Published online Mar 16, 2022. doi: 10.12998/wjcc.v10.i8.2457
Peer-review started: July 27, 2021
First decision: October 22, 2021
Revised: November 4, 2021
Accepted: January 29, 2022
Article in press: January 29, 2022
Published online: March 16, 2022
Coronavirus disease 2019 (COVID-19) has become a worldwide pandemic and significant public health issue. The effectiveness of extracorporeal membrane oxygenation (ECMO) in treating COVID-19 patients has been called into question.
To conduct a meta-analysis on the mortality of COVID-19 patients who require ECMO.
This analysis adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes 2020 (PRISMA) and has been registered at the International Prospective Register of Systematic Reviews (number CRD42020227414). A quality assessment for all the included articles was performed by the Newcastle-Ottawa Scale (NOS). Studies with tenor more COVID-19 patients undergoing ECMO were included. The random-effects model was used to obtain the pooled incidence of mortality in COVID-19 patients receiving ECMO. The source of heterogeneity was investigated using subgroup and sensitivity analyses.
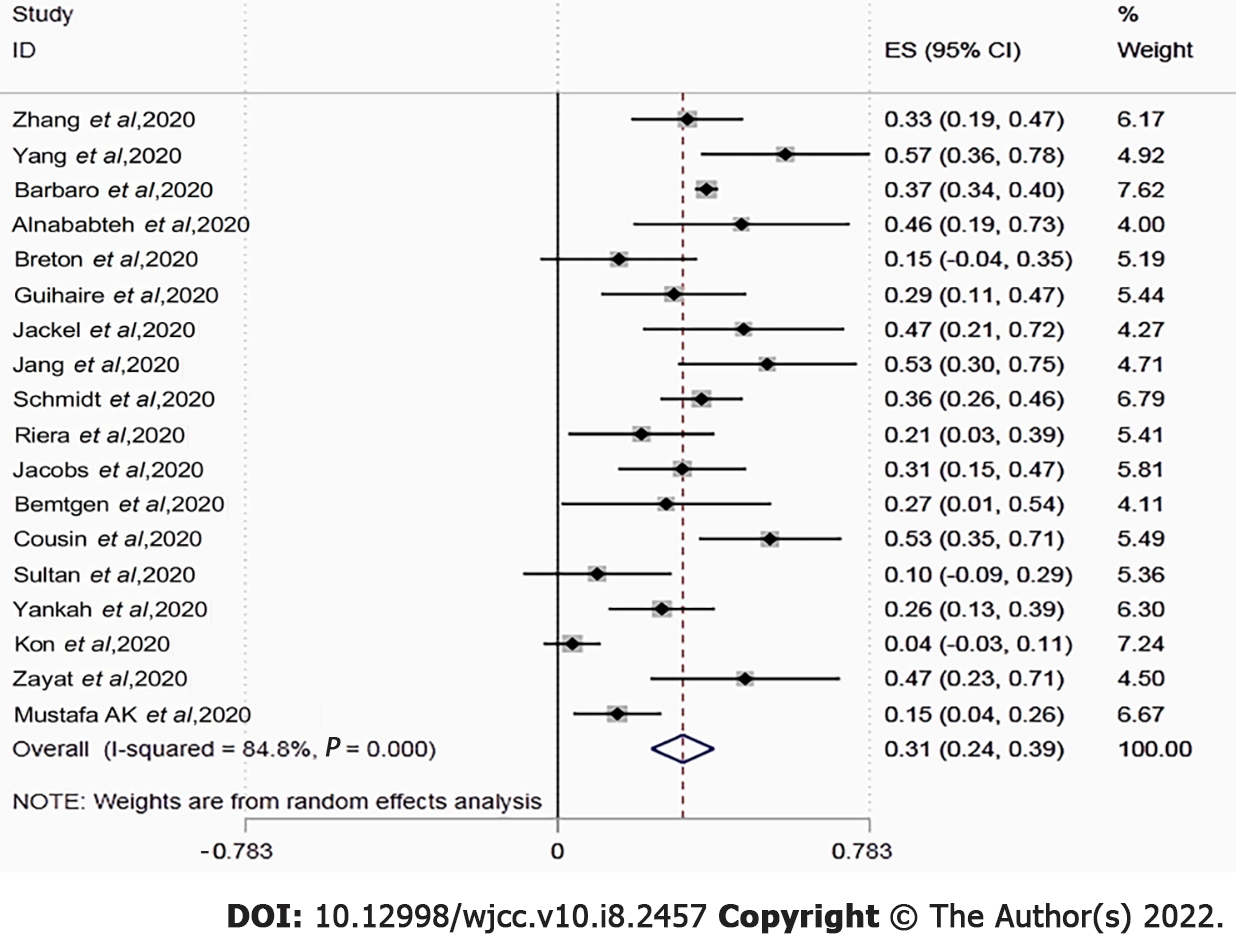
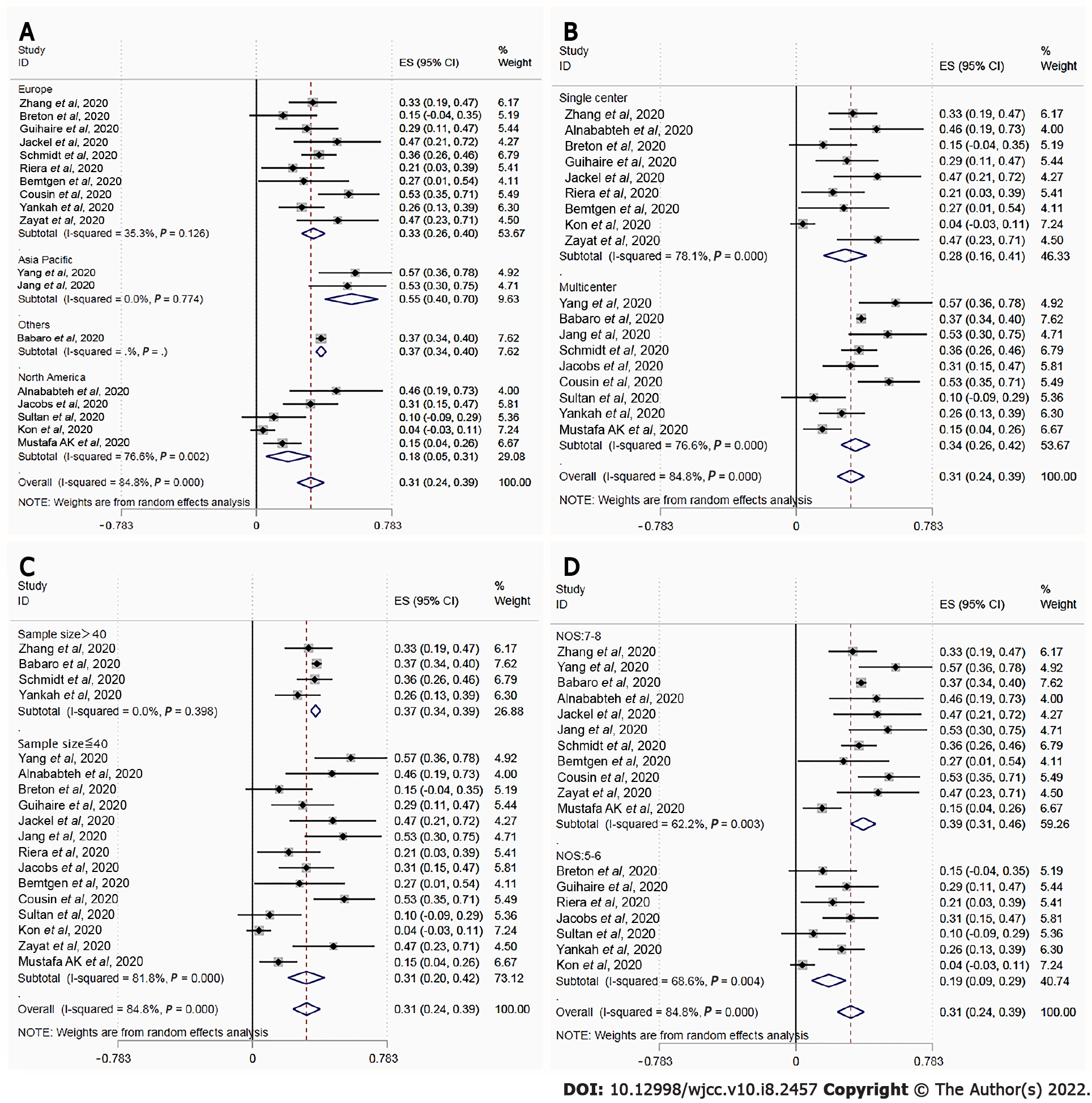
We identified 18 articles with 1494 COVID-19 patients who were receiving ECMO. The score of the quality assessment ranged from 5 to 8 on the NOS. The majority of patients received veno-venous ECMO (93.7%). Overall mortality was estimated to be 0.31 [95% confidence interval (CI): 0.24-0.39; I2 = 84.8%] based on random-effect pooled estimates. There were significant differences in mortality between location groups (33.0% vs 55.0% vs 37.0% vs 18.0%, P < 0.001), setting groups (28.0% vs 34.0%, P < 0.001), sample size (37.0% vs 31.0%, P < 0.001), and NOS groups (39.0% vs 19.0%, P < 0.001). However, both subgroup analyses based on location, setting, and sample size, and sensitivity analysis failed to identify the source of heterogeneity. The funnel plot indicated no evident asymmetry, and the Egger's (P = 0.95) and Begg's (P = 0.14) tests also revealed no significant publication bias.
With more resource assessment and risk-benefit analysis, our data reveal that ECMO might be a feasible and effective treatment for COVID-19 patients.
