Emerging and Established Histological Techniques for the Analysis of Thrombosis in COVID-19 Lungs
- 1Department of Medicine, University of Illinois College of Medicine, Rockford, IL, United States
- 2Department of Pediatrics, Lung and Vascular Biology Program, Stanley Manne Children's Research Institute, Ann & Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, United States
- 3Department of Pediatrics, Division of Critical Care, Northwestern University Feinberg School of Medicine, Chicago, IL, United States
Coronavirus disease 2019 (COVID-19) is the potentially lethal disease that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Patients with COVID-19 have an increased risk of thrombosis, but the role of thrombosis in the pathogenesis and progression of severe COVID-19 remains unclear. A better understanding of the contribution of thrombosis to the development and progression of COVID-19 could lead to the development of novel COVID-19 treatments. For this reason, established and emerging histological techniques have recently been used to analyze COVID-19 lungs quantitatively and visually and in two and three dimensions. The gold standard and novel state-of the-art histological techniques that have been used to assess thrombosis in COVID-19 lungs are described in this Mini Review.
Background
Coronavirus disease 2019 (COVID-19) is the highly contagious and potentially debilitating disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is associated with increased levels of pulmonary thrombosis (1–3). The COVID-19 pandemic has resulted in massive global suffering (4) including job losses, travel restrictions (5), mental and physical illness (6, 7), and mortality (7). Several treatments for COVID-19 have been given emergency use authorization by the FDA, but these treatments are less than optimal. Remdesivir, for example, improves time to recovery in hospitalized patients with moderate disease (NIAID ACTT-1 trial), but has not been shown to improve survival (8, 9). A major limitation of Remdesivir is its requirement for intra-venous delivery. Another example, Dexamethasone, reduces mortality in persons with severe COVID-19 (RECOVERY trial) (10), but corticosteroid treatment in patients with SARS-CoV, MERS, and influenza can result in increased mortality, incidence of secondary infections, and impaired viral clearance. Convalescent plasma is another treatment granted emergency use authorization by the FDA for hospitalized COVID-19 patients, but a mortality benefit of this treatment has not yet been shown (11). The FDA has also granted emergency use authorization to monoclonal antibodies, for the treatment of non-hospitalized mild to moderate COVID-19. These treatments have importantly shown a reduction in hospitalization in those at high risk for disease progression (BLAZE-1 trial) (12, 13). However, monoclonal antibodies are not approved for hospitalized or critically ill individuals requiring mechanical ventilation The REMAP-CAP and RECOVERY trials are also assessing the application of Tocilizumab and Sarilumab, which are interleukin 6 receptor antagonists, in critically ill COVID-19 patients (14). Another immunomodulator, Baricitinib, which inhibits januse kinase 1 and 2, has been shown to improve survival in COVID-19 patients receiving Baricitinib plus Remdesivir versus Remdesivir alone (15). Regarding the use of Ivermectin, current evidence is inconclusive (16, 17), but additional randomized controlled trials are underway.
While clinical studies of COVID-19 treatments crucial are ongoing, including tests of anti-coagulant therapies (18, 19), an improved understanding of the pathogenesis and pathological characteristics of COVID-19 may lead to the development of novel treatment options. To this end, several studies have used established or emerging histological techniques to characterize the pathological features of lung injury in COVID-19 patients, including widespread pulmonary thrombosis. Such techniques can also be useful for disease diagnosis or retrospective tissue analysis. For example, a recent study has demonstrated the potential for high-resolution cleared-tissue microscopy in the 3-dimensional (3D) analysis of thrombosis in COVID-19 lungs (20). Although this proof-of-concept study was limited to a single patient, such emerging technological advances enable for the analysis of thrombosis in COVID-19 lungs in cubic millimeter volumes and provide novel insights into COVID-19 disease pathogenesis. While the organ-specific pathological characteristics of COVID-19 have been reviewed elsewhere (21–26), this mini-review describes the 2D and 3D histological studies of COVID-19 lungs, with a focus on thrombosis and vascular abnormalities. To this end, we searched PubMed and Google Scholar for articles that included the following terms: “coagulation or thrombosis or thrombus” and “COVID-19 or COVID19” and “histological or histology” and “lung or pulmonary”.
