Approach to the management of COVID-19 patients: When home care can represent the best practice
Abstract
BACKGROUND:
The pandemic that began around February 2020, caused by the viral pathogen SARS-CoV-2 (COVID-19), has still not completed its course at present in June 2022.
OBJECTIVE:
The open research to date highlights just how varied and complex the outcome of the contagion can be.
METHOD:
The clinical pictures observed following the contagion present variabilities that cannot be explained completely by the patient’s age (which, with the new variants, is rapidly changing, increasingly affecting younger patients) nor by symptoms and concomitant pathologies (which are no longer proving to be decisive in recent cases) in relation to medium-to-long term sequelae. In particular, the functions of the vascular endothelium and vascular lesions at the pre-capillary level represent the source of tissue hypoxia and other damage, resulting in the clinical evolution of COVID-19.
RESULTS:
Keeping the patient at home with targeted therapeutic support, aimed at not worsening vascular endothelium damage with early and appropriate stimulation of endothelial cells, ameliorates the glycocalyx function and improves the prognosis and, in some circumstances, could be the best practice suitable for certain patients.
CONCLUSION:
Clinical information thus far collected may be of immense value in developing a better understanding of the present pandemic and future occurrences regarding patient safety, pharmaceutical care and therapy liability.
1.Introduction
The impact of the current SARS-CoV-2 (COVID-19) pandemic on several fronts is there for all to see. In the various fields of scientific research, we are still witnessing unprecedented output. Despite this, to date it is not easy to determine relationships between the risk factors predisposing people to a more severe reaction to COVID-19 infection. A recent paper addresses, in depth, COVID-19 mortality, “identifying frail (not limited to older, multimorbid, symptomatic) persons at risk of poor outcomes [1]”. Frailty represents biological age, rather than chronological age, and can be defined as a state of vulnerability to poor resolution of homeostasis after a stressor event [2–4].
On the other hand, genetic alterations suggest interesting ideas regarding multi-gene expression, especially on large specific chromosomes for epithelial membrane proteins in the lungs, according to recent papers on this topic [5]. A recent paper stated that “a combination of multiple genetic and non-genetic factors contributes to an individual’s unique immune response and susceptibility to SARS-CoV-2 infection [6]”.
Correct interpretation of these aspects opens important avenues of research for the development of a precision pharmaceutical approach to the COVID-19 pandemic [7]. This suggests that recognizing the totality of factors that contribute to COVID-19 individual susceptibility and subsequent pathophysiology would enable improvements in the design of clinical trials and improved precision in medical interventions in the treatment of the various presentations of the disease [8].
In Table 1, the great deal information to be collected and interpreted in order to orientate among the different manifestations of COVID-19, both in the sense of predicting severity and therapeutic intervention in the various clinical expressions, are schematized [9–12].
Table 1
Risk factors, symptoms and signs for interpreting the staging criteria of COVID-19
| Risk factors | Symptoms and signs |
| Age | Cellular and tissue senescence determining the biological age are responsible for different responses to COVID-19: more the senescence, more the severity of the disease |
| ∙ Cardiovascular disease (cardiac arrest, arrhythmia, acute heart failure, acute myocarditis, myocardial infarction, myocardic signs in Kawasaki disease) | |
| ∙ Diabetes mellitus | |
| ∙ Hypertension | |
| ∙ Lung disease (COPD) | |
| Comorbidities | ∙ Cancer |
| ∙ Chronic kidney disease | |
| ∙ Obesity | |
| ∙ Smoking | |
| ∙ Anticancer chemotherapy | |
| (i) blood cell count and bone marrow activity for hematopoiesis (RBC; WBC, with lymphocyte immune phenotype; thrombocytes; RDW; reticulocytes) | |
| (ii) inflammatory activity (ESR, CRP, SOD, cytokines and chemokines) | |
| Blood analysis laboratory findings | (iii) coagulation activity (ATIII, fibrinogen, D-dimer) |
| (iv) kidney function (creatinine; uricemia; ACR; eGFR) | |
| (v) miscellaneous (haptoglobin, ferritin, procalcitonin, troponin, plasmatic proteins, LDH, BNP, NT-pro-BNP, electrolytes, blood gas analysis; GAGs) | |
| ∙ Respiratory symptoms (acute respiratory failure requiring noninvasive or invasive ventilation, with SpO2 measurements, tachypnea, labored breathing, mucous) | |
| ∙ Arrhythmia | |
| ∙ Neurocognitive symptoms (headache, lethargy, confusion, loss of taste) | |
| ∙ Gastrointestinal symptoms (abdominal pain, vomiting, diarrhea) | |
| ∙ Sore throat | |
| ∙ Persistent fever (>37.5 °C) | |
| Clinical findings | ∙ Dermatological signs (signs of subepithelial hemorrhage) and symptoms |
| ∙ Conjunctivitis | |
| ∙ Serositis (small pleural, pericardial, and ascitic effusions) | |
| ∙ Myalgias | |
| ∙ Swollen hands/feet | |
| ∙ Lymphadenopathy | |
| ∙ Hepatomegaly | |
| ∙ Encephalopathy, seizures, coma, or meningoencephalitis | |
| (i) Lung (ground-glass opacification; small pleural effusions, patchy consolidations, focal consolidation, and atelectasis) | |
| Imaging findings (diagnostic service with thorax echographia; ECG; Thorax X-ray; MRI) | (ii) Abdominal (nonspecific, including free fluid, ascites, bowel and mesenteric inflammation, including terminal ileitis, mesenteric adenopathy/adenitis, and pericholecystic edema) |
| (iii) Heart (Coronary artery dilation/aneurysm, mitral regurgitation and pericardial effusion) | |
| ABO phenotype | |
| Genetic factors | HLA |
| MHC | |
| G6PD deficit |
Table 1 (Continued).
