Abstract
Background
There are currently no evidence-based guidelines that provide standardized criteria for the discharge of COVID-19 patients from the hospital.
Objective
To address this gap in practice guidance, we reviewed published guidance and collected discharge protocols and procedures to identify and synthesize common practices.
Design
Rapid review of existing guidance from US and non-US public health organizations and professional societies and qualitative review using content analysis of discharge documents collected from a national sample of US academic medical centers with follow-up survey of hospital leaders
Setting and Participants
We reviewed 65 websites for major professional societies and public health organizations and collected documents from 22 Academic Medical Centers (AMCs) in the US participating in the HOspital MEdicine Reengineering Network (HOMERuN).
Results
We synthesized data regarding common practices around 5 major domains: (1) isolation and transmission mitigation; (2) criteria for discharge to non-home settings including skilled nursing, assisted living, or homeless; (3) clinical criteria for discharge including oxygenation levels, fever, and symptom improvement; (4) social support and ability to perform activities of daily living; (5) post-discharge instructions, monitoring, and follow-up.
Limitations
We used streamlined methods for rapid review of published guidance and collected discharge documents only in a focused sample of US academic medical centers.
Conclusion
AMCs studied showed strong consensus on discharge practices for COVID-19 patients related to post-discharge isolation and transmission mitigation for home and non-home settings. There was high concordance among AMCs that discharge practices should address COVID-19-specific factors in clinical, functional, and post-discharge monitoring domains although definitions and details varied.
Similar content being viewed by others
INTRODUCTION
The pandemic caused by the coronavirus disease of 2019 (COVID-19) has placed unprecedented strain on hospitals around the world. Recent research on COVID-19 care delivery has focused on challenges related to critical care capacity (ICU beds, ventilators, and providers)1 and staffing for general medical wards,2 particularly in the early phase of the pandemic (March–June 2020).3 This period was characterized by massive surges that overwhelmed health systems in population centers like Wuhan, China4 or New York City in the USA5 or entire regions such as Lombardy in Italy.6 Other cities and regions that experienced a less intense initial surge were able to manage the volume of COVID-19 patients with reductions in hospitalizations for other (non-COVID-19) populations. As the pandemic has progressed, total hospital volume has increased7 and second and third surges of COVID-19 have required new strategies to continue with elective procedures and admissions.8 For such strategies to be effective, hospitals must be able to safely and quickly transition COVID-19 patients from inpatient care to other settings.
Unfortunately, the variable clinical course of COVID-19 complicates transitions of care because although some patients improve quickly, some worsen after a period of clinical stability9 and some require weeks to recover completely.10 Initially, observational data helped to identify clinical and socio-demographic factors associated with disease severity requiring critical care versus ward-level care. More recent analyses have focused on predicting a wider range of adverse events such as in-hospital mortality,11 increased LOS,12 critical illness,13, 14 or other adverse15 or favorable outcomes within a 96-hour window.16 These advances notwithstanding, there is still limited evidence on outcomes after hospital discharge and wide variation in guidance about discharge decision-making across countries with high rates of COVID-19 around the world.17 Furthermore, there are currently no evidence-based clinical practice guidelines to inform discharge processes unique to COVID-19, including isolation protocols; monitoring for disease progression and follow-up care; and care for social and functional needs during the post-discharge isolation period.
To address these gaps, we leveraged the COVID-19 Response Team of the HOspital MEedicine Reengineering Network18 (HOMERuN), which is a national network of hospitalist leaders at US academic medical centers. Since the beginning of the pandemic, HOMERUN has applied the Institute for Healthcare Improvement (IHI) Breakthrough Series approach19 to enable rapid assessments of current challenges and disseminate best practices on the front lines of care at US hospitals. We also utilized rapid evidence synthesis techniques20 developed by the Center for Evidence Based Practice (CEP) at the University of Pennsylvania to review and update published guidance from major public health organizations and professional societies. CEP has developed and disseminated numerous rapid guidance reports for COVID-1921 to guide operational decisions.22 Our objective was to conduct a rapid, mixed-methods review of current practices for COVID-19 patients to inform discharge decision-making at a time when COVID-19 hospitalizations are rising again.