Core Tip: Coronavirus disease 2019 (COVID-19) has become a worldwide pandemic and significant public health issue. The effectiveness of extracorporeal membrane oxygenation (ECMO) in treating COVID-19 patients has been called into question. Therefore, we conducted this meta-analysis on the mortality of COVID-19 patients who require ECMO. We identified 18 articles with 1494 COVID-19 patients who were receiving ECMO. With more resource assessment and risk-benefit analysis, our data reveal that ECMO might be a feasible and effective treatment for COVID-19 patients.
- Citation: Zhang Y, Wang L, Fang ZX, Chen J, Zheng JL, Yao M, Chen WY. Mortality in patients with COVID-19 requiring extracorporeal membrane oxygenation: A meta-analysis. World J Clin Cases 2022; 10(8): 2457-2467
- URL: https://www.wjgnet.com/2307-8960/full/v10/i8/2457.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i8.2457
Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has become a global pandemic. As of October 25, 2021, there have been over 243 million infections and approximately 4.9 million deaths[1]. It was characterized by rapid disease progression and a high risk of acute respiratory distress syndrome (ARDS) due to severe or fatal pneumonia[2]. Although the majority of COVID-19 patients have very moderate disease, current studies suggest that approximately 18% of them have serious disease[3] with a mortality rate of up to 53%-67%[4,5].
Extracorporeal membrane oxygenation (ECMO), which can support gas exchange in patients failing conventional mechanical ventilation[6], has been proven to be effective in the treatment of severe respiratory failure and cardiovascular compromise caused by ARDS, based on the experience of previous viral outbreaks, such as Middle East Respiratory Syndrome and H1N1 influenza[7-9]. There are two primary ECMO therapy methods: Veno-venous ECMO (VV-ECMO) and veno-arterial ECMO (VA-ECMO). For the vast majority of COVID-19 patients who need ECMO treatment, we adopted VV-ECMO. The World Health Organization (WHO) and Chinese experts have recommended that ECMO can be used as a salvage therapy for severe COVID-19 patients who are not responding to conventional ARDS treatments[10-14]. Although there are still numerous complications, research has revealed that 63% of patients recovered from ARDS and were weaned from ECMO.
