedicenti, S.; Bahador, A.; Sălăgean, T.; Bordea, I.R. The effect of antimicrobial photodynamic therapy using chlorophyllin–Phycocyanin mixture on Enterococcus faecalis: The influence of different light sources. Appl. Sci. 2020, 10, 4290. [Google Scholar] [CrossRef]
- Popa, D.; Bordea, I.-R.; Burde, A.V.; Crişan, B.; Câmpian, R.S.; Constantiniuc, M. Surface modification of Zir-conia after laser irradiation. Optoelectron. Adv. Mater. Rapid Commun. 2016, 10, 785–788. [Google Scholar]
- Bordea, I.R.; Lucaciu, P.O.; Crişan, B.; Mirza, C.; Popa, D.; Mesaroș, A.Ș.; Pelekanos, S.; Campian, R.S. The influence of chromophore presence in an experimental bleaching gel on laser assisted tooth whitening efficiency. Studia Univ. Babes-Bolyai Chemia 2016, 61, 215–223. [Google Scholar]
- Chen, Z.-M.; Fu, J.-F.; Shu, Q.; Chen, Y.-H.; Hua, C.-Z.; Li, F.-B.; Lin, R.; Tang, L.-F.; Wang, T.-L.; Wang, W.; et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J. Pediatr. 2020, 16, 240–246. [Google Scholar] [CrossRef] [Green Version]
- Samson, N.; Fink, B.; Matts, P.J.; Dawes, N.C.; Weitz, S. Visible changes of female facial skin surface topography in relation to age and attractiveness perception. J. Cosmet. Dermatol. 2010, 9, 79–88. [Google Scholar] [CrossRef]
- Patra, S.; Kerry, R.G.; Maurya, G.K.; Panigrahi, B.; Kumari, S.; Rout, J.R. Emerging molecular prospective of SARS-CoV-2: Feasible nanotechnology based detection and inhibition. Front. Microbiol. 2020, 11, 2098. [Google Scholar] [CrossRef]
- Kaufman, E.; Lamster, I.B. The diagnostic applications of saliva—A review. Crit. Rev. Oral Biol. Med. 2002, 13, 197–212. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin. J. Biomol. Struct. Dyn. 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Millet, J.K.; Whittaker, G.R. Host cell proteases: Critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015, 202, 120–134. [Google Scholar] [CrossRef]
- Earar, K.; Arbune, M.; Schipor, O.; Dorobat, C.M.; Stefanescu, V.; Gurau, G.; Indrei, L.L.; Fulga, I.; Pavel, L.L.; Giuroiu, C.-L. Oral Mucosa- Gate for COVID-19 Infection and Correlation with Chemical Structures of the Biocides. Rev. Chim. 2020, 71, 410–415. [Google Scholar] [CrossRef]
- Bidra, A.S.; Pelletier, J.S.; Westover, J.B.; Frank, S.; Brown, S.M.; Tessema, B. Comparison of in vitro inactivation of SARS CoV-2 with hydrogen peroxide and povidone-iodine oral antiseptic rinses. J. Prosthodont. 2020, 29, 599–603. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Yip, C.C.-Y.; Chan, K.-H.; Wu, T.-C.; Chan, J.M.-C.; Leung, W.-S.; Chik, T.S.-H.; Choi, C.Y.-C.; Kandamby, D.H.; et al. Consistent detection of 2019 novel coronavirus in saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [Green Version]
- Herron, J.; Hay-David, A.; Gilliam, A.; Brennan, P. Personal protective equipment and Covid 19- a risk to healthcare staff? Br. J. Oral Maxillofac. Surg. 2020, 58, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-Z.; Cheng, X.-Q.; Li, J.-Y.; Zhang, P.; Yi, P.; Xu, X.; Zhou, X.-D. Saliva in the diagnosis of diseases. Int. J. Oral Sci. 2016, 8, 133–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fini, M.B. What dentists need to know about COVID-19. Oral Oncol. 2020, 105, 104741. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Cui, B.; Duan, X.; Zhang, P.; Zhou, X.; Yuan, Q. Saliva: Potential diagnostic value and transmission of 2019-nCoV. Int. J. Oral Sci. 2020, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, N.; Wong, D.T. Saliva: Diagnostics and therapeutic perspectives. Oral Dis. 2010, 17, 345–354. [Google Scholar] [CrossRef] [Green Version]
- Gu, H.; Xie, Z.; Li, T.; Zhang, S.; Lai, C.; Zhu, P.; Wang, K.; Han, L.; Duan, Y.; Zhao, Z.; et al. Angiotensin-converting enzyme 2 inhibits lung injury induced by respiratory syncytial virus. Sci. Rep. 2016, 6, 19840. [Google Scholar] [CrossRef]
- Centorrino, F. Infezione da Coronavirus SARS-Cov2 ed evoluzione della patologia: Un’analisi del Recettore ACE2 (prima parte). Microbiol. Ital. 2020. Available online: https://www.microbiologiaitalia.it/virologia/infezione-da-coronavirus-sars-cov2-ed-evoluzione-della-patologia-unanalisi-del-recettore-ace2/ (accessed on 4 January 2021).