METHODS
Assessment of Existing Recommendations
The rapid worldwide onset of the pandemic compels novel methods for rapid synthesis of existing practice guidance.23 Given the novelty of COVID-19, we anticipated there would not be enough evidence from clinical trials to conduct a full systematic review. Instead, we compiled a list of web sites from public health agencies such as the US and the European Centers for Disease Prevention and Control, professional societies such as the Infectious Disease Society of America, evidence clearinghouses such as the Centre for Evidence-Based Medicine at Oxford University, and major US academic medical centers that made their clinical guidance publicly available. With urgent need for guidance at the front lines, we considered this an acceptable compromise, given the lack of direct evidence from published clinical trials and the wide range of clinical considerations for COVID-19 discharge decisions that will likely confound and delay robust clinical trials in the near future.
The list of sites scanned by the CEP team grew from about 30 at the outset of our project in late March to 65 by July (Appendix 1). We did not exclude sites from our list due to the absence of any discharge relevant guidance; however, some sites referred to other sources in lieu of providing their own recommendations. In such cases, we focused on the referred site for updates. Once sources were scanned, we qualitatively summarized their recommendations into a table organized by date and source of guidelines from public health agencies and professional societies. For each source, we attempted to determine the type and level of evidence on which recommendations were based (secondary or primary studies or case reports and expert opinion). Findings from this rapid review were published online (April 2020) and revised for this report (November 2020).
Collection of Novel Data
We collected novel data in two steps. First, given the limited details about discharge practices found in the existing recommendations above, we asked site leads at HOMERuN institutions to submit clinical protocols, procedure manuals, documentation templates, and other documents related to the hospital discharge of patients with COVID-19. We received documents from 22 institutions (March–April 2020): Cleveland Clinic, University of Michigan, Northwestern University, Ohio State University, University of Pennsylvania, University of California San Francisco, Cornell University, University of Wisconsin, Medical College of Wisconsin, University of Kentucky, Johns Hopkins University, Cristiana Care Medical Center, University of Colorado, Mount Sinai, University of Missouri, University of Miami, University of Pittsburg, Tulane University, University of Washington, Oregon Health Sciences University, Brigham and Women’s Hospital, Vanderbilt University. Second, after completing thematic analysis of these documents to identify major clinical practices (described below), we created a follow-up survey comprised of 21 questions (Appendix 2) and distributed this via email to site leads at the 22 institutions that had submitted discharge documents (May–June 2020). The purpose of this follow-up survey was to create a uniform assessment of practices across all sites, recognizing that some practices might be common across sites but not uniformly addressed in discharge documents. Each question in the follow-up survey represented a category from the analysis of discharge documents described below with binary response (yes/no) to indicate if the practice in that category was present or not. Responses to this follow-up survey were received from 21 of 22 institutions.
Analysis of Novel Data
Two authors with experience in qualitative research (JLS, SRG) individually reviewed all documents submitted from each site by applying techniques from thematic analysis of qualitative data.24, 25 While thematic analysis was originally used to analyze texts transcribed from interviews, it has more recently been applied to other texts including medical records to facilitate discovery of themes to better understand a phenomenon of interest.26, 27 Accordingly, we followed steps in thematic analysis to interpret discharge documents. First, we created descriptive categories for practices addressed by the documents. These categories, modeled from initial codes in thematic analysis, were intended to sort different areas of discharge planning into categories with face value for clinicians. For example, discharge documents addressing fever (e.g., how long a patient should be afebrile prior to discharge) were sorted to an initial category of “temperature.” Similarly, documents addressing oxygen saturation levels or supplementation were categorized “oxygen.” Some initial categories were broader; for example discharge criteria for ferritin, D-dimer, and LDH were not each given their own category but were instead grouped together as “laboratory markers.” These categories were refined through negotiated consensus with authors who were the HOMERuN site-leads representing their institutions (JNG, RW, MB), and who have experience discharging COVID-19 patients at their institutions. This resulted in a list of 22 categories analogous to a final code book in thematic analysis. The Principal Investigator for HOMERuN (ADA) joined this group in identifying 5 major domains (analogous to themes) for discharge practice that emerged from the categories. Discrepancies in coding between authors were resolved through negotiated consensus, and all authors agreed on the final code structure and 5 major themes.