Two-Dimensional Histology of COVID-19 Lungs
Features of Coagulopathy
The first open autopsy histological analysis of dissected lung used hematoxylin and eosin (H&E) staining, fluorescence/immunostaining, and electron microscopy in a thorough 2-dimensional (2D) analyses of 10 COVID-19 patients (27). In their study, Fox et al. showed pulmonary thrombotic microangiopathy including platelet aggregation, platelet-rich microthrombi formation, fibrin deposition, and hemorrhage, with evidence of the activation of megakaryocytes contributing to small vessel clot formation (27). The pulmonary megakaryocytes exhibited nuclear hyperchromasia and atypia, were located in alveolar capillaries, and were found to be producing platelets (27). CD4+ lymphocytes were also found to aggregate around small blood vessels, some of which appeared to contain platelets and microthrombi (27). Notably, the presence of microthrombi was specific to the lung tissue and the SARS-CoV-2 pandemic (27). In another study of 6 postmortem lung samples from COVID-19 patients, Eckermann et al. showed conventional histopathological evidence of microthrombi formation and fibrin deposition (28). Microthrombi formation, fibrin deposition, and hemorrhage were also shown in another histopathological study of 31 deceased COVID-19 patients (29). Ackermann et al. examined the lungs of 7 patients who died from COVID-19 by seven-color immunohistochemical analysis, micro–computed tomographic imaging, scanning electron microscopy, and corrosion casting (30). In their study, pulmonary microthrombi formation and fibrin deposition was found (30). In a post-mortem examination study of 7 COVID-19 patients, Rapkiewicz et al. employed H&E staining, immunostaining, and electron microscopy to demonstrate evidence of platelet-rich thrombi in the pulmonary microvasculature and thrombus formation in large pulmonary arteries (31). By studying H&E-stained lung sections from 8 autopsy cases from patients with COVID-19, Kianzad et al. also found histological evidence of pulmonary thrombosis (32). Similarly, Romanova et al. (33), Bryce et al. (34), Bidari Zerehpoosh et al. (35), Mauad et al. (36), Bruce-Brand (37), Elsoukkary et al. (38), and Grosse et al. (39) used H&E staining to show evidence of lung thrombosis in COVID-19 autopsy samples. H&E staining and immunofluorescence were also employed by Nicolai et al. (40) and Oprinca et al. (41) in their demonstrations of pulmonary thrombosis in COVID-19 lungs. A combination of H&E staining, immunostaining, and electron microscopy were used by Falasca et al. (42) and Carsana et al. (43), to show pulmonary microthrombi, fibrin deposition, and hemorrhage in COVID-19 patients at postmortem. Microthrombi containing neutrophil extracellular traps were also shown using immunofluorescence staining, by Middleton et al., in 3 COVID-19 lung autopsies (44).