- Wu, C.; Zheng, S.; Chen, Y.; Zheng, M. Single-Cell RNA Expression Profiling of ACE2, the Putative Receptor of Wuhan 2019-NCoV, in the Nasal Tissue. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Sungnak, W.; Network, H.L.B.; Huang, N.; Bécavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Kang, Z.; Gong, H.; Xu, D.; Wang, J.; Li, Z.; Cui, X.; Xiao, J.; Meng, T.; Zhou, W.; et al. The digestive system is a potential route of 2019-nCov infection: A bioinformatics analysis based on single-cell transcriptomes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Liang, W.; Feng, Z.; Rao, S.; Xiao, C.; Xue, X.; Lin, Z.; Zhang, Q.; Qi, W. Diarrhoea may be underestimated: A missing link in 2019 novel coronavirus. Gut 2020, 69, 1141–1143. [Google Scholar] [CrossRef] [Green Version]
- Zou, X.; Chen, K.; Zou, J.; Han, P.; Hao, J.; Han, Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020, 14, 185–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage after 2019-NCoV Infection | BioRxiv. Available online: https://www.biorxiv.org/content/10.1101/2020.02.03.931766v1 (accessed on 4 January 2021).
- De Cicco, R.L.; Watson, J.C.; Bassi, D.E.; Litwin, S.; Klein-Szanto, A.J. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clin. Cancer Res. 2004, 10, 4480–4488. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Li, Y.; Gan, F.; Du, Y.; Yao, Y. Salivary glands: Potential reservoirs for COVID-19 asymptomatic infection. J. Dent. Res. 2020, 99, 989. [Google Scholar] [CrossRef] [Green Version]
- High Expression of ACE2 Receptor of 2019-NCoV on the Epithelial Cells of Oral Mucosa | International Journal of Oral Science. Available online: https://www.nature.com/articles/s41368-020-0074-x (accessed on 4 January 2021).
- Liu, L.; Wei, Q.; Alvarez, X.; Wang, H.; Du, Y.; Zhu, H.; Jiang, H.; Zhou, J.; Lam, P.; Zhang, L.; et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J. Virol. 2011, 85, 4025–4030. [Google Scholar] [CrossRef] [Green Version]
- Spencer, J.P.; Pawlowski, R.H.T.; Thomas, S. Vaccine adverse events: Separating myth from reality. Am. Fam. Physician 2017, 95, 786–794. [Google Scholar]
- Krammer, F. SARS-CoV-2 vaccines in development. Nat. Cell Biol. 2020, 586, 1–16. [Google Scholar] [CrossRef]
- Mantovani, A.; Netea, M.G. Trained innate immunity, Epigenetics, and Covid-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [Green Version]
- Kurtz, J.; Franz, K. Evidence for memory in invertebrate immunity. Nat. Cell Biol. 2003, 425, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection in Children and Adolescents. JAMA Pediatr. 2020, 174, 882. [Google Scholar] [CrossRef] [Green Version]
- Escobar, L.E.; Molina-Cruz, A.; Barillas-Mury, C. BCG vaccine protection from severe coronavirus disease 2019 (COVID-19). Proc. Natl. Acad. Sci. USA 2020, 117, 17720–17726. [Google Scholar] [CrossRef] [PubMed]
- De Laval, B.; Maurizio, J.; Kandalla, P.K.; Brisou, G.; Simonnet, L.; Huber, C.; Gimenez, G.; Matcovitch-Natan, O.; Reinhardt, S.; David, E.; et al. C/EBPβ-dependent epigenetic memory induces trained immunity in hematopoietic stem cells. Cell Stem Cell 2020, 26, 657–674.e8. [Google Scholar] [CrossRef]
- Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R.; The Severe Covid-19 GWAS Group; et al. Genomewide association study of severe Covid-19 with respiratory failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [CrossRef]
- WHO. DRAFT Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 29 October 2020).