To characterize the degree that individual categories were described across all sites, we adapted the evidence synthesis technique of creating a concordance table where each column represents a category, and each site is a row (Appendix 3). Boxes are shaded to show concordance and footnotes are used to describe details (e.g., which specific lab values are described in the “laboratory markers” category). This concordance table was first created using discharge documents analyzed and then updated with responses from the follow-up survey to update the concordance table with different shading denoting either description in the discharge documents (green) or response from the survey (yellow). Finally, to summarize findings, we organized categories into key practice domains, analogous to the grouping of codes into themes for reporting qualitative data.
RESULTS
Guidance from the rapid review of public health organizations and professional societies based on expert opinion is presented in Table 1. We did not find any recommendations that included other sources of data such as case reports, primary, or secondary studies. Overall the guidance from these sources emphasized isolation and transmission mitigation strategies, although several addressed broad clinical criteria. To facilitate comparison of this guidance with data collected from HOMERuN sites, we included a column to cross-reference the 5 key practice domains described below as relevant.
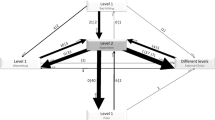
We integrated data collected from 22 US centers that provided written discharge protocols for analysis and responses to our follow-up survey from 21 centers into Figure 1. Practices in 5 domains with the greatest reported consensus are summarized in the figure, with specific considerations for each practice domain described in detail below.
-
1.
Isolation and transmission mitigation for discharge to home: use of isolation guidelines was the area of greatest consensus in this study with most (95%, 21/22) citing the Centers for Disease Control (CDC) and a few sites (32%, 7/22) also citing their state’s department of health guidance. CDC guidance calls for isolation by patients who have tested positive for COVID-19 as staying home and separated from others until the following criteria are met: 10 days since the onset of symptoms, lack of fever for 24 h without fever reducing medications, and improvement in symptoms.28 Accordingly, most hospitals included the ability for patients to socially isolate at home (e.g., separate bedrooms or bathrooms while under home isolation) as discharge criteria. Furthermore, most sites (82%, 18/22) required use of Personal Protective Equipment (PPE) in transportation from the hospital and 73% (16/22) gave PPE (masks) to patients for use at home.
-
2.
Specific discharge considerations for settings other than home: most sites (86%, 19/22) addressed discharge to Skilled Nursing Facilities, Inpatient Rehabilitation, or Long-Term Acute Care, although details of specific requirements were often set by the accepting facilities. Most sites (73%, 16/22) also gave specific guidance for homeless patients (often recommending a respite facility or similar) and many (68%, 15/22) addressed congregate or assisted living settings. Some hospitals reserved their strictest criteria (e.g., two negative COVID-19 tests or until the end of isolation per CDC guidelines) for patients being discharged to these non-home settings.
-
3.