| Risk factors | Symptoms and signs |
| Dietary habits | |
| Psychosocial/socioeconomic factors | Caloric restriction |
| Vitamin deficiencies | |
| Air pollution, with particular reference to NO2 and PMs |
[i] ACR: albumin-to-creatinine ratio, ATIII: antithrombin, BNP: B-type natriuretic peptide, CRP: C-reactive protein, ECG: electrocardiogram, eGFR: estimated glomerular filtration rate, ESR: erythrocyte sedimentation rate, G6PD: glucose-6-phosphate dehydrogenase, GAGs: glycosaminoglycans, HLA: human leukocyte antigen, LDH: lactate dehydrogenase, MHC: major histocompatibility complex, MRI: magnetic resonance imaging, NO: nitric oxide, NT-pro-BNP N-terminal pro b-type natriuretic peptide, PM: particulate matters, RBC: red blood cells, RDW: red cell distribution width, SOD: superoxide dismutase, WBC: white blood cells.
Their management should enable the realization of one mode of differential diagnosis of COVID-19 patients. Obviously, to catalog and analyze so many large datasets, it would be necessary to build valid information technology support for their correct interpretation [13–16]. It would also be of fundamental importance to consider those cases presenting persistent and prolonged effects after acute COVID-19 [17].
A careful observation of the therapeutic guidelines, issued by the relevant international organizations, does not state any specific therapies, at least at the moment. It does enable us to highlight the evolution, and to critically review the management indicated [18]. Moreover, clinical trials of promising molecules (antivirals, anti-inflammatories, monoclonal antibodies, dietary supplements, etc.) lack sufficient evidence of proven efficacy [19–22]. Clinical trials of repurposed drugs also failed to demonstrate full evidence of efficacy [23].
From the very beginning, it has been possible to ascertain that the pulmonary symptoms, although severe, have never been the only cause of fatal outcomes. On the contrary, the epidemiology and severity of the cases have shown that the cause of death is attributable to disturbances in patients’ vascular systems [24]. Regarding previous statements, it is of paramount importance to focus on endotheliitis as a disfunction of the vascular endothelium in the clinical evolution of COVID-19 [25,26].
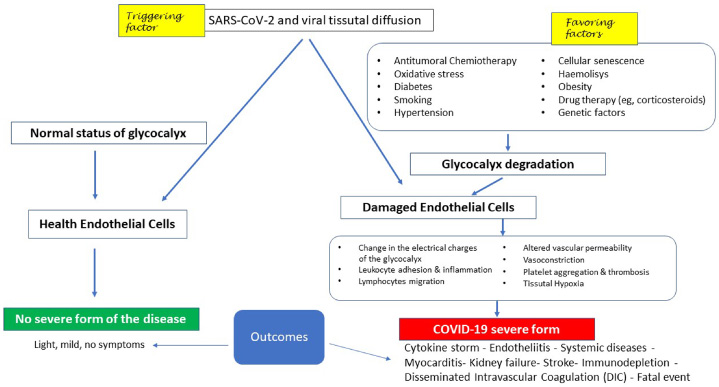
For an overview, Fig. 1 schematizes the following: in the presence of a normal condition of the glycocalyx, both the viral contagion and the tissue diffusion of the virus affecting other systems, in addition to the pulmonary and upper airways, are not able to develop COVID-19, even if it releases cytokines into the vascular system (left side). Conversely, the pre-existing condition of glycocalyx damage (right side), by reducing the ability of the endothelial cell to maintain its homeostatic functions, leads to the development of all those phases typical of the severe form of COVID-19, in which 2% has resulted in the patient’s death. From here, it follows that the severity of the disease is not linked to the triggering factor represented by the virus as such, but to pre-existing endothelial lesions as a favoring factor.