- BioNTech SE. Study to Describe the Safety, Tolerability, Immunogenicity, and Efficacy of RNA Vaccine Candidates against COVID-19 in Healthy Individuals. ClinicalTrials.gov NCT04368728; 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04368728 (accessed on 4 January 2021).
- Mahase, E. Covid-19: Moderna vaccine is nearly 95% effective, trial involving high risk and elderly people shows. BMJ Br. Med. J. 2020, 371. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: UK government asks regulator to assess Oxford vaccine as questions are raised over interim data. BMJ 2020, 371, m4670. [Google Scholar] [CrossRef]
- Lundgren, J.D. ClinicalTrials.gov. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04760132 (accessed on 4 January 2021).
- Callaway, E. What Pfizer’s landmark COVID vaccine results mean for the pandemic. Nat. Cell Biol. 2020. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Vaccine candidate may be more than 90% effective, interim results indicate. BMJ 2020, 371, m4347. [Google Scholar] [CrossRef] [PubMed]
- Staff, W.N. Three COVID Vaccines Compared. Available online: https://www.webmd.com/vaccines/covid-19-vaccine/news/20201214/closer-look-at-three-covid-19-vaccines (accessed on 4 January 2021).
- Liu, Y.; Liu, J.; Xia, H.; Zhang, X.; Fontes-Garfias, C.R.; Swanson, K.A.; Cai, H.; Sarkar, R.; Chen, W.; Cutler, M. Neutralizing Activity of BNT162b2-Elicited Serum—Preliminary Report. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Staff, R. AstraZeneca CEO Expects to Run New Global Trial of COVID-19 Vaccine—Bloomberg. Reuters 2020. Available online: https://www.bloomberg.com/news/articles/2020-11-26/astra-likely-to-run-fresh-global-covid-vaccine-trial-ceo-says (accessed on 4 January 2021).
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Vaccino Covid, Oxford-Irbm-Astrazeneca: “Efficacia al 90% Con Una Dose e Mezza. Costo Di 2,80 Euro e 200 Milioni Di Dosi a Fine 2020”—Il Fatto Quotidiano. Available online: https://www.ilfattoquotidiano.it/2020/11/23/vaccino-covid-oxford-irbm-astrazeneca-efficacia-al-90-con-una-dose-e-mezza-costo-confermato-a-280-euro/6012974/ (accessed on 4 January 2021).
- COVID-19, Il Vaccino Astra Zeneca Efficace “per Errore” | Univadis. Available online: https://www.univadis.it/viewarticle/covid-19-il-vaccino-astra-zeneca-efficace-per-errore-734126 (accessed on 4 January 2021).
- Shulla, A.; Gallagher, T. Role of spike protein endodomains in regulating coronavirus entry. J. Biol. Chem. 2009, 284, 32725–32734. [Google Scholar] [CrossRef] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; Macary, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- S.p.A, S.E.A. È Bresciano Lo Spray Che Fa Da Barriera al Virus. Available online: https://www.bresciaoggi.it/territori/citt%c3%a0/e-bresciano-lo-spray-che-fa-da-barriera-al-virus-1.8272678 (accessed on 4 January 2021).
- Salute, M. Della Notizie. Available online: http://www.salute.gov.it/portale/news/p3_2_1.jsp?lingua=italiano&menu=notizie&dataa=2020/12/31&datada=2016/01/01¬izie.page=12 (accessed on 4 January 2021).
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Ergoren, M.C.; Paolacci, S.; Manara, E.; Dautaj, A.; Dhuli, K.; Anpilogov, K.; Camilleri, G.; Suer, H.K.; Sayan, M.; Tuncel, G.; et al. A Pilot Study on the Preventative Potential of Alpha-Cyclodextrin and Hydroxytyrosol against SARS-CoV-2 Transmission. Acta Bio Med. Atenei Parm. 2020, 91, e2020022. [Google Scholar] [CrossRef]
- Baglivo, M.; Baronio, M.; Natalini, G.; Beccari, T.; Chiurazzi, P.; Fulcheri, E.; Petralia, P.P.; Michelini, S.; Fiorentini, G.; Miggiano, G.A.; et al. Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: A possible strategy for reducing SARS-COV-2 infectivity? Acta Bio Med. Atenei Parm. 2020, 91, 161–164. [Google Scholar]
- Paolacci, S.; Ceccarini, M.R.; Codini, M.; Manara, E.; Tezzele, S.; Percio, M.; Capodicasa, N.; Kroni, D.; Dundar, M.; Ergoren, M.C.; et al. Pilot study for the evaluation of safety profile of a potential inhibitor of SARS-CoV-2 endocytosis. Acta Bio Med. Atenei Parm. 2020, 91, e2020009. [Google Scholar] [CrossRef]
- Dallavilla, T.; Bertelli, M.; Morresi, A.; Bushati, V.; Stuppia, L.; Beccari, T.; Chiurazzi, P.; Marceddu, G. Bioinformatic Analysis Indicates That SARS-CoV-2 Is Unrelated to Known Artificial Coronaviruses. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4558–4564. [Google Scholar] [CrossRef] [PubMed]
- WHO/Europe Discusses How to Deal with Pandemic Fatigue. Available online: https://www.who.int/news-room/feature-stories/detail/who-europe-discusses-how-to-deal-with-pandemic-fatigue (accessed on 4 January 2021).