Specific clinical criteria for discharge: most sites (82%, 18/22) addressed COVID-19-specific clinical features as criteria for discharge, although definitions and details varied considerably. A few sites (18%, 4/22) gave little or no guidance (e.g., use clinical judgment); a few sites (14%, 3/22) gave very specific guidance, using detailed algorithms based on age, comorbidities, immunocompromise, lab values, need for supplementary oxygen, stability of vital signs, and time since symptom onset, but most sites were between these extremes. For specific criteria, most sites addressed symptom improvement, temperature, and oxygen requirement, although parameters varied. For example, some sites specified that patients be afebrile for a given time (range: 24–72 h) while others simply required patients to be afebrile at discharge. Similarly, some sites specified specific oxygen saturation levels (range >90–94%) or supplementation levels (range: 2–4 L) while others simply required these to be stable or at baseline at the time of discharge. Relatively few sites (36%, 8/22) incorporated laboratory criteria, age (36%, 8/22), high-risk comorbidities (32%, 7/22), or infectious disease consultation (18%, 4/22) as considerations for the decision to discharge, e.g., requiring a longer period of stability or time since symptom onset for patients with high-risk comorbidities.
-
4.
Specific discharge considerations for social support and functional status: most sites (77%, 17/22) assessed for level of social support (variably defined) available to patients during recovery, and many (59%, 13/22) specifically assessed for patient ability to perform activities of daily living independently while under home isolation. A few sites (27%, 6/22) reported addressing Durable Medical Equipment and access to food, medication, or supplies in ways that were specific for COVID-19 patients.
-
5.
Approaches to post-discharge monitoring, instructions, and follow up: most hospitals (77%, 17/22) provided home monitoring programs and/or virtual follow-up care, which ranged from daily messaging via texting or patient portals, phone calls by a nurse, and, less commonly, the use of home pulse oximeters and/or thermometers. Many hospitals (59%, 13/22) created COVID-19-specific brochures, discharge instructions, and other materials to cover topics such as use of PPE, travel restrictions, social distancing, home isolation duration, and symptoms to watch for and what to do if they occur.
DISCUSSION
This multi-center report identifies 5 key domains of discharge practice for patients hospitalized with COVID-19. Consensus was greatest for discharge practices related to isolation and transmission mitigation. This is especially important given the resurgence pattern seen in the USA as well as other countries throughout the pandemic. Hospitals must ensure patients returning to the community are not infecting others, especially as most Americans have not yet been exposed to the SARS Co-V2 virus and herd immunity is still elusive.29, 30 Unfortunately, as many as 1 in 5 Americans may not have living quarters suitable for isolation.31 Isolation and mitigation concerns are also heightened for patients discharging to settings other than home. While specific criteria for discharge to these settings were more variable than discharge to home and often set by the receiving facility, nearly all hospitals addressed additional considerations for discharge to settings such as skilled nursing facilities, inpatient rehabilitation, homeless shelters, and assisted living.
Beyond these crucial considerations of isolation and transmission mitigation by discharge setting, we found concordance on important domains of clinical, social, and functional considerations as well as approaches to post-discharge monitoring. For clinical criteria, we found high concordance that symptom improvement, oxygen requirements, and fever curves were the most important features. While individual hospitals (just like individual clinicians) may differ in their level of comfort with specific parameters (e.g., no supplemental 02 vs minimal; afebrile 24 vs 72 h), it seems clear that oxygen and temperature should be part of the discharge decision. Similarly, while specific approaches and definitions may vary, it seems clear that some formal assessment of social support and ability to perform activities of daily living, especially while under isolation, should be performed for all COVID-19 discharges, and there should be some COVID-19-specific form of home-monitoring and/or follow-up. Given the prolonged recovery period,32 these post-discharge functional and follow-up issues may be the most important factors from a patient-centered perspective,33 analogous to isolation and transmission mitigation being the most important considerations from a public health perspective. While we did not inquire specifically about discharge practices to improve caregiver engagement,34 ensure patient-caregiver understanding,35 and improve the patient-caregiver experience,36 we note there is great opportunity for improvement with respect to the discharge of COVID patients and communication with their caregivers.