Fig. 1.
Potential clinical evolution following infection by SARS-CoV-2 in relation to the conditions of the vascular endothelium at the glycocalyx level (see text for further explanation).
To obtain an optimal therapeutic treatment of patients, the following cases must be complied:
We suggest specific home care for the COVID-19 patient, capable of allowing adequate protection of the glycocalyx and, therefore, a more favorable development of the clinical evolution linked to severe acute respiratory syndrome (SARS). This involves deploying a task force with the bare minimum of instrumental examinations, leaving hospitalization only for the most serious situations that cannot be managed at home, such as severe forms of hypoxia, or myocardial or renal complications.
Given the circumstances, it follows that to optimize a therapeutic approach to COVID-19 patients, positive outcomes related to the management of the patient at home should be realized. Starting from being able to determine the health status of the vascular endothelium and easily apply novel approaches to the treatment of endothelial diseases, as well as by being able to define the presence of genetic and epigenetic factors associated with severe clinical outcomes of COVID-19, we may introduce an opportunity to implement beneficial home care for the patient. These points will be discussed.
2.Discussion
2.1.Patient management at home
The open question, based on the many ideas correlating with clinical and therapeutic experience, concerns the optimization of home therapy for the COVID-19 patient, assuming increased responsibility by home doctors. The authors believe the evaluation of respiratory function and radiological imaging are not binding parameters for hospital admission. This conviction is corroborated by the fact that people who have undergone forced breathing through intubation, at best, have suffered irreversible damage to the lungs and increased vascular disease [29,30]. Once pulmonary compromise caused by an infection with SARS-CoV-2 has been ascertained, in cases where there is no imminent danger of death from other concomitant diseases (such as risk of cardiac arrest, myocarditis, acute renal failure, severe anemia) “home hospitalization” with integrated assistance is desirable, provided it is appropriate and monitored consistently [31]:
routine instrumental tests, such as an ECG to monitor damage to the myocardium;
oxygen saturation checks to maintain the correct intake, potentially with supportive Oxygen Therapy through nasal high-flow or Venturi mask;
routine blood-chemical tests to evaluate the general state of health;
more importantly, continuous and watchful care for the patient.
All the above should diminish the “over treatments” that caused an elevated rate of deaths and more severe outcomes in the initial period of the pandemic.
At this point, it is appropriate to change the behavior of family doctors and medical task forces in the interests of providing the most suitable therapy and assistance for the patient at home to obtain a greater survival rate and a less severe occurrence of sequelae (post illness), as well as not overburden hospital wards. There are numerous cases in which doctors have cared patients at home, ensuring that basic conditions for managing a viral disease like SARS are maintained; that is, absolute rest, reduction in psychological stress, maintenance of vital capacity, administering oxygen at home even with low levels of O2 saturation, use of non-steroidal anti-inflammatory drugs instead of reckless use of massive doses of cortisone (especially in the viremic phase), sufficient nutrition and hydration, and continuous control and monitoring of critical parameters. In some of these cases, recoveries have occurred even with the threat of severe outcomes [31]. What should be emphasized is the current lack of conformity in the behavior of first contact doctors (family doctors) with WHO instructions to keep patients at home as much as possible.
2.2.Vascular endothelium
Regarding the subject of vascular endothelium, the information reported on the ABO blood group loci associated with severe SARS-CoV-2 infection may find a more complete and significant interpretation in this direction [32,33], as also may be the case regarding the integrity of endothelial glycoproteins [34].
At this point, a question arises: “Can we determine the greatest common divisor from which to orient ongoing medical research?”. Determining the state of health of the vascular endothelial cell, with reference to the glycocalyx is, in our opinion and with many pointers indicated by other authors, the best answer [35].
Blood flow patterns and associated shear stresses play important roles in modulating both glycocalyx dimensions and the signaling activities of the cytoskeletal fibrils contained therein [36,37]. Moreover, vascular endothelial glycocalyx contributes to an atheroprotective function [38] with specific characteristics, depending on the anatomical location [39] and pathological conditions [40].
Growing evidence suggests that senescent or damaged endothelial cells can acquire a particular phenotype through multifaceted pathways, such as reduced NO availability, oxidative stress-induced DNA damage, mitochondrial dysfunction, impaired angiogenesis, and senescent endothelial progenitor cell-associated imbalance between vascular endothelial damage and repair [41].
The most efficient way to ensure patient safety is to protect the integrity of the vascular system, with particular reference to microcirculation. In this regard, compliance with the WHO’s appeal to avoid the use of cortisone derivatives in the viremia phase becomes of fundamental importance. In fact, the two main effects consequent to the administration of glucocorticoids are:
reduction of the immune response against the viral attack;
induced acute hyperglycemia.