Features of Vasculopathy
An early case report of post-mortem lung biopsy using 2D H&E staining revealed diffuse alveolar damage, hyaline membrane formation, interstitial lymphocyte infiltration, and multinucleated syncytial cells in alveolar spaces showing viral cytopathic-like changes (45). Diffuse alveolar damage, hyaline membrane formation, and edema was also found in 2D analyses of dissected lung from open autopsy COVID-19 patients (27). These lungs showed inflammatory cell infiltration (i.e., CD4+ and CD8+ lymphocytes) in the interstitial spaces and adjacent to large bronchioles and blood vessels (27). Furthermore, RNA imaging and electron microscopy demonstrated fused pneumocytes within alveolar spaces, which contained substantial amounts of RNA and may represent virally infected cells (27). Using H&E staining and scanning electron microscopy, Varga et al. assessed the lung tissue of 3 COVID-19 patients at post-mortem (46). Histological analyses revealed inflammatory cell accumulation in association with the endothelium, which exhibited evidence of viral elements (46). Evidence of endothelial cell apoptotic bodies, small lung vessel congestion, and septal thickening was also found (46). Diffuse alveolar damage and hyaline membrane formation, as well as evidence of emphysema, was also found in 2D slices of COVID-19 lungs by Eckermann et al. (28). Meanwhile, Sadegh Beigee et al. (29) found evidence of the following microscopic features in a cohort of 31 deceased COVID-19 patients: hyaline membrane formation; interstitial leukocyte infiltration; and edema. Ackermann et al. (30) also found evidence of the following features in a cohort of 7 COVID-19 post-mortem lung samples: diffuse alveolar damage; edema; angiotensin-converting enzyme 2 (ACE2) expression in epithelial and endothelial cells; endothelial damage and viral infection; and T-cell infiltration. In an autopsy study by Rapkiewicz et al., lungs showed diffuse alveolar damage with hyaline membranes, pneumocyte hyperplasia, perivascular lymphocyte infiltration, and viral inclusions in macrophages and epithelial cells (31). More recently, Qin et al. used H&E staining and immunofluorescence to show histological evidence of endothelial dysfunction (in the form of VCAM1 staining) in an autopsied lung collected from a severe COVID-19 patient, as well as extensive edema, and evidence of viral staining (47). In H&E-stained lung sections from autopsy cases from COVID-19 patients, Kianzad et al. also found histological evidence of edema, neutrophil infiltration, and diffuse alveolar damage (32). Similarly, Romanova et al. (33), Bryce et al. (34), Bidari Zerehpoosh et al. (35), Mauad et al. (36), Bruce-Brand (37), and Elsoukkary et al. (38) used H&E staining to demonstrate diffuse alveolar damage and leukocyte infiltration in COVID-19 autopsy samples. H&E staining and immunofluorescence were also employed by Nicolai et al. (40) and Radermecker et al. (48) in their demonstrations of neutrophil infiltration and neutrophil extracellular trap formation in COVID-19 lungs. Oprinca et al. used H&E and immunohistochemical staining to show hyaline membrane formation and diffuse alveolar damage in 3 COVID-19 autopsy samples (41). A combination of H&E staining, immunostaining, and electron microscopy were used by Falasca et al. (42) and Carsana et al. (43) in COVID-19 patients at postmortem, to show pulmonary hyaline membranes, inflammatory cell infiltration, diffuse alveolar damage, and vasculitis.
Three-Dimensional Histology of COVID-19 Lungs
Features of Coagulopathy
In a study by Li et al., 3D images of lung autopsy tissues were rendered from a single COVID-19 patient (20). This study represents the first report of 3D microscopy images from optically cleared lung tissues from a COVID-19 patient autopsy (20). These authors showed extensive evidence of platelet-rich clotting with adherent mononuclear cells in branching vessels and extensive fibrin clotting in small capillaries (20). The 3D technique used by Li et al. was also able to confirm the finding of activated mature megakaryocytes in small lung vessels, as shown by large, multiple, lobular nuclei (20). Eckermann et al. used multi-scale phase contrast x-ray tomography to analyze postmortem lung samples from 6 COVID-19 patients in 3D (28). In their study, autopsy samples of up to 8 mm thickness were scanned and reconstructed at a resolution that enabled the segmentation of individual cells (28). These studies showed evidence of microthrombi formation and fibrin deposition (28). Ackermann et al. also showed evidence of vessel occlusion by 3D micro computed tomography (30).
Features of Vasculopathy
In the study by Eckermann et al., 3D virtual histology was used to visualize diffuse alveolar damage, emphysema, hyaline membrane formation, septal thickening, and leukocyte infiltration (28). Li et al. (20) used 3D virtual histology to observe multi-nucleated cells with evidence of viral cytopathic changes, scattered hyaline-fibrin aggregates and inflammatory cells in the alveolar spaces, and diffuse alveolar damage. In the study by Ackermann et al., scanning electron microscopy and microvascular corrosion casting were used to demonstrate structurally deformed capillaries and aberrant angiogenesis in COVID-19 lungs (30).