- Whitelaw, S.; Mamas, M.A.A.; Topol, E.; Van Spall, H.G. Applications of digital technology in COVID-19 pandemic planning and response. Lancet Digit. Health 2020, 2, e435–e440. [Google Scholar] [CrossRef]
- Emary, K.R.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.J.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S. Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7). 2021. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00628-0/fulltext (accessed on 4 January 2021).
- Pinho, A.C. COVID-19 Vaccine AstraZeneca: PRAC Preliminary View Suggests No Specific Issue with Batch Used in Austria. Available online: https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-prac-preliminary-view-suggests-no-specific-issue-batch-used-austria (accessed on 12 March 2021).
- AIFA Dispone Divieto di Utilizzo di un Lotto AstraZeneca. Accertamenti in Corso in Coordinamento Con EMA. Available online: https://aifa.gov.it/-/aifa-dispone-divieto-di-utilizzo-di-un-lotto-astrazeneca-accertamenti-in-corso-in-coordinamento-con-ema (accessed on 12 March 2021).
- Burgos, R.M.; Badowski, M.E.; Drwiega, E.; Ghassemi, S.; Griffith, N.; Herald, F.; Johnson, M.; Smith, R.O.; Michienzi, S.M. The race to a COVID-19 vaccine: Opportunities and challenges in development and distribution. Drugs Context 2021, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Haynes, B.F.; Corey, L.; Fernandes, P.; Gilbert, P.B.; Hotez, P.J.; Rao, S.; Santos, M.R.; Schuitemaker, H.; Watson, M.; Arvin, A. Prospects for a safe COVID-19 vaccine. Sci. Transl. Med. 2020, 12, eabe0948. [Google Scholar] [CrossRef] [PubMed]
- Vaccines, J. A Study of AdCOVS for the Prevention of SARS-CoV-2-Mediated COVID-19 in Adult Partici-pants (ENSEMBLE). ClinicalTrials.Gov NCT04505722. Vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7); 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04614948 (accessed on 4 January 2021). [CrossRef]
- FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine (accessed on 11 March 2021).
- Statement from NIH and BARDA on the FDA Emergency Use Authorization of the Janssen COVID-19 Vac-cine | National Institutes of Health (NIH). Available online: https://www.nih.gov/news-events/news-releases/statement-nih-barda-fda-emergency-use-authorization-janssen-covid-19-vaccine (accessed on 2 March 2021).
- Tatsis, N.; Ertl, H.C. Adenoviruses as vaccine vectors. Mol. Ther. 2004, 10, 616–629. [Google Scholar] [CrossRef]
- Aggiornamento Sulla Revisione Ciclica del Vaccino COVID-19 di Astrazeneca. Available online: https://www.aifa.gov.it/web/guest/-/aggiornamento-sulla-revisione-ciclica-del-vaccino-covid-19-di-astrazeneca (accessed on 4 January 2021).