Both have fundamental importance in the worsening of the disease.
In particular, we consider hyperglycemia consequent to high dose supplementation of corticosteroids (dexamethasone up to 48 mg/day) to have a devastating effect on the gelled portion of the matrix, but not on the fibrils responsible for the mechanosensitive function of the vascular endothelium [42]. Analogously, extreme caution must also be exercised in the use of heparin-like anticoagulants. Recently, it has been reported that heparin can prevent glycocalyx shedding in COVID-19 patients [43]. However, this is only true if the glycocalyx is present in undamaged form. In fact, as AT-III activators, it is possible to predict their enhancement in terms of increased risk of disseminated intravascular coagulation (DIC), through interaction with the process of fibrinolysis and dissolution of thrombi, without preventing parietal micro-coagulation of the platelets due to contact with fibrils uncovered by the gelled portion of the glycocalyx [44].
It is worth mentioning that damage is induced at the erythrocyte level with the introduction of a precapillary arterial hemolysis, with decreases in both hemoglobin and erythrocyte values, despite a strong bone marrow response and increased haptoglobin to counteract the increased presence of circulating oxyhemoglobin. The point of discussion is represented by lymphopenia shown in all patients with severe COVID-19. One hypothesis correlates the decrease in serum Ca2+ ions, as calcium is responsible for activating the actin contained in the internal fibrils of the glycocalyx when these strongly bind lymphocytes, platelets, and erythrocytes to both the vascular wall and to the precapillary endothelial cells. This sequestration of corpuscular elements at the precapillary arteriolar level, correlated with predisposing conditions of the damaged glycocalyx, is the basis of systemic vasculitis due to an anomalous trans-endothelial migration of lymphocytes [36]. To summarize, cellular interaction mechanisms at the precapillary level are linked to glycocalyx integrity. In detail, normal glycocalyx shows physiological sliding of the corpuscular elements of the blood, and leukocytes and lymphocytes are temporarily attached, recognized, and released. On the other hand, partial damage leads a reduction in the thickness of the glycocalyx, with a strong leukocyte and lymphocyte interaction, and potential induction of trans-endothelial migration, together with initial platelet adhesion. Eventually, massive damage to the glycocalyx implies strong adhesion with leukocytes and lymphocytes actin-Ca2+-mediated, and the appearance of trans-endothelial migration phenomena affecting lymphocytes, mainly CD8+ T cells as a critical subpopulation of MHC class I-restricted T cell and mediators of adaptive immunity. The loss of glycocalyx also causes a reduction in antithrombin III, with a consequent increase in intrinsic endovascular coagulation, in addition to platelet adhesion, with the onset of parietal thrombosis.
2.3.Host genetic and epigenetic factors associated with clinical outcomes of COVID-19
The search for host genetic factors in coronavirus susceptibility is producing interesting results [45–48]. In this regard, we must also consider a break in the “epigenetic information” which leads to a failure to repair cell damage, even in the presence of an intact and functioning DNA code. In fact, where this can be re-established with the restoration of cellular osmosis thanks to correct “hormetic” therapy, also known as “post-translational oxidative eustress [49]”, a healing process likely takes place. Ultimately, it is necessary as much as possible to not interrupt the chain of epigenetic analogical information, finding a way to re-establish the correct conditions at the glycocalyx and its fibrils at the vascular endothelial level.
Knowledge of the host’s genetic polymorphism could allow personalized adjuvant therapies. We must consider that if the genetic polymorphism regulates expression of the glycocalyx in terms of size, quality, specificity and at the same time indicates the degree of severity of the disease, it is useful to look for any polymorphism in the HLA, MHC systems and in the genome of the glycocalyx itself [50]. So, we hypothesize that targeted oxidative eustress acts as an epigenetic factor with effects on the composition of the glycocalyx. Treatments in this direction are made in different trials using an oxygen-ozone mixture or other inductors of oxidative stress in the vascular compartment [51]. More importantly, we think that these treatments can restore normal homeostasis of the endothelial cell by modulating the gene expression, restoring the normal antithrombotic and anti-inflammatory functions of the vascular endothelium, either through the transcriptional pathways of the self-healing of cells or by means of activation of the antioxidant response element in the nucleus of cells [52,53].
2.4.Novel approaches in diagnosis and treatment of endothelial diseases
There are many articles on endothelial damage from COVID-19 in the literature [54–57]. On the other hand, ethical considerations and technical difficulties limit the study of endothelial cells from different vascular regions both in healthy persons and in patients with different type of diseases [58]. A deeper understanding of endothelial cell biology is essential for the elucidation of several key factors, among which are:
specific mechanisms important both in defense against toxic and infectious diseases that interfere with the maintenance of a physiological lining of the vascular endothelium, and in tissue engineering and regenerative medicine;
the regulation of immune responses and inflammation [59];
novel approaches in the treatment of hypoxia-based diseases [60].