Methodological Considerations and Future Perspectives
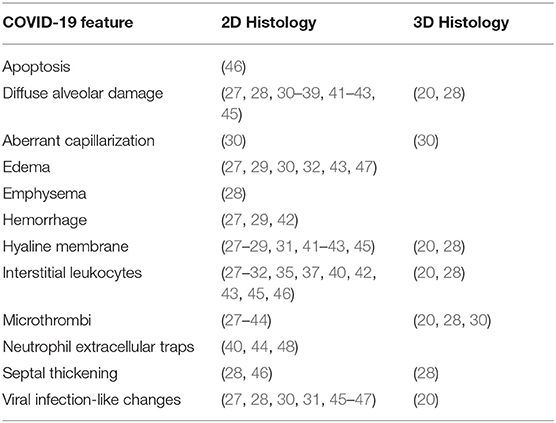
While both 2D and 3D histological techniques remain temporally limited by the analysis of one timepoint and spatially limited by the amount of tissue that can be imaged, such techniques can provide valuable insights into COVID-19 disease pathology. Often, 2D techniques are quicker and cheaper to perform compared with 3D techniques, but the 3D techniques yield more information and provide improved visualization of disease features. In the manuscript by Li et al. (20), for instance, 3D renderings from a 7.8 mm × 5.9 mm × 0.9 mm formalin-fixed block of lung tissue from the left peripheral upper lobe were generated, which enabled the visualization of lung features from millimeter dimension vessels to single cell nuclei. However, 3D histology from serial sectioning and whole slide scanning with image reconstruction is expensive and time consuming. In future, it is likely that established 2D histological techniques will be complemented with emerging 3D virtual histology. While the histological studies of COVID-19 patients are often limited in sample size, the key findings from studies of COVID-19 lung that have used 2D and 3D techniques appear to be congruent so far (Table 1). However, it remains to be seen what (if any) additional information will be uniquely generated from each type of histological technique. Furthermore, it is important to note that the findings from histological studies are observational and are usually limited to the demonstration of associations between pathological features and death in diseased patients.
Analysis of 2D lung tissue sections remains the gold-standard for histological examination, but 2D tissue sectioning can create cutting artifacts that confuse interpretation. Pathological interpretation may be improved in future studies by 3D analysis of tissue microstructure (49). Emerging histological techniques have used fluorescence contrast to simulate H&E staining (50, 51) and optical tissue clearing and high throughput sectioning microscopes to acquire 3D virtual histology of different tissue types (49, 52–54). Such 3D virtual imaging techniques could be improved by: (i) increasing resolution with higher aperture waveguide optics, enhanced pixel detector technology, and improvements in holographic reconstruction; (ii) including more than two zoom levels; and (iii) developing cell-specific markers coupled to radiocontrast agents (28). In both 2D and 3D histology, the consistency and reliability of novel and emerging histological techniques should be an area of focus in future studies. It would also be beneficial if 2D and 3D histological techniques could be upscaled and carried out in multiple different patient sub-groups and at various stages of disease severity. Furthermore, it could be useful if histological imaging studies in future could be performed in an automated manner, perhaps eventually in living patients. Methodological factors pertaining to both 2D and 3D histology should be carefully considered when designing and carrying out histological studies of COVID-19 lungs (55, 56). Nevertheless, histological studies will likely continue to improve understanding of COVID-19 pathogeneses and progression, which will add to understanding of COVID-19 from other pre-clinical and clinical observations, and hopefully lead to the development of novel therapies and treatment strategies.
Conclusions
Novel treatments for COVID-19 patients and an improved understanding of COVID-19 pathogenesis may arise from in-depth analyses of the histological features of COVID-19 lungs, which include enhanced levels of thrombosis. Such analyses will likely be facilitated by novel histological techniques for high-resolution and large-volume imaging of lung structure and pulmonary thrombosis at the microvascular level.