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatulin, A.I.; Dzharullaeva, A.S.; Tukhvatulina, N.M.; Shcheblyakov, D.V.; Shmarov, M.M.; Tokarskaya, E.A.; Simakova, Y.V.; Egorova, D.A.; et al. Safety and immunogenicity of GamEvac-Combi, a heterologous VSV- and Ad5-vectored Ebola vaccine: An open phase I/II trial in healthy adults in Russia. Hum. Vaccines Immunother. 2017, 13, 613–620. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Palacios, R.; Patiño, E.G.; Piorelli, R.D.O.; Conde, M.T.R.P.; Batista, A.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; Ockenhouse, C.F.; et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac—PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 1–3. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Ayatollahi, J.; Aljabali, A.A.; Shastri, M.D.; Shukla, S.D.; Chellappan, D.K.; Jha, N.K.; Anand, K.; Katari, N.K.; Mehta, M.; et al. An overview of vaccine development for COVID-19. Ther. Deliv. 2021, 12, 235–244. [Google Scholar] [CrossRef] [PubMed]










| Phase 3 Trials SARS-CoV-2 Vaccines | |||
|---|---|---|---|
| Manufacturer | Type of Vaccine | General Characteristics | Trial Code |
| Sinopharm/China National Biotec Group Co/Wuhan Institute of Biological Product | Inactivated Virus | SARS-CoV-2 (Vero Cell) | CNCTR2000034780 |
| CNCTR2000039090 | |||
| NCT04510207 | |||
| NCT04612972 | |||
| Sinopharm/China National Biotec Group Co/Beijing Institute of Biological Product | Inactivated Virus | SARS-CoV-2 (Vero Cell) | NCT04560881 |
| NCT04510207 | |||
| CanSino Biological Inc/Beijing Institute of Biological Product | Non-replicating vector | Adenovitus type S vector | NCT04526990 |
| NCT04540419 | |||
| Garnaleya Research Institute/Russian Health Ministry | Non-replicating vector | Gam-COVID-Vac Adeno-based (Ad26-RHAd5-S) | NCT0453096 |
| NCT0564716 | |||
| NCT04642339 | |||
| NCT04656613 | |||
| Janssen Pharmacheutical | Non-replicating vector | Ad26COV2S | NCT04505722ù |
| NCT04614948 | |||
| Novavax | Proteic Sub-unit | SARS-CoV-2 rS/Matrix M1-Adjuvant | NCT04611802 |
| EUCTR2020-004123-16-GB | |||
| NCT045583995 | |||
| Anhui Zhaifei Longcom Biopharmaceutical/Institute of Microbiology Chinese Academy of Sciences | Proteic Sub-unit | Recombinant SARS-CoV-2 (CHO cell) | NCT04646590 |
| CureVac AG | Rna Vaccine | CVnCoV Vaccine | NCT04674189 |
| Institute of Medical Biology/Chinese Academy of Medical Sciences | Inactivated Virus | SARS-CoV-2 (Vero Cell) | NCT04659239 |
| Research Institute for Biological Safety Problem/Kazakhistan | Inactivated Virus | QarCovid-in | NCT04691908 |
| Cadila Healthcare Ltd. | Dna Vaccine | NCov Vaccine | CTR/2020/07/026352 |
| Bharat Biotech International Ltd. | Inactivated Virus | SARS-CoV-2 virion | NCT04543481 |
| Phase 4 Trials SARS-CoV2 Vaccines | |||
|---|---|---|---|
| Manufacturer | Type of Vaccine | General Characteristics | Trial Code |
| Pfizer/BioNTech + Fosun Pharma | RNA Vaccine | BNT162 (3 LNP-mRNAs} | NCT04760132 |
| EUCTR2O 21-0000412-28 | |||
| EUCTR2O 21-0000412-28 | |||
| Sinovac Research and Development Ltd. | Inactivated Virus | SARS-CoV-2 vaccine | NCTO445€595 NCTO4508075 |
| NCTO4502344 NCTO4617483 | |||
| NCTO465179O | |||
| Astra Zeneca + University of Oxford | Non-replicating vector | ChAdOxI-S (AZD1222) (Covishield) | ISRCTN89951424 NCTO4516746 NCT04540393 NCT045380S1 |
| EUCTR2020-00 52 26-28-DE | |||
| Moderna + National institute of Allergy and Infectious Diseases (NIAID) | RNA Vaccine | mRNA-1273 | NCT04470427 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms 2021, 9, 793. https://doi.org/10.3390/microorganisms9040793
Inchingolo AD, Inchingolo AM, Bordea IR, Malcangi G, Xhajanka E, Scarano A, Lorusso F, Farronato M, Tartaglia GM, Isacco CG, et al. SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs. Microorganisms. 2021; 9(4):793. https://doi.org/10.3390/microorganisms9040793
Chicago/Turabian StyleInchingolo, Alessio Danilo, Angelo Michele Inchingolo, Ioana Roxana Bordea, Giuseppina Malcangi, Edit Xhajanka, Antonio Scarano, Felice Lorusso, Marco Farronato, Gianluca Martino Tartaglia, Ciro Gargiulo Isacco, and et al. 2021. "SARS-CoV-2 Disease through Viral Genomic and Receptor Implications: An Overview of Diagnostic and Immunology Breakthroughs" Microorganisms 9, no. 4: 793. https://doi.org/10.3390/microorganisms9040793