Current research concerning oxidative eustress/distress balance of anti-inflammatory treatments fits well in this pleiotropic context [61–63].
3.Conclusions and future directions
In this work, we have focused on the benefits that appropriate home care services as opposed to standard hospitalization can offer to patients with COVID-19. In our opinion, in this disease the home care makes possible to safeguard patients by better preserving endothelial glycocalyx, the functional jelly-like layer covering the luminal surface of the vascular endothelium. It is a target that has been shown to be the cause of deaths and severe forms of COVID-19. The physiological functions, protection, damage, and regeneration strategies of endothelial glycocalyx have important clinical implications [64]. Many physicians may not be aware of recent advances in the understanding of its critical role in health and vascular diseases.
On the other hand, the organism of higher animals relates to the stability of the internal cell milieu with many daily rhythms, such as body temperature, secretion of hormones and many others. Over time, the importance of the organism adapting to various stimuli has become increasingly evident as well as the importance of the response of each organism in relation to its own specificity in terms of the search for, or maintenance of, balance and functional integrity. In such a context, allostasis represents a more accurate concept of homeostasis (remaining stable by staying the same). Based on the multi-point property of allostasis, we could image the allostatic system as a special “buffering system”.
The term “stress” refers to an event or series of events that evoke, among others, a set of physiological responses. Targeted oxidative stress is well described as “oxidative eustress” and elicits adaptive responses in cells. On the other hand, “oxidative distress” is the result of chronic, uncontrolled overproduction of noxious metabolites that can result in tissue damage. Such opposing effects are expected due to differences in temporal (acute vs. chronic) and spatial (subcellular setting vs. bloodstream) formation of reactive oxygen and nitrogen species.
Both the correct use and dosage of chemical agents with pro-oxidant action are the basis of therapeutic approaches characterized by a paradoxical effect on the organism. All these actions are conducted mainly in relation to the polysaccharide matrix coating of vascular endothelial cells, the integrity of which modifies their behavior, giving rise to positive responses capable of quickly re-establishing the necessary quantity of glycocalyx, while its damage may result in its total inability to maintain the normal cellular functions of the vascular tree. In fact, a well-controlled, mild, and transitory eustress could activate a healthy response in an organism toward diseases where the oxidative stress is able to heavily damage the glycocalyx. This is more evident in pathological conditions such as COVID-19 and so-called “long COVID”, which affects different organs, and where conditions of vascular damage are long-lasting and find little benefit from traditional therapies [65,66]. Epigenetics, translational research, and regenerative medicine, as well as personalized medicine, are the main avenues for future research directions, capitalizing on what we have learned because of the terrible losses during this pandemic; reaffirming the importance of patient safety, pharmaceutical care, and medical liability once the real etiopathogenesis of COVID-19 is understood.
Acknowledgements
We kindly thank Robert Bannan, Joseph Nova, and David Seaman for the critical linguistic revisions of the manuscript.
Conflict of interest
None to report.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
All clinical information relating to the at-home treatment of COVID-19 patients who generated these reflections is available from the corresponding author upon reasonable request.
References
[1] | Polidori MC, Sies H, Ferrucci L, Benzing T. COVID-19 mortality as a fingerprint of biological age. Ageing Res Rev. (2021) ;67: :101308. doi:10.1016/j.arr.2021.101308. |
[2] | Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) ;381: :752. doi:10.1016/S0140-6736(12)62167-9. (Erratum in: Lancet. 2013;382:1328). |