Our findings suggest urgent need for more research on clinical factors that predict poor post-discharge outcomes. Understanding these outcomes with respect to different discharge criteria will help balance length of stay with readmission risk, especially given preliminary data showing shorter length of stay may be associated with readmission in patients with COVID-19.37, 38 Additionally, the role of social determinants of health in post-hospital outcomes was generally acknowledged by our sites, but further research is needed to develop these approaches to address disparities in community-level outcomes.39, 40 Finally, beyond evidence for the utility of specific approaches within clinical practice domains, there is a need to understand the sustainability of approaches we identified, such as post-discharge monitoring. As we await empirical data to build a broader evidence base, discharge practices with high consensus in key domains can provide useful guidance for hospitals to consider as they develop and refine protocols for a prolonged COVID-19 pandemic. While hospitals and providers will naturally vary in the specifics of their discharge practices, this study provides examples of best practices that can help improve standards for the management of this disease.
This study has several limitations. First, we received data on discharge practices only from hospitalist leaders at major academic medical centers participating in HOMERuN; practices at US community hospitals and hospitals in other countries may be different. Second, we do not have outcomes data to demonstrate the impact of discharge practices reported here on outcomes such as readmission or post-discharge mortality. While this highlights a clear need for future research, we believe the discharge practices reported here provide face validity while we await outcomes data. Third, we report on discharge practices as described in discharge documents and reported by clinical leaders at the AMCs we studied; we do not have direct data from observations of clinician actions or indirect data from chart reviews to characterize variations in daily practice. Finally, while we began this study during the first (spring) surge and updated our data during the second (summer) surge, a third (winter) surge is underway at the time of this submission and practices may change. Thus, our results may be a reflection of what was known, what guidance was available, and hospital, regional, or national priorities at the time we collected our data.
In conclusion, we found that the 22 US Academic Medical Centers in our study showed general consensus on discharge practices for COVID-19 patients related to post-discharge isolation and transmission mitigation for home and non-home settings. There was high concordance among these US AMCs that discharge practices should address COVID-19-specific factors in social, functional, and post-discharge monitoring domains, although definitions and details varied. More research is needed to determine optimal clinical criteria for discharge.
References
Jenke AT, Mei H, Rothenberg C, et al. Analysis of hospital resource availability and COVID-19 mortality across the United States. J Hosp Med. In press, Dec 2020.
Auerbach A, O'Leary KJ, Greysen SR, et al for the HOMERuN COVID-19 Collaborative Group. Hospital Ward Adaptation During the COVID-19 Pandemic: a National Survey of Academic Medical Centers. J Hosp Med. 2020 Aug;15(8):483-488. doi: https://doi.org/10.12788/jhm.3476
Fraher EP, Pittman P, Frogner BK, et al. Ensuring and Sustaining a Pandemic Workforce. N Engl J Med. 2020;382(23):2181-2183. doi:https://doi.org/10.1056/NEJMp2006376
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020; 323: 1061–9.
Richardson, S. et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA (2020) doi:https://doi.org/10.1001/jama.2020.6775.
Grasselli, G. et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA (2020) doi:https://doi.org/10.1001/jama.2020.5394.
Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The Impact of the COVID-19 Pandemic on Hospital Admissions in the United States. Health Aff (Millwood). 2020 Nov;39(11):2010-2017. doi: https://doi.org/10.1377/hlthaff.2020.00980. Epub 2020 Sep 24.
Khullar D, Bond AM, Schpero WL. COVID-19 and the Financial Health of US Hospitals. JAMA. 2020;323(21):2127–2128. doi:https://doi.org/10.1001/jama.2020.6269
Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults with COVID-19 in an Integrated Health Care System in California. JAMA. 2020;323(21):2195-2198. doi:https://doi.org/10.1001/jama.2020.7202
Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020; e2012603. https://doi.org/10.1001/jama.2020.12603
Chen, R. et al. Risk Factors of Fatal Outcome in Hospitalized Subjects with Coronavirus Disease 2019 from a Nationwide Analysis in China. Chest (2020) doi:https://doi.org/10.1016/j.chest.2020.04.010.