Despite such optimism for a possible role for ECMO in COVID-19, concerns have been raised due to the high mortality rate observed in studies[15]. Venerable age, late ECMO initiation time, and multiple complications (diabetes, heart disease, obesity, etc.) are all independent risk factors that increase the 90-d mortality rate. The role of ECMO in the treatment of diseases caused by this new virus is still uncertain and controversial. Currently, there is insufficient worldwide evidence to assess the effectiveness of ECMO. The majority of prior studies were based on retrospective cohort studies and case reports in a specific population, making it difficult to analyze the impact of ECMO on COVID-19 patients in a systematic manner[6]. Therefore, we conducted a meta-analysis focusing on the mortality of COVID-19 patients requiring ECMO in order to guide current clinical practice and future research efforts.
To identify articles concerning ECMO and COVID-19, a comprehensive literature search was undertaken utilizing the PubMed, EMBASE, Web of Science, Cochrane Library, and Clinical Trials databases. On December 9, 2020, all searches were updated, and the search strategy was divided into two categories: (1) COVID-19; and (2) ECMO. The following keywords were used: “COVID-19”, “2019 novel coronavirus disease”, “2019-nCoV”, and “Extracorporeal membrane oxygenation.” The articles were limited to English literature. To prevent missing relevant records, reference lists from the selected studies and systematic reviews were thoroughly searched. Furthermore, this systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines, and it was registered at the International Prospective Register of Systematic Reviews (number CRD42020227414) prior to submission.
Original studies that matched the following criteria were included in the meta-analysis: (1) Studies with tenor more patients; (2) Patients with laboratory-confirmed COVID-19 receiving ECMO; (3) Studies with primary data; and (4) Studies with more than 5 points on the Newcastle-Ottawa scale (NOS). The exclusion criteria were: (1) Duplicate publication or dataset; and (2) Animal and in vitro experiments, case reports, letters, editorials, comments, conference summaries, meta-analyses, and reviews were excluded.
Data from the included articles were extracted, including authors' names, publication year, study location, study design, sample size, patient baseline characteristics, clinical parameters, management, primary outcome, and complications. The article search, selection, data extractions, and quality assessment were all conducted independently by two reviewers (ZY and CWY), and any disagreements were resolved after consensus.
The NOS was applied for a quality assessment on all of the included papers. All included papers were classified as poor (scores 0–3), moderate (scores 4–6), or high (scores 7–9) quality studies, with papers with more than 5 points being considered for further analysis.
The pooled estimates of the event rate were obtained using the random-effects model. Each study had its own underlying effect size. The random-effects model assumes that there is a mean population effect size that varies depending on the study. We used the inconsistency statistic (I2) to evaluate the extent of heterogeneity. An I2 value greater than 50% was considered to indicate substantial heterogeneity. The Egger's and Begg's tests were used to assess publication bias and a visual funnel plot for asymmetry was presented. Sensitivity analysis and subgroup analysis were also utilized to investigate the source of heterogeneity and a two-sided test at the 5% level was considered statistically significant. Moreover, Stata 15.1 (Stata Corporation, College Station, TX, United States) was utilized to conduct the meta-analysis.
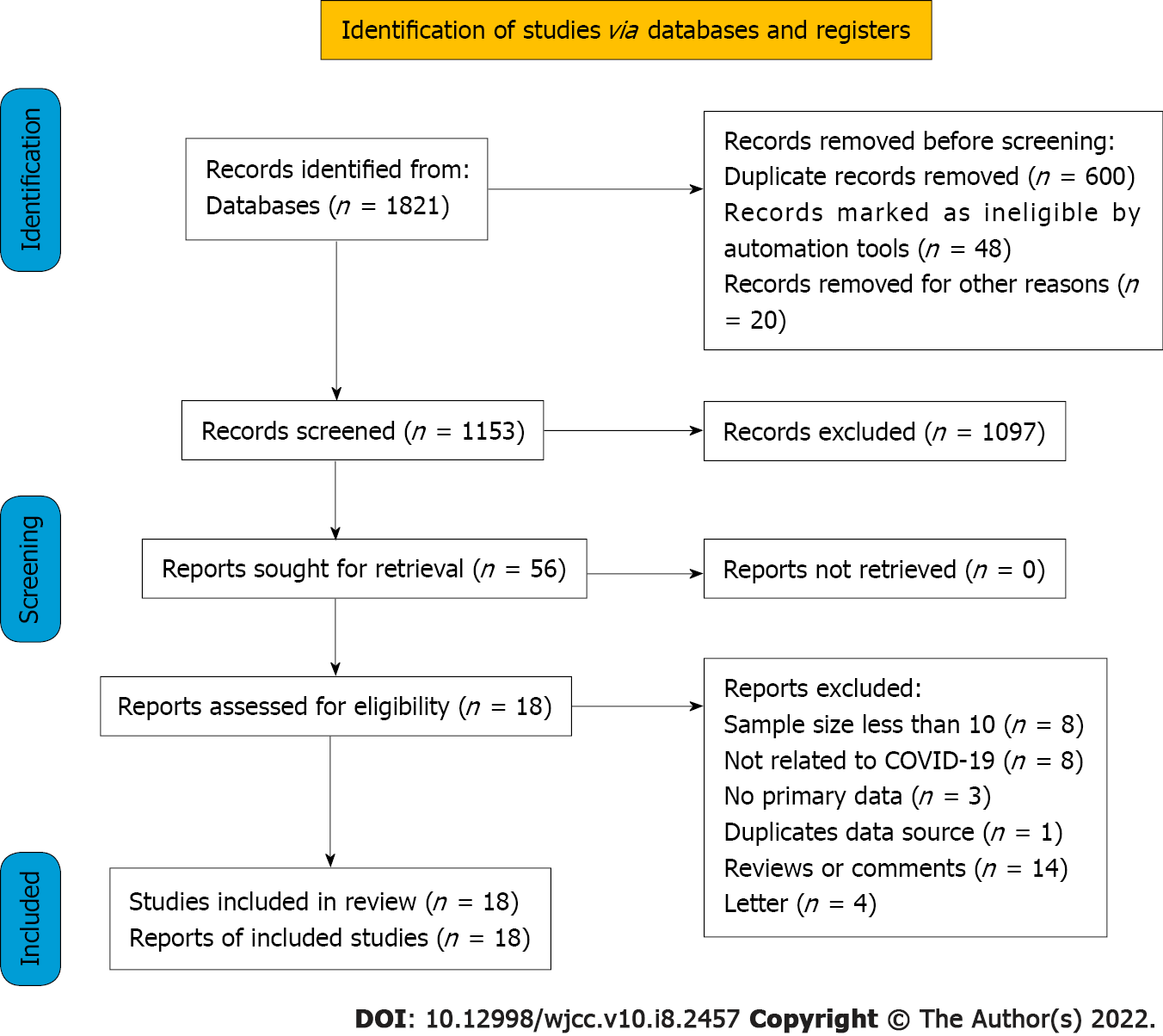