[3] | Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) ;14: (6):392. doi:10.1016/j.jamda.2013.03.022. |
[4] | Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. (2016) ;26: :53. doi:10.1016/j.arr.2015.12.003. |
[5] | Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D Genetic mechanisms of critical illness in COVID-19. Nature. (2021) ;591: (7848):92. doi:10.1038/s41586-020-03065-y. |
[6] | Ballow M, Haga CL. Why do some people develop serious COVID-19 disease after infection, while others only exhibit mild symptoms? J Allergy Clin Immunol Pract. (2021) ;9: (4):1442. doi:10.1016/j.jaip.2021.01.012. |
[7] | Pereira NL, Ahmad F, Byku M, Cummins NW, Morris AA, Owens A COVID-19: understanding inter-individual variability and implications for precision medicine. Mayo Clin Proc. (2021) ;96: (2):446. doi:10.1016/j.mayocp.2020.11.024. |
[8] | Álvarez-Mon M, Ortega MA, Gasulla Ó, Fortuny-Profitós J, Mazaira-Font FA, Saurina P A predictive model and risk factors for case fatality of COVID-19. J Pers Med. (2021) ;11: (1):36. doi:10.3390/jpm11010036. |
[9] | Signorini C, Pignatti P, Coccini T. How do inflammatory mediators, immune response and air pollution contribute to COVID-19 disease severity? A lesson to learn. Life (Basel). (2021) ;11: (3):182. doi:10.3390/life11030182. |
[10] | McIntosh K . Coronaviruses, In: Hirsch MS, Bloom A (eds) UpToDate. Waltham, Mass.: UpToDate, 2021. [updated 2022 February 24; cited 2022 April 28]. Available from: https://www.uptodate.com/contents/coronaviruses. |
[11] | Görlinger K, Levy JH. COVID-19-associated coagulopathy. Anesthesiology. (2021) ;134: (3):366. doi:10.1097/ALN.0000000000003688. |
[12] | Cappanera S, Palumbo M, Kwan SH, Priante G, Martella LA, Saraca LM When does the cytokine storm begin in COVID-19 patients? A quick score to recognize it. J Clin Med. (2021) ;10: (2):297. doi:10.3390/jcm10020297. |
[13] | Pham Q, Nguyen DC, Huynh-The T, Hwang W, Pathirana PN. Artificial Intelligence (AI) and big data for coronavirus (COVID-19) pandemic: a survey on the state-of-the-arts. IEEE Access. (2020) ;8: :130820. doi:10.1109/ACCESS.2020.3009328. |
[14] | Pradana AR, Madjid SR, Prayitno HJ, Utami RD, Dharmawan Y. Potential applications of big data for managing the COVID-19 pandemic. J Phys: Conf Ser. (2021) ;1720: :012002. doi:10.1088/1742-6596/1720/1/012002. |
[15] | Jin C, Chen W, Cao Y, Xu Z, Tan Z, Zhang X Development and evaluation of an artificial intelligence system for COVID-19 diagnosis. Nat Commun. (2020) ;11: (1):5088. doi:10.1038/s41467-020-18685-1. |
[16] | Galindez G, Matschinske J, Rose TD, Sadegh S, Salgado-Albarrán M, Späth J Lessons from the COVID-19 pandemic for advancing computational drug repurposing strategies. Nat Comput Sci. (2021) ;1: :33. doi:10.1038/s43588-020-00007-6. |
[17] | Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS Post-acute COVID-19 syndrome. Nat Med. (2021) ;27: (4):601. doi:10.1038/s41591-021-01283-z. |
[18] | Cennimo DJ, Bergman SJ, Olsen KM. Coronavirus Disease 2019 (COVID-19) guidelines. Medscape. (2021) , [updated 2022 April 25; cited 2022 April 28]. Available from: https://emedicine.medscape.com/article/2500114-guidelines. |
[19] | Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V Remdesivir for the treatment of COVID-19. Cochrane Database Syst Rev. (2021) ;8: (8):CD014962. doi:10.1002/14651858.CD014962. |
[20] | Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. (2021) ;8: (8):CD014963. doi:10.1002/14651858.CD014963. |
[21] | Andreas M, Piechotta V, Skoetz N, Grummich K, Becker M, Joos L Interventions for palliative symptom control in COVID-19 patients. Cochrane Database Syst Rev. (2021) ;8: (8):CD015061. doi:10.1002/14651858.CD015061. |
[22] | Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database Syst Rev. (2021) ;5: (5):CD015043. doi:10.1002/14651858.CD015043. |
[23] | Al-Saleem J, Granet R, Ramakrishnan S, Ciancetta NA, Saveson C, Gessner C Knowledge graph-based approaches to drug repurposing for COVID-19. J Chem Inf Model. (2021) ;61: (8):4058. doi:10.1021/acs.jcim.1c00642. |
[24] | Chen W, Pan JY. Anatomical and pathological observation and analysis of SARS and COVID-19: microthrombosis is the main cause of death. Biol Proced Online. (2021) ;23: (1):4. doi:10.1186/s12575-021-00142-y. |
[25] | Yamaoka-Tojo M. Vascular endothelial glycocalyx damage in COVID-19. Int J Mol Sci. (2021) ;21: :9712. doi:10.3390/ijms21249712. |