Hong, Y. et al. Clinical characteristics of coronavirus disease 2019 and development of a prediction model for prolonged hospital length of stay. Ann. Transl. Med. 8, 443 (2020).
Liang, W. et al. Development and Validation of a Clinical Risk Score to Predict the Occurrence of Critical Illness in Hospitalized Patients with COVID-19. JAMA Intern. Med. (2020) doi:https://doi.org/10.1001/jamainternmed.2020.2033.
Cheng, F.-Y. et al. Using machine learning to predict ICU transfer in hospitalized COVID-19 patients. J. Clin. Med. Res. 9, 1668 (2020).
Li, L. et al. COVID-19-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 92, 577–583 (2020).
Razavian N, Major VJ, Sudarshan M, et al. A Validated, Real-Time Prediction Model for Favorable Outcomes in Hospitalized COVID-19 Patients. NPJ Digit Med. 2020 Oct 6; 3:130. https://doi.org/10.1038/s41746-020-00343-x. PMID: 33083565; PMCID: PMC7538971.
Sze S, Pan D, Williams CML, et al. The need for improved discharge criteria for hospitalised patients with COVID-19-implications for patients in long term care facilities. Age Ageing. 2020 Sep 19: afaa206. https://doi.org/10.1093/ageing/afaa206. Epub ahead of print. PMID: 32951032; PMCID: PMC7543250.
Auerbach AD, Patel MS, Metlay JP, et al. The Hospital Medicine Reengineering Network (HOMERuN): a learning organization focused on improving hospital care. Acad Med. 2014;89(3):415-420.
The Breakthrough Series: IHI’s Collaborative Model for Achieving Breakthrough Improvement White Paper. Boston: Institute for Healthcare Improvement; 2003. Accessed Dec 2, 2020 at: http://www.ihi.org/resources/Pages/IHIWhitePapers/TheBreakthroughSeriesIHIsCollaborativeModelforAchievingBreakthroughImprovement.aspx
Penn Medicine Center for Evidence-based Practice, Methods Documents – Rapid Guidance Summary methods document. Accessed Dec 2, 2020 at: http://www.uphs.upenn.edu/cep/methods/index.html
Penn Medicine COVID-19 Guidance Summaries, Center for Evidence-based Practice – Criteria for Discharging Patients from Inpatient Care. Accessed Dec 2, 2020 at: http://www.uphs.upenn.edu/cep/COVID/indexCOVID.html
COVID-19 Guidance Collaborative. Agency for Healthcare Research and Quality. Accessed Dec 2, 2020 at: https://digital.ahrq.gov/covid-acts
Mitchell M, Flores E, Connolly J, Goel R, Gottschalk A, Kavanagh N, Philipson B, Wang S, Wood C, Mull N. Ultra-rapid synthesis of secondary sources for hospital COVID-19 guidance. Advances in Evidence Synthesis: special issue. Cochrane Database of Systematic Reviews 2020;(9 Suppl 1):440 https://doi.org/10.1002/14651858.CD202001
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101.
Giacomini MK, Cook DJ; Evidence-Based Medicine Working Group. Users’ guides to the medical literature: XXIII: qualitative research in health care A: are the results of the study valid? JAMA. 2000;284(3):357-362.
Wong SPY, Vig EK, Taylor JS, et al. Timing of initiation of maintenance dialysis: a qualitative analysis of the electronic medical records of a national cohort of patients from the Department of Veterans Affairs. JAMA Intern Med. 2016;176(2):228-235.
Butler CR, Wightman A, Richards CA, et al. Thematic analysis of the health records of a national sample of US Veterans with advanced kidney disease evaluated for transplant. JAMA Intern Med. Published online November 23, 2020.