In the initial search, 1821 potentially relevant articles were identified. After eliminating duplicates, 1153 studies were assessed based on title and abstract. Following a full-text review of the remaining 56 studies, 38 studies were excluded. Finally, the meta-analysis comprised 18 articles representing 1494 verified COVID-19 patients treated with ECMO (Figure 1). Since EuroELSO was a subset of centers participating in the international ELSO registry, we eliminated the previous study on EuroELSO to avoid data duplication[16,17].
The characteristics of these studies are summarized in Table 1. All of the studies were observational in design, with the majority of them being retrospective (94.2%). The score of the quality assessment ranged from 5 to 8 on the NOS. All studies were rated as “moderate to high quality.” In the majority of cases, VV-ECMO was utilized (93.8%). Table 2 summarizes the sample characteristics of the included studies.
| Ref. | Year | Location | Study design | Sample size | Type of ECMO (VV%) | Follow-up | NOS score |
| Zhang et al[31] | 2020 | United Kingdom | Retrospective/Single center | 43 | 100% | In-hospital | 7 |
| Yang et al[21] | 2020 | China | Retrospective/Multicenter | 21 | NA | In-hospital | 8 |
| Barbaro et al[16] | 2020 | ELSO | Retrospective/Multicenter | 1035 | 94.49% | 3 mo | 7 |
| Alnababteh et al[32] | 2021 | United States | Retrospective/Single center | 13 | 100% | In-hospital | 8 |
| Le Breton et al[27] | 2020 | France | Retrospective/Single center | 13 | 100% | In-hospital | 5 |
| Guihaire et al[33] | 2020 | France | Retrospective/Single center | 24 | 100% | In-hospital | 5 |
| Jäckel et al[34] | 2020 | Germany | Retrospective/Single center | 15 | 100% | 1 mo | 8 |
| Jang et al[19] | 2020 | Korea | Retrospective/Multicenter | 19 | 84.21% | In-hospital | 7 |
| Schmidt et al[35] | 2020 | France | Retrospective/Multicenter | 83 | 97.59% | 3 mo | 7 |
| Riera et al[36] | 2020 | Spain | Retrospective/Single center | 19 | 100% | In-hospital | 5 |
| Jacobs et al[24] | 2020 | United States | Retrospective/Multicenter | 32 | 78.13% | In-hospital | 6 |
| Bemtgen et al[37] | 2021 | German | Prospective/ Single center | 11 | 100% | 28 d | 8 |
| Cousin et al[20] | 2021 | France | Retrospective/Multicenter | 30 | 100% | 3 mo | 8 |
| Sultan et al[28] | 2020 | United States | Retrospective/Multicenter | 10 | 100% | 26 d | 5 |
| Yankah et al[38] | 2021 | German | Retrospective/Multicenter | 42 | 100% | 1 mo | 5 |
| Kon et al[29] | 2021 | United States | Retrospective/Single center | 27 | 100% | In-hospital | 6 |
| Zayat et al[39] | 2021 | German | Retrospective/Single center | 17 | 94.12% | In-hospital | 7 |
| Mustafa et al[40] | 2020 | United States | Retrospective/Multicenter | 40 | 100% | In-hospital | 7 |
| Ref. | Age (yr) | Proportion of male (%) | Smoke | SOFA | PH | PaO2/FiO2 (mmHg) | Comorbidities | ECMO duration (d) | Pre-ECMO MV (d) | Complications |
| Zhang et al[31] | 46 (35.5-52.5) | 76.7% | NA | 7 (4-10) | 7.30 (7.19–7.36) | 67.5 (58.9–77.8) | (1), (2), (3), (4), (5) | 13 (8-20) | 5 (2-6) | 1, 2, 4, 5 |
| Yang et al[18] | 45.2 ± 14.5 | 57.1% | NA | 6.5 (4.8-8.0) | 7.30 (7.19–7.41) | 60 (55.6-72.0) | (3), (4), (6) | 9.1 (5.9-24.8) | 1.5 (0.5-3.5) | 1, 2, 6 |
| Barbaro et al[16] | 49 (41–57) | 73.8% | NA | NA | NA | 72 (59-94) | (2), (3), (4), (5), (6) | 13.9 (7.8-23.3) | 4 (1.8-6.4) | 1, 3, 7, 8 |
| Alnababteh et al[32] | 44.5 ± 9.5 | 61.5% | 1 | 9.3 ± 4.5 | 7.32 (7.23-7.39) | 81.3 ± 20.3 | (1), (2) | 12 (10.4-16.2) | NA | 1, 2, 6 |
| Le Breton et al[27] | 49.3 | 69.2% | 4 | 9.9 | 7.28 | 59 | (1) | 13 (3-34) | 6 | 1, 2 |
| Guihaire et al[33] | 48.8 ± 8.9 | 83.3% | 2 | NA | NA | 67 (52–78) | (1), (2) | 19 ± 10.1 | 6.3 (1-11) | 1, 3 |
| Jäckel et al[34] | 60.8 (54.1-67.0) | 26.7% | 3 | 10 (8-11) | 7.30 (7.23-7.40) | NA | (1), (2), (3), (4) | 11.3 (7.8-23.8) | NA | NA |
| Jang et al[19] | 63 (60-66) | 79.0% | 3 | NA | 7.30 (7.20-7.40) | 92 (62.4-138.7) | (1), (2), (3), (4), (5), (6) | 15.9 (7.7-28.2) | 3.1 (0.8-5.5) | 1, 4, 5, 6, 8 |
| Schmidt et al[35] | 49 (41-56) | 73.5% | 2 | 12 (9-13) | 7.32 (7.24–7.38) | 60 (54–68) | (1), (3), (4), (5), (6), (7) | NA | 4 (3-6) | 1, 2, 3, 5, 8 |
| Riera et al[36] | 50.5 (31-64) | 84.2% | NA | NA | 7.2 (6.9–7.4) | 70.8 (57–118) | (1), (3) | 10.7 (2-33) | NA | 1, 3 |