[26] | Tricarico G, Zavan B, Travagli V. Clinical evidence and therapeutic treatments at the time of the coronaviruses responsible for SARS: a perspective and points of view with a focus on vascular endothelium. Coronaviruses. (2021) ;2: (11):e130921191743. doi:10.2174/2666796702666210223155317. |
[27] | Chen YJ, Jian WH, Liang ZY, Guan WJ, Liang WH, Chen RC Earlier diagnosis improves COVID-19 prognosis: a nationwide retrospective cohort analysis. Ann Transl Med. (2021) ;9: (11):941. doi:10.21037/atm-20-7210. |
[28] | Layachi WM-A, Sevilla-García M, García-Romero A, Soguero-Ruiz C, Jiménez IM. On the statistical differences in the pharmacological treatment of COVID-19 patients 2021 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI) (2021) . pp. 1–5. |
[29] | Akin S, van Hooven D, Ince C, Jansen T. Veno-arterial thrombosis and microcirculation imaging in a patient with COVID-19. Respir Med Case Rep. (2021) ;33: :101428. doi:10.1016/j.rmcr.2021.101428. |
[30] | Hibbert KA, Goiffon RJ, Fogerty AE. Case 18-2021: an 81-year-old man with cough, fever, and shortness of breath. N Engl J Med. (2021) ;384: (24):2332. doi:10.1056/NEJMcpc2100283. |
[31] | Sitammagari K, Murphy S, Kowalkowski M, Chou SH, Sullivan M, Taylor S Insights from rapid deployment of a “virtual hospital” as standard care during the COVID-19 pandemic. Ann Intern Med. (2021) ;174: (2):192. doi:10.7326/M20-4076. |
[32] | Anastassopoulou C, Gkizarioti Z, Patrinos GP, Tsakris A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum Genomics. (2020) ;14: :40. doi:10.1186/s40246-020-00290-4. |
[33] | Reilly JP, Meyer NJ, Shashaty MG, Anderson BJ, Ittner C, Dunn TG The ABO histo-blood group, endothelial activation, and acute respiratory distress syndrome risk in critical illness. J Clin Invest. (2021) ;131: (1):e139700. doi:10.1172/JCI139700. |
[34] | Sabioni L, De Lorenzo A, Lamas C, Muccillo F, Castro-Faria-Neto HC, Estato V Systemic microvascular endothelial dysfunction and disease severity in COVID-19 patients: evaluation by laser Doppler perfusion monitoring and cytokine/chemokine analysis. Microvasc Res. (2021) ;134: :104119. doi:10.1016/j.mvr.2020.104119. |
[35] | Hahn RG, Patel V, Dull RO. Human glycocalyx shedding: systematic review and critical appraisal. Acta Anaesthesiol Scand. (2021) ;65: (5):590. doi:10.1111/aas.13797. |
[36] | Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat Immunol. (2011) ;13: (1):67. doi:10.1038/ni.2173. |
[37] | Witjas FMR, van den Berg BM, van den Berg CW, Engelse MA, Rabelink TJ. Concise review: The endothelial cell extracellular matrix regulates tissue homeostasis and repair. Stem Cells Transl Med. (2019) ;8: (4):375. doi:10.1002/sctm.18-0155. |
[38] | Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. (2011) ;91: :327. doi:10.1152/physrev.00047.2009. |
[39] | Ruane-O’Hora T, Markos F. The arteriolar glycocalyx plays a role in the regulation of blood flow in the iliac of the anaesthetised pig. Physiol Res. (2018) ;67: :41. doi:10.33549/physiolres.933630. |
[40] | Becker BF, Chappell D, Bruegger D, Annecke T, Jacob M. Therapeutic strategies targeting the endothelial glycocalyx: acute deficits, but great potential. Cardiovasc Res. (2010) ;87: :300. doi:10.1093/cvr/cvq137. |
[41] | Toya T, Ahmad A, Attia Z, Cohen-Shelly M, Ozcan I, Noseworthy PA Vascular aging detected by peripheral endothelial dysfunction is associated with ECG-derived physiological aging. J Am Heart Assoc. (2021) ;10: :e018656. doi:10.1161/JAHA.120.018656. |
[42] | Mazori AY, Bass IR, Chan L, Mathews KS, Altman DR, Saha A Hyperglycemia is associated with increased mortality in critically ill patients with COVID-19. Endocr Pract. (2021) ;27: (2):95. doi:10.1016/j.eprac.2020.12.015. |
[43] | Potje SR, Costa TJ, Fraga-Silva TFC, Martins RB, Benatti MN, Almado CEL Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci. (2021) ;276: :119376. doi:10.1016/j.lfs.2021.119376. |
[44] | Kruip MJHA, Cannegieter SC, Ten Cate H, van Gorp ECM, Juffermans NP, Klok FA Caging the dragon: research approach to COVID-19-related thrombosis. Res Pract Thromb Haemost. (2021) ;5: (2):278. doi:10.1002/rth2.12470. |