Author Contributions
CE and AS wrote and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
CE is supported in part by an American Heart Association Career Development Award (19CDA34500000).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
2D, 2-dimensional; 3D, 3-dimensional; COVID-19, coronavirus disease 2019.
References
1. Page EM, Ariëns RAS. Mechanisms of thrombosis and cardiovascular complications in COVID-19. Thromb Res. (2021) 200:1–8. doi: 10.1016/j.thromres.2021.01.005
2. Middeldorp S, Coppens M, van Haaps TF, Foppen M, Vlaar AP, Müller MCA, et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost. (2020) 18:1995–2002. doi: 10.1111/jth.14888
3. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. (2020) 120:998–1000. doi: 10.1055/s-0040-1714350
4. Xu B, Gutierrez B, Mekaru S, Sewalk K, Goodwin L, Loskill A, et al. Epidemiological data from the COVID-19 outbreak, real-time case information. Sci Data. (2020) 7:106. doi: 10.1038/s41597-020-0448-0
5. Iacus SM, Natale F, Santamaria C, Spyratos S, Vespe M. Estimating and projecting air passenger traffic during the COVID-19 Coronavirus outbreak and its socio-economic impact. Saf Sci. (2020) 129:104791. doi: 10.1016/j.ssci.2020.104791
6. Wang C, Pan R, Wan X, Tan Y, Xu L, Ho CS, et al. Immediate psychological responses and associated factors during the initial stage of the 2019 coronavirus disease (COVID-19) epidemic among the general population in China. Int J Environ Res Public Health. (2020) 17:1729. doi: 10.3390/ijerph17051729
7. Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. (2020) 382:2372–4. doi: 10.1056/NEJMc2010419
8. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. (2020) 383:1813–26. doi: 10.1056/NEJMoa2007764
9. Spinner CD, Gottlieb RL, Criner GJ, Arribas López JR, Cattelan AM, Soriano Viladomiu A, et al. Effect of remdesivir vs. standard care on clinical status at 11 days in patients with moderate COVID-19: a randomized clinical trial. JAMA. (2020) 324:1048–57. doi: 10.1001/jama.2020.16349
10. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. (2020) 384:693–704. doi: 10.1101/2020.06.22.20137273
11. Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. (2020) 95:1888–97. doi: 10.1016/j.mayocp.2020.09.032
12. Chen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, et al. SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N Engl J Med. (2020) 384:229–237. doi: 10.1056/NEJMoa2029849
13. Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, Bhore R, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. (2021) 384:238–51. doi: 10.1056/NEJMoa2035002
14. REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. (2021) 384:1491–502. doi: 10.1056/NEJMoa2100433
15. Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. (2021) 384:795–807. doi: 10.1056/NEJMoa2031994
16. Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther. (2021) 28:e299–318. doi: 10.1097/MJT.0000000000001377
17. Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, et al. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. (2021) 28:e434–60. doi: 10.1097/MJT.0000000000001402
18. REMAP-CAP Investigators, ACTIV-4a Investigators, ATTACC Investigators, Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. (2021) 385:777–89. doi: 10.1056/NEJMoa2103417
19. ATTACC Investigators, ACTIV-4a Investigators, REMAP-CAP Investigators, Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. (2021) 385:790–802. doi: 10.1056/NEJMoa2105911
20. Li G, Fox SE, Summa B, Hu B, Wenk C, Akmatbekov A, et al. Multiscale 3-dimensional pathology findings of COVID-19 diseased lung using high-resolution cleared tissue microscopy. bioRxiv. (2020). doi: 10.1101/2020.04.11.037473