| Jacobs et al[24] | 52.4 ± 12.5 | 68.8% | NA | NA | NA | NA | (2), (3), (4), (5) | 7.3 ± 3.3 | 4.3 ± 2.4 | NA |
| Bemtgen et al[37] | 59.4 (49.8-61.1) | 63.6% | NA | 14 (13–16) | NA | NA | NA | 17.9 (7.8-23.8) | NA | NA |
| Cousin et al[20] | 57 (47-62) | 80.0% | 1 | 10 (7-12) | 7.37 (7.32-7.41) | 69 (63-75) | (1), (3), (4), (5), (7) | 11 (7-14) | 6 (4-9) | 1, 2, 3, 6, 8 |
| Sultan et al[28] | NA | 70.0% | 2 | NA | NA | NA | (3), (4) | 11 (4-14) | NA | 6 |
| Yankah et al[38] | 51 (25-73) | NA | NA | NA | NA | NA | NA | 10.6 | NA | NA |
| Kon et al[29] | 40 (30.5-47) | 85.2% | NA | NA | 7.28 (7.22-7.39) | 84 (70-118) | (1), (3), (4), (5), (6), (7) | 11 (10-14) | 2 (1-4) | 3, 4, 6 |
| Zayat et al[39] | 57 (39-73) | 64.7% | 4 | 11.9 ± 9.4 | 7.3 (7.2-7.4) | 53-75 | (1), (3), (5), (6), (7) | 16 (11-21) | 3 (3-15) | 1, 3, 5, 8 |
| Mustafa et al[40] | 48.4 (22-62) | 75% | 7 | NA | NA | NA | (1), (2), (3), (4), (5), (6) | 13.0 ± 2.6 | 4.0 ± 0.5 | NA |
The in-hospital or short-term mortality ranged between 0.04 and 0.57. Overall pooled mortality was estimated to be 0.31 [95% confidence interval (CI): 0.24-0.39; I2 = 84.8%] using random-effect pooled estimates (Figure 2). Two studies found that the mortality rate in the ECMO group was lower than that in the mechanical ventilator group (57.1% vs 63.2% and 46.2% vs 47.8%).
Figure 3 depicts the outcomes of the random effects model and subgroup analysis. There were significant differences in mortality between location groups (Figure 3A; 33.0% vs 55.0% vs 37.0% vs 18.0%, P < 0.001), setting groups (Figure 3B; 28.0% vs 34.0%, P < 0.001), sample size group (Figure 3C; 37.0% vs 31.0%, P < 0.001), and NOS groups (Figure 3D; 39.0% vs 19.0%, P < 0.001). There was no source of heterogeneity in any of the subgroups.
Funnel plots showed no obvious asymmetry. The Egger’s (P = 0.95) and Begg’s tests (P = 0.14) further revealed no significant publication bias. The recalculated pooled results did not change significantly after removing each study sequentially, showing that there was no outlier study that influenced the overall result.
ECMO is an essential instrument for treating severe respiratory and heart failure, and it was widely employed in the treatment of critically ill patients during the COVID-19 pandemic. However, previous research found that the impact of ECMO in critically ill COVID-19 patients was controversial due to the high mortality rate. Therefore, we conducted this meta-analysis that included 18 original independent studies to examine the effectiveness of ECMO in the treatment of COVID-19. The mortality rate of COVID-19 patients treated with ECMO in our investigation was 31%, which was lower than the mortality rate of severe COVID-19 patients in the prior study. These data imply that ECMO treatment may decrease the mortality rate of COVID-19 patients who are severely ill.
Although some studies reported that the mortality rate of COVID-19 patients treated with ECMO is as high as 53%-83.3%[18-22], which is substantially higher than the results of this study, we believe such high mortality may be due to other reasons rather than the ECMO treatment being ineffective. For example, many previous studies conducted in the early stage of the COVID-19 outbreak used a small sample size with a high proportion of patients having pre-existing comorbidities, which may be an important factor contributing to the high mortality[23]. Additionally, due to a lack of experience in treating COIVD-19 at the beginning of the outbreak, ECMO treatment may have been initiated too late. The secondary infection and other complications may also lead to high mortality. These possible reasons of increased mortality are consistent with prior results obtained by certain researchers who discovered that variables such as advanced age (> 65 years old), gender (male), pre-existing diseases, and acute or chronic organ failure may contribute to a poor prognosis[24-26]. Considering these factors, our findings may provide evidence for the future application of ECOM in treating severe COVID-19 patients.