[45] | LoPresti M, Beck DB, Duggal P, Cummings DAT, Solomon BD. The role of host genetic factors in coronavirus susceptibility: review of animal and systematic review of human literature. Am J Hum Genet. (2020) ;107: :381. doi:10.1016/j.ajhg.2020.08.007. |
[46] | Wang F, Huang S, Gao R, Zhou Y, Lai C, Li Z Initial whole-genome sequencing and analysis of the host genetic contribution to COVID-19 severity and susceptibility. Cell Discov. (2020) ;6: (1):83. doi:10.1038/s41421-020-00231-4. |
[47] | Andolfo I, Russo R, Lasorsa VA, Cantalupo S, Rosato BE, Bonfiglio F Common variants at 21q22.3 locus influence MX1 and TMPRSS2 gene expression and susceptibility to severe COVID-19. iScience. (2021) ;24: (4):102322. doi:10.1016/j.isci.2021.102322. |
[48] | Yildirim Z, Sahin OS, Yazar S, Bozok Cetintas V. Genetic and epigenetic factors associated with increased severity of Covid-19. Cell Biol Int. (2021) ;45: (6):1158. doi:10.1002/cbin.11572. |
[49] | Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. (2020) ;21: :363. doi:10.1038/s41580-020-0230-3. |
[50] | Chaqour B. Caught between a “Rho” and a hard place: are CCN1/CYR61 and CCN2/CTGF the arbiters of microvascular stiffness? J Cell Commun Signal. (2020) ;14: :21. doi:10.1007/s12079-019-00529-3. |
[51] | Tricarico G, Travagli V. The relationship between ozone and human blood in the course of a well-controlled, mild, and transitory oxidative eustress. Antioxidants (Basel). (2021) ;10: (12):1946. doi:10.3390/antiox10121946. |
[52] | Hiebert P. The Nrf2 transcription factor: a multifaceted regulator of the extracellular matrix. Matrix Biol Plus. (2021) ;10: :100057. doi:10.1016/j.mbplus.2021.100057. |
[53] | Tretter V, Hochreiter B, Zach ML, Krenn K, Klein KU. Understanding cellular redox homeostasis: a challenge for precision medicine. Int J Mol Sci. (2021) ;23: (1):106. doi:10.3390/ijms23010106. |
[54] | Queisser KA, Mellema RA, Middleton EA, Portier I, Manne BK, Denorme F COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight. (2021) ;6: (17):e147472. doi:10.1172/jci.insight.147472. |
[55] | Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. (2021) ;21: (5):319–329. doi:10.1038/s41577-021-00536-9. |
[56] | Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis. (2021) ;24: (4):755–788. doi:10.1007/s10456-021-09805-6. |
[57] | Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. (2021) ;17: (1):46–64. doi:10.1038/s41581-020-00357-4. |
[58] | Drost CC, Rovas A, Kümpers P. Protection and rebuilding of the endothelial glycocalyx in sepsis—Science or fiction? Matrix Biol Plus. (2021) ;12: :100091. doi:10.1016/j.mbplus.2021.100091. |
[59] | Hattori Y, Hattori K, Machida T, Matsuda N. Vascular endotheliitis associated with infections: its pathogenetic role and therapeutic implication. Biochem Pharmacol. (2022) ;9: :114909. doi:10.1016/j.bcp.2022.114909. |
[60] | Krüger-Genge A, Blocki A, Franke RP, Jung F. Vascular endothelial cell biology: an update. Int J Mol Sci. (2019) ;20: :4411. doi:10.3390/ijms20184411. |
[61] | Oyaizu T, Enomoto M, Yamamoto N, Tsuji K, Horie M, Muneta T Hyperbaric oxygen reduces inflammation, oxygenates injured muscle, and regenerates skeletal muscle via macrophage and satellite cell activation. Sci Rep. (2018) ;8: :1288. doi:10.1038/s41598-018-19670-x. |
[62] | Scassellati C, Galoforo AC, Bonvicini C, Esposito C, Ricevuti G. Ozone: a natural bioactive molecule with antioxidant property as potential new strategy in aging and in neurodegenerative disorders. Ageing Res Rev. (2020) ;63: :101138. doi:10.1016/j.arr.2020.101138. |
[63] | Fratta Pasini AM, Stranieri C, Cominacini L, Mozzini C. Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-Cov-2 complications. Antioxidants (Basel). (2021) ;10: :272. doi:10.3390/antiox10020272. |
[64] | Pillinger NL, Kam P. Endothelial glycocalyx: basic science and clinical implications. Anaesth Intensive Care. (2017) ;45: (3):295–307. doi:10.1177/0310057X1704500305. |
[65] | Arévalos V, Ortega-Paz L, Rodríguez-Arias JJ, Calvo López M, Castrillo-Golvano L, Salazar-Rodríguez A Acute and chronic effects of COVID-19 on the cardiovascular system. J Cardiovasc Dev Dis. (2021) ;8: (10):128. doi:10.3390/jcdd8100128. |
[66] | Vollenberg R, Tepasse PR, Ochs K, Floer M, Strauss M, Rennebaum F Indications of persistent glycocalyx damage in convalescent COVID-19 patients: a prospective multicenter study and hypothesis. Viruses. (2021) ;13: (11):2324. doi:10.3390/v13112324. |