21. Scimeca M, Urbano N, Bonfiglio R, Montanaro M, Bonanno E, Schillaci O, et al. Imaging diagnostics and pathology in SARS-CoV-2-Related diseases. Int J Mol Sci. (2020) 21:6960. doi: 10.3390/ijms21186960
22. Fakhoury HMA, Kvietys PR, Shakir I, Shams H, Grant WB, Alkattan K. Lung-centric inflammation of COVID-19: potential modulation by vitamin D. Nutrients. (2021) 13:2216. doi: 10.3390/nu13072216
23. Maruhashi T, Higashi Y. Pathophysiological association of endothelial dysfunction with fatal outcome in COVID-19. Int J Mol Sci. (2021) 22:5131. doi: 10.3390/ijms22105131
24. Vinayagam S, Sattu K. SARS-CoV-2 and coagulation disorders in different organs. Life Sci. (2020) 260:118431. doi: 10.1016/j.lfs.2020.118431
25. Vasquez-Bonilla WO, Orozco R, Argueta V, Sierra M, Zambrano LI, Muñoz-Lara F, et al. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol. (2020) 105:74–83. doi: 10.1016/j.humpath.2020.07.023
26. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. (2020) 26:1017–32. doi: 10.1038/s41591-020-0968-3
27. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from new orleans. Lancet Res Med. (2020) 8:681–6. doi: 10.1016/S2213-2600(20)30243-5
28. Eckermann M, Frohn J, Reichardt M, Osterhoff M, Sprung M, Westermeier F, et al. 3D virtual pathohistology of lung tissue from Covid-19 patients based on phase contrast X-ray tomography. Elife. (2020) 9:e60408. doi: 10.7554/eLife.60408.sa2
29. Sadegh Beigee F, Pourabdollah Toutkaboni M, Khalili N, Nadji SA, Dorudinia A, Rezaei M, et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract. (2020) 216:153228. doi: 10.1016/j.prp.2020.153228
30. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. (2020) 383:120–8. doi: 10.1056/NEJMoa2015432
31. Rapkiewicz AV, Mai X, Carsons SE, Pittaluga S, Kleiner DE, Berger JS, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClin Med. (2020) 24:100434. doi: 10.1016/j.eclinm.2020.100434
32. Kianzad A, Meijboom LJ, Nossent EJ, Roos E, Schurink B, Bonta PI, et al. COVID-19: histopathological correlates of imaging patterns on chest computed tomography. Respirology. (2021) 26:869–77. doi: 10.1111/resp.14101
33. Romanova ES, Vasilyev VV, Startseva G, Karev V, Rybakova MG, Platonov PG. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J Intern Med. (2021) 290:655–65. doi: 10.1111/joim.13300
34. Bryce C, Grimes Z, Pujadas E, Ahuja S, Beasley MB, Albrecht R, et al. Pathophysiology of SARS-CoV-2: the Mount Sinai COVID-19 autopsy experience. Mod Pathol. (2021) 34:1456–67. doi: 10.1038/s41379-021-00793-y
35. Bidari Zerehpoosh F, Sabeti S, Bahrami-Motlagh H, Mokhtari M, Naghibi Irvani SS, Torabinavid P, et al. Post-mortem histopathologic findings of vital organs in critically ill patients with COVID-19. Arch Iran Med. (2021) 24:144–51. doi: 10.34172/aim.2021.23
36. Mauad T, Duarte-Neto AN, da Silva LFF, de Oliveira EP, de Brito JM, do Nascimento ECT, et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir Res. (2021) 22:32. doi: 10.1186/s12931-021-01628-9
37. Bruce-Brand C, Allwood BW, Koegelenberg CFN, Lalla U, Louw E, Diacon AH, et al. Postmortem lung biopsies from four patients with COVID-19 at a tertiary hospital in Cape Town, South Africa. S Afr Med J. (2020) 110:1195–200. doi: 10.7196/SAMJ.2020.v110i12.15290
38. Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A, et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. (2021) 88:56–68. doi: 10.1159/000511325
39. Grosse C, Grosse A, Salzer HJF, Dünser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. (2020) 49:107263. doi: 10.1016/j.carpath.2020.107263