COVID-19 Quarantine vs. Isolation. Centers for Disease Control. Site accessed on Dec 1, 2020 at: https://www.cdc.gov/coronavirus/2019-ncov/downloads/COVID-19-Quarantine-vs-Isolation.pdf
Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Intern Med. Published online November 24, 2020. doi:https://doi.org/10.1001/jamainternmed.2020.7976
Omer SB, Yildirim I, Forman HP. Herd Immunity and Implications for SARS-CoV-2 Control. JAMA. 2020;324(20):2095–2096. doi:https://doi.org/10.1001/jama.2020.20892
Sehgal AR, Himmelstein DU, Woolhandler S. Feasibility of separate rooms for home isolation and quarantine for COVID-19 in the United States. Ann Intern Med. 2020 Jul 21:M20-4331.
Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-Day Outcomes Among Patients Hospitalized With COVID-19. Ann Intern Med. 2020 Nov 11. https://pubmed.ncbi.nlm.nih.gov/33175566. Epub ahead of print.
Bowles KH, McDonald M, Barrón Y, Kennedy E, O'Connor M, Mikkelsen M. Surviving COVID-19 After Hospital Discharge: Symptom, Functional, and Adverse Outcomes of Home Health Recipients. Ann Intern Med. 2020 Nov 24. https://pubmed.ncbi.nlm.nih.gov/33226861. Epub ahead of print.
Rodakowski J, Rocco PB, Ortiz M et al. Caregiver integration during discharge planning for older adults to reduce resource use: a metaanalysis. J Am Geriatr Soc 2017; 65: 1748-1755.
Greysen SR, Harrison JD, Kripalani S, Vasilevskis E, Robinson E, Metlay J, Schnipper JL, Meltzer D, Sehgal N, Ruhnke GW, Williams MV, Auerbach AD. Understanding patient-centred readmission factors: a multi-site, mixed-methods study. BMJ Qual Saf. 2017 Jan;26(1):33-41.
Cancino RS, Manasseh C, Kwong L, Mitchell SE, Martin J, Jack BW. Project RED Impacts Patient Experience. J Patient Exp. 2017 Dec;4(4):185-190. doi: https://doi.org/10.1177/2374373517714454. Epub 2017 Jun 16.
Somani SS, Richter F, Fuster V, et al. Characterization of Patients Who Return to Hospital Following Discharge from Hospitalization for COVID-19. J Gen Intern Med. 2020 Oct;35(10):2838-2844. https://doi.org/10.1007/s11606-020-06120-6. Epub 2020 Aug 19. PMID: 32815060; PMCID: PMC7437962.
Parra Ramírez LM, Caballero MC, Morrás de la Torre I, et al. Hospital Readmissions of Discharged Patients with COVID-19. medRxiv preprint https://doi.org/10.1101/2020.05.31.20118455. Site accessed Dec 2, 2020.
Adhikari S, Pantaleo NP, Feldman JM, Ogedegbe O, Thorpe L, Troxel AB. Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas. JAMA Netw Open. 2020;3(7):e2016938. doi:https://doi.org/10.1001/jamanetworkopen.2020.16938
Gu T, Mack JA, Salvatore M, et al. Characteristics Associated with Racial/Ethnic Disparities in COVID-19 Outcomes in an Academic Health Care System. JAMA Netw Open. 2020;3(10): e2025197.
Acknowledgements
Data from this paper was presented at the 2020 Society of Hospital Medicine Annual Meeting (Virtual).
Funding
This project was funded by the Gordon and Betty Moore Foundation. The funder had no role in the design or interpretation of this study or the interpretation of results.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to conceptualization, initial drafting and revisions of the manuscript, and all authors approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Greysen, S.R., Auerbach, A.D., Mitchell, M.D. et al. Discharge Practices for COVID-19 Patients: Rapid Review of Published Guidance and Synthesis of Documents and Practices at 22 US Academic Medical Centers. J GEN INTERN MED 36, 1715–1721 (2021). https://doi.org/10.1007/s11606-021-06711-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11606-021-06711-x