According to the studies with a low mortality rate among patients who received ECMO treatment, we recommend that ECMO should be combined with the use of other therapies, such as antiviral, immunosuppressive, anticoagulant, and supportive therapies, as well as the collaboration of professional multidisciplinary teams and professional nursing[27-29]. Additionally, the timing of ECMO intervention may also be a critical factor affecting the prognosis. Previous studies have confirmed that early ECMO intervention after mechanical ventilation can enhance the survival rate of patients with HINI pneumonia and ARDS[26]. The majority of patients with severe ARDS and severe SARS-CoV-2 pneumonia received delayed treatment and rapidly worsened. Yang et al[21] showed that among COVID-19 patients treated with ECMO, the average time from mechanical ventilation to ECMO in the survival group was shorter than that in the death group. The use of early ECMO may be associated with a better prognosis[18]. Despite the fact that the PaO2/FiO2 ratio is less than 80 mm Hg and the MV treatment has been administered, we believe that ECMO should be initiated as soon as possible. Moreover, research has shown that prolonged ECMO treatment may be associated with an increased risk of death and multiple organ failure[30]. Therefore, both the timing and duration of ECMO treatment are critical to consider when treating critically ill patients with COVID-19.
We acknowledge that our study has several limitations. To begin with, the power of the study is limited by the small sample size. Biases in patient selection and indication may have existed due to the small sample size. Additionally, the majority of the included studies were observational with retrospective designs, and more than half of the studies were single-center ones, which may lead to selection and reporting bias. Finally, the survival analyses for mortality were based on a short-term follow-up and varied death observation time, which might have underestimated the reported prevalence of mortality and complications.
Despite the fact that ECMO is approved as a rescue treatment for severe COVID-19, its application during the COVID-19 pandemic is currently restricted due to high treatment costs and an unclear usage procedure. Our findings suggested that ECMO might be a feasible and effective treatment for COVID-19 patients with more resource evaluation and risk-benefit analysis. Further research with a large sample size and multi-center designs is needed to examine the effectiveness of ECMO in the treatment of COVID-19 patients.
Patients with moderate to severe coronavirus disease 2019 (COVID-19) infection often have severe acute respiratory distress syndrome, with a poor prognosis, limited treatment options, and high mortality. Extracorporeal membrane oxygenation (ECMO) has been proven to have the advantages of improving symptoms and reducing mortality in previous studies. However, existing studies lack strong evidence and disagreement whether ECMO can reduce the mortality of patients with moderate to severe COVID-19 infection. This article intends to summarize the mortality of COVID-19 patients treated with ECMO in order to evaluate the efficacy of ECMO in COVID-19 patients.
To summarize the mortality and comorbidities of COVID-19 patients treated with ECMO, to evaluate the efficacy and adverse reactions of ECMO inCOVID-19 patients, and to clarify the effectiveness of ECMO treatment, in order to provide a basis for future treatment options for patients with moderate to severe COVID-19.
To evaluate the efficacy and adverse reactions of ECMO inCOVID-19 patients.
This research was conducted through the method of meta-analysis. A random effects model was adopted to assess the mortality of COVID-19 patients treated with ECMO.
The mortality rate of COVID-19 patients treated with ECMO was 31%, which was lower than the mortality rates of severe COVID-19 patients in previous studies, suggesting that ECMO treatment can reduce the mortality rate of severe COVID-19 patients. Previous studies have not yet given clear answers to when ECMO should be used and the duration of ECMO treatment.
ECMO may be a feasible and effective treatment for COVID-19 patients.
Prospective, large-sample, multi-center studies are needed to have a more comprehensive under
We would like to thank all authors of the included primary studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam VA S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | WHO. Coronavirus Disease (COVID-19) Situation Reports. [cited 6 February 2021]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. [Cited in This Article: ] |
| 2. | Singhal T. A review of coronavirus disease-2019 (COVID-19). Indian J Ped. 2020;87:281-286. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2006] [Cited by in F6Publishing: 1477] [Article Influence: 369.3] [Reference Citation Analysis (0)] |
| 3. | Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: A single arm meta-analysis. J Med Virol. 2020;92:612-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 292] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 4. | Xu J, Yang X, Yang L, Zou X, Wang Y, Wu Y, Zhou T, Yuan Y, Qi H, Fu S, Liu H, Xia J, Xu Z, Yu Y, Li R, Ouyang Y, Wang R, Ren L, Hu Y, Xu D, Zhao X, Yuan S, Zhang D, Shang Y. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: a multicenter retrospective study from Wuhan, China. Crit Care. 2020;24:394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 138] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 5. | Xie J, Wu W, Li S, Hu Y, Hu M, Li J, Yang Y, Huang T, Zheng K, Wang Y, Kang H, Huang Y, Jiang L, Zhang W, Zhong M, Sang L, Zheng X, Pan C, Zheng R, Li X, Tong Z, Qiu H, Du B. Clinical characteristics and outcomes of critically ill patients with novel coronavirus infectious disease (COVID-19) in China: a retrospective multicenter study. Intensive Care Med. 2020;46:1863-1872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 6. | Hu BS, -Z Hu M, Jiang LX, Yu J, Chang Y, Cao Y, Dai ZP. Extracorporeal membrane oxygenation (ECMO) in patients with COVID-19: a rapid systematic review of case studies. Eur Rev Med Pharmacol Sci. 2020;24:11945-11952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 7. | Roch A, Lepaul-Ercole R, Grisoli D, Bessereau J, Brissy O, Castanier M, Dizier S, Forel JM, Guervilly C, Gariboldi V, Collart F, Michelet P, Perrin G, Charrel R, Papazian L. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med. 2010;36:1899-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, Mourvillier B, Ara-Somohano C, Bastien O, Zogheib E, Clavel M, Constan A, Marie Richard JC, Brun-Buisson C, Brochard L; REVA Research Network. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med. 2013;187:276-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 351] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 9. | Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, Shalhoub S, Almotairi A, Al Khatib K, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Al Mekhlafi GA, Al Harthy A, Kharaba A, Ahmadi MA, Sadat M, Mutairi HA, Qasim EA, Jose J, Nasim M, Al-Dawood A, Merson L, Fowler R, Hayden FG, Balkhy HH; Saudi Critical Care Trial Group. Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit Care Med. 2017;45:1683-1695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Barrasa H, Rello J, Tejada S, Martín A, Balziskueta G, Vinuesa C, Fernández-Miret B, Villagra A, Vallejo A, San Sebastián A, Cabañes S, Iribarren S, Fonseca F, Maynar J; Alava COVID-19 Study Investigators. SARS-CoV-2 in Spanish Intensive Care Units: Early experience with 15-day survival in Vitoria. Anaesth Crit Care Pain Med. 2020;39:553-561. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 144] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 11. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32663] [Cited by in F6Publishing: 28479] [Article Influence: 7119.8] [Reference Citation Analysis (3)] |
| 12. | Tang X, Du RH, Wang R, Cao TZ, Guan LL, Yang CQ, Zhu Q, Hu M, Li XY, Li Y. Comparison of hospitalized patients with ARDS caused by COVID-19 and H1N1. Chest. 2020;158:195-205. [DOI] [Cited in This Article: ] [Cited by in Crossref: 235] [Cited by in F6Publishing: 220] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 13. | Zangrillo A, Beretta L, Scandroglio AM, Monti G, Fominskiy E, Colombo S, Morselli F, Belletti A, Silvani P, Crivellari M, Monaco F, Azzolini ML, Reineke R, Nardelli P, Sartorelli M, Votta CD, Ruggeri A, Ciceri F, De Cobelli F, Tresoldi M, Dagna L, Rovere-Querini P, Serpa Neto A, Bellomo R, Landoni G; COVID-BioB Study Group. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;. [PubMed] [Cited in This Article: ] |
| 14. | WHO. Clinical management of COVID-19. [cited 6 February 2021]. Available from: https://www.who.int/publications-detail-redirect/clinical-management-of-covid-19. [Cited in This Article: ] |
| 15. | Ñamendys-Silva SA. ECMO for ARDS due to COVID-19. Heart Lung. 2020;49:348-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Barbaro RP, MacLaren G, Boonstra PS, Iwashyna TJ, Slutsky AS, Fan E, Bartlett RH, Tonna JE, Hyslop R, Fanning JJ, Rycus PT, Hyer SJ, Anders MM, Agerstrand CL, Hryniewicz K, Diaz R, Lorusso R, Combes A, Brodie D; Extracorporeal Life Support Organization. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 577] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 17. | Marullo AG, Cavarretta E, Biondi Zoccai G, Mancone M, Peruzzi M, Piscioneri F, Sartini P, Versaci F, Morelli A, Miraldi F, Frati G. Extracorporeal membrane oxygenation for critically ill patients with coronavirus-associated disease 2019: an updated perspective of the European experience. Minerva Cardioangiol. 2020;68:368-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Yang X, Cai S, Luo Y, Zhu F, Hu M, Zhao Y, Zheng R, Li X, Hu B, Peng Z. Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome: A Multicenter Descriptive Study. Crit Care Med. 2020;48:1289-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 19. | Jang WS, Kim J, Baek J, Jung H, Jang JS, Park JS, Oh TH, Jang SY, Kim YS, Kwon YS. Clinical course of COVID-19 patients treated with ECMO: A multicenter study in Daegu, South Korea. Heart Lung. 2021;50:21-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Cousin N, Bourel C, Carpentier D, Goutay J, Mugnier A, Labreuche J, Godeau E, Clavier T, Grange S, Tamion F, Durand A, Moussa MD, Duburcq T; Lille Intensive Care COVID-19 Group. SARS-CoV-2 Versus Influenza-associated Acute Respiratory Distress Syndrome Requiring Veno-venous Extracorporeal Membrane Oxygenation Support. ASAIO J. 2021;67:125-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6231] [Cited by in F6Publishing: 6349] [Article Influence: 1587.3] [Reference Citation Analysis (0)] |
| 22. | Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2604] [Cited by in F6Publishing: 3048] [Article Influence: 762.0] [Reference Citation Analysis (0)] |
| 23. | Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, Curtis HJ, Mehrkar A, Evans D, Inglesby P, Cockburn J, McDonald HI, MacKenna B, Tomlinson L, Douglas IJ, Rentsch CT, Mathur R, Wong AYS, Grieve R, Harrison D, Forbes H, Schultze A, Croker R, Parry J, Hester F, Harper S, Perera R, Evans SJW, Smeeth L, Goldacre B. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4343] [Cited by in F6Publishing: 3766] [Article Influence: 941.5] [Reference Citation Analysis (0)] |