40. Nicolai L, Leunig A, Brambs S, Kaiser R, Joppich M, Hoffknecht ML, et al. Vascular neutrophilic inflammation and immunothrombosis distinguish severe COVID-19 from influenza pneumonia. J Thromb Haemost. (2021) 19:574–81. doi: 10.1111/jth.15179
41. Oprinca GC, Muja LA. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int J Legal Med. (2021) 135:329–39. doi: 10.1007/s00414-020-02406-w
42. Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, et al. Postmortem findings in italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. (2020) 222:1807–15. doi: 10.1093/infdis/jiaa578
43. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. (2020) 20:1135–40. doi: 10.1016/S1473-3099(20)30434-5
44. Middleton EA, He XY, Denorme F, Campbell RA, Ng D, Salvatore SP, et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. (2020) 136:1169–79. doi: 10.1182/blood.2020007008
45. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Res Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
46. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
47. Qin Z, Liu F, Blair R, Wang C, Yang H, Mudd J, et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics. (2021) 11:8076–91. doi: 10.7150/thno.61810
48. Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d'Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. (2020) 217:e20201012. doi: 10.1084/jem.20201012
49. Glaser AK, Reder NP, Chen Y, McCarty EF, Yin C, Wei L, et al. Light-sheet microscopy for slide-free non-destructive pathology of large clinical specimens. Nat Biomed Eng. (2017) 1:0084. doi: 10.1038/s41551-017-0084
50. Giacomelli MG, Husvogt L, Vardeh H, Faulkner-Jones BE, Hornegger J, Connolly JL, et al. Virtual hematoxylin and eosin transillumination microscopy using epi-fluorescence imaging. PLoS ONE. (2016) 11:e0159337. doi: 10.1371/journal.pone.0159337
51. Elfer KN, Sholl AB, Wang M, Tulman DB, Mandava SH, Lee BR, et al. DRAQ5 and eosin ('D&E') as an analog to hematoxylin and eosin for rapid fluorescence histology of fresh tissues. PLoS ONE. (2016) 11:e0165530. doi: 10.1371/journal.pone.0165530
52. Reder NP, Glaser AK, McCarty EF, Chen Y, True LD, Liu JTC. Open-top light-sheet microscopy image atlas of prostate core needle biopsies. Arch Pathol Lab Med. (2019) 143:1069–75. doi: 10.5858/arpa.2018-0466-OA
53. Glaser AK, Reder NP, Chen Y, Yin C, Wei L, Kang S, et al. Multi-immersion open-top light-sheet microscope for high-throughput imaging of cleared tissues. Nat Commun. (2019) 10:2781. doi: 10.1038/s41467-019-10534-0
54. Hu B, Bolus D, Brown JQ. Improved contrast in inverted selective plane illumination microscopy of thick tissues using confocal detection and structured illumination. Biomed Opt Exp. (2017) 8:5546–59. doi: 10.1364/BOE.8.005546
55. Skok K, Vander K, Setaffy L, Kessler HH, Aberle S, Bargfrieder U, et al. COVID-19 autopsies: procedure, technical aspects and cause of fatal course. Experiences from a single-center. Pathol Res Pract. (2021) 217:153305. doi: 10.1016/j.prp.2020.153305
Keywords: thrombosis, lung, histology, pulmonary, COVID - 19
Citation: Spier AB and Evans CE (2021) Emerging and Established Histological Techniques for the Analysis of Thrombosis in COVID-19 Lungs. Front. Cardiovasc. Med. 8:745906. doi: 10.3389/fcvm.2021.745906
Received: 22 July 2021; Accepted: 26 August 2021;
Published: 21 September 2021.
Edited by:
Hugo Ten Cate, Maastricht University Medical Centre, NetherlandsReviewed by:
Mathilde Nijkeuter, University Medical Center Utrecht, NetherlandsCédric Duval, University of Leeds, United Kingdom
Copyright © 2021 Spier and Evans. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Colin E. Evans, colinevans@northwestern.edu
 Addie B. Spier1
Addie B. Spier1  Colin E. Evans
Colin E. Evans