| 24. | Jacobs JP, Stammers AH, St Louis J, Hayanga JWA, Firstenberg MS, Mongero LB, Tesdahl EA, Rajagopal K, Cheema FH, Coley T, Badhwar V, Sestokas AK, Slepian MJ. Extracorporeal Membrane Oxygenation in the Treatment of Severe Pulmonary and Cardiac Compromise in Coronavirus Disease 2019: Experience with 32 Patients. ASAIO J. 2020;66:722-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 137] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 25. | Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, Brenner SK, Leonberg-Yoo A, Schenck EJ, Radbel J, Reiser J, Bansal A, Srivastava A, Zhou Y, Sutherland A, Green A, Shehata AM, Goyal N, Vijayan A, Velez JCQ, Shaefi S, Parikh CR, Arunthamakun J, Athavale AM, Friedman AN, Short SAP, Kibbelaar ZA, Abu Omar S, Admon AJ, Donnelly JP, Gershengorn HB, Hernán MA, Semler MW, Leaf DE; STOP-COVID Investigators. Factors Associated With Death in Critically Ill Patients With Coronavirus Disease 2019 in the US. JAMA Intern Med. 2020;180:1436-1447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 608] [Article Influence: 152.0] [Reference Citation Analysis (0)] |
| 26. | Sukhal S, Sethi J, Ganesh M, Villablanca PA, Malhotra AK, Ramakrishna H. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: A systematic review and meta-analysis. Ann Card Anaesth. 2017;20:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Le Breton C, Besset S, Freita-Ramos S, Amouretti M, Billiet PA, Dao M, Dumont LM, Federici L, Gaborieau B, Longrois D, Postel-Vinay P, Vuillard C, Zucman N, Lebreton G, Combes A, Dreyfuss D, Ricard JD, Roux D. Extracorporeal membrane oxygenation for refractory COVID-19 acute respiratory distress syndrome. J Crit Care. 2020;60:10-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sultan I, Habertheuer A, Usman AA, Kilic A, Gnall E, Friscia ME, Zubkus D, Hirose H, Sanchez P, Okusanya O, Szeto WY, Gutsche J. The role of extracorporeal life support for patients with COVID-19: Preliminary results from a statewide experience. J Card Surg. 2020;35:1410-1413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 29. | Kon ZN, Smith DE, Chang SH, Goldenberg RM, Angel LF, Carillo JA, Geraci TC, Cerfolio RJ, Montgomery RA, Moazami N, Galloway AC. Extracorporeal Membrane Oxygenation Support in Severe COVID-19. Ann Thorac Surg. 2021;111:537-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166-E176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 145] [Reference Citation Analysis (0)] |
| 31. | Zhang J, Merrick B, Correa GL, Camporota L, Retter A, Doyle A, Glover GW, Sherren PB, Tricklebank SJ, Agarwal S, Lams BE, Barrett NA, Ioannou N, Edgeworth J, Meadows CIS. Veno-venous extracorporeal membrane oxygenation in coronavirus disease 2019: a case series. ERJ Open Res. 2020;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Alnababteh M, Hashmi MD, Vedantam K, Chopra R, Kohli A, Hayat F, Kriner E, Molina E, Pratt A, Oweis E, Zaaqoq AM. Extracorporeal membrane oxygenation for COVID-19 induced hypoxia: Single-center study. Perfusion. 2021;36:564-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Guihaire J, Owyang CG, Madhok J, Laverdure F, Gaillard M, Girault A, Lebreton G, Mercier O. Specific Considerations for Venovenous Extracorporeal Membrane Oxygenation During Coronavirus Disease 2019 Pandemic. ASAIO J. 2020;66:1069-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Jäckel M, Rilinger J, Lang CN, Zotzmann V, Kaier K, Stachon P, Biever PM, Wengenmayer T, Duerschmied D, Bode C, Staudacher DL, Supady A. Outcome of acute respiratory distress syndrome requiring extracorporeal membrane oxygenation in Covid-19 or influenza: A single-center registry study. Artif Organs. 2021;45:593-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Schmidt M, Hajage D, Lebreton G, Monsel A, Voiriot G, Levy D, Baron E, Beurton A, Chommeloux J, Meng P, Nemlaghi S, Bay P, Leprince P, Demoule A, Guidet B, Constantin JM, Fartoukh M, Dres M, Combes A; Groupe de Recherche Clinique en REanimation et Soins intensifs du Patient en Insuffisance Respiratoire aiguE (GRC-RESPIRE) Sorbonne Université; Paris-Sorbonne ECMO-COVID investigators. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. 2020;8:1121-1131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 305] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 36. | Riera J, Argudo E, Martínez-Martínez M, García S, García-de-Acilu M, Santafé M, Díaz C, Contreras S, Cortina A, Bonilla C, Pacheco A, Resta P, Palmer N, Castro MÁ, Ferrer R. Extracorporeal Membrane Oxygenation Retrieval in Coronavirus Disease 2019: A Case-Series of 19 Patients Supported at a High-Volume Extracorporeal Membrane Oxygenation Center. Crit Care Explor. 2020;2:e0228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Bemtgen X, Zotzmann V, Benk C, Rilinger J, Steiner K, Asmussen A, Bode C, Wengenmayer T, Maier S, Staudacher DL. Thrombotic circuit complications during venovenous extracorporeal membrane oxygenation in COVID-19. J Thromb Thrombolysis. 2021;51:301-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 38. | Yankah CA, Trimlett R, Sandoval E, Lotz C, Ledot S, Pomar JL, Price S, Meybohm P. COVID-19 Pulmonary Failure and Extracorporeal Membrane Oxygenation: First Experience from Three European Extracorporeal Membrane Oxygenation Centers. Thorac Cardiovasc Surg. 2021;69:259-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Zayat R, Kalverkamp S, Grottke O, Durak K, Dreher M, Autschbach R, Marx G, Marx N, Spillner J, Kersten A. Role of extracorporeal membrane oxygenation in critically Ill COVID-19 patients and predictors of mortality. Artif Organs. 2021;45:E158-E170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Mustafa AK, Alexander PJ, Joshi DJ, Tabachnick DR, Cross CA, Pappas PS, Tatooles AJ. Extracorporeal Membrane Oxygenation for Patients With COVID-19 in Severe Respiratory Failure. JAMA Surg. 2020;155:990-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |