Collaborative and Structured Network for Maintenance of Mechanical Ventilators during the SARS-CoV-2 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mechanical Ventilator
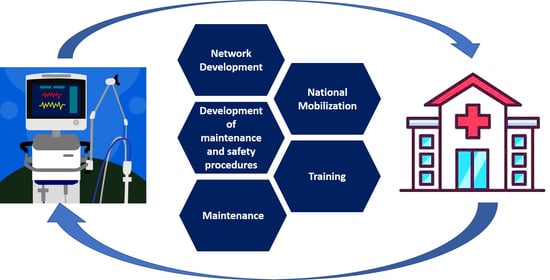
2.2. Maintenance Collaborative Network
2.3. Maintenance Process Lines
3. Results and Discussion
3.1. Network Mobilization
3.2. Training Sessions
3.3. Network Mobilization
3.4. Maintenance Numbers
4. Conclusions
- The first maintenance unit was launched in three days with all required protocols and safety
- The engagement of SENAI institutes in the initiative allowed quick setup of new units once the institutes were present in all states in Brazil. Additionally, the interest of volunteers and industrial companies in supporting the initiative allowed knowledge transfer and financial support.
- The 2516 ventilators the initiative returned to healthcare units supported 30,192 human lives in the worst situation of the COVID-19 pandemic, considering a theoretical number calculated at the maximum continuous capacity.
- There was support in improving the infrastructure of the state hospital network and training of specialized labor for this type of action.
- The biggest challenges were the logistics, specialized manpower, and availability of spare parts to perform the maintenance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Procedures | Documents | Link |
|---|---|---|
| Reception | Receiving protocol | https://doi.org/10.6084/m9.figshare.14737848 (accessed on 5 June 2021) |
| Hygiene/Disinfection | Basic cleaning protocol | https://doi.org/10.6084/m9.figshare.14737848 |
| Screening | Operation and service manuals | Operations Manuals: https://consultas.anvisa.gov.br/#/saude/ (accessed on 5 June 2021) Service Manuals: Available for the Open Project Medtronic PB560. Link: https://www.medtronic.com/us-en/e/open-files.html (accessed on 5 June 2021) For other ventilators, please, consult the manufacturer. |
| Electronic maintenance | Basic requirements for ventilator maintenance | https://doi.org/10.6084/m9.figshare.14737848 |
| Mechanical maintenance | Basic requirements for ventilator maintenance | https://doi.org/10.6084/m9.figshare.14737848 |
| Calibration and safety tests | Operation and service manuals | Operations Manuals: https://consultas.anvisa.gov.br/#/saude/ Service Manuals: Available for the Open Project Medtronic PB560. Link: https://www.medtronic.com/us-en/e/open-files.html (accessed on 5 June 2021) For other ventilators, please, consult the manufacturer. |
| Expedition and delivery | Devolution protocol | https://doi.org/10.6084/m9.figshare.14737848 |
| Type | Link |
|---|---|
| International live | https://www.youtube.com/watch?v=ELX_tzKlalQ (accessed on15 March 2021) https://www.youtube.com/watch?v=0DHysjNTa2k (accessed on 15 March 2021) https://www.youtube.com/watch?v=Uy6DYu2pdvM (accessed on 15 March 2021) |
| Clinical engineering live | https://www.youtube.com/watch?v=7pOarJOt7vY (accessed on 15 March 2021) https://www.youtube.com/watch?v=QCd-NkFFKEA (accessed on 15 March 2021) |
References
- Zhang, B.; Zhou, H.; Zhou, F. Study on SARS-CoV-2 transmission and the effects of control measures in China. PLoS ONE 2020, 15, e0242649. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolny, H.M. Modeling the role of asymptomatics in infection spread with application to SARS-CoV-2. PLoS ONE 2020, 15, 1–14. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Decaro, N.; Lorusso, A. Novel human coronavirus (SARS-CoV-2): A lesson from animal coronaviruses. Vet. Microbiol. 2020, 244, 108693. [Google Scholar] [CrossRef] [PubMed]
- Johns Hopkins University & Medicine COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 15 March 2021).
- The Lancet. COVID-19 in Brazil: “So what?”. Lancet 2020, 395, 1461. [Google Scholar] [CrossRef]
- De Souza, W.M.; Buss, L.F.; da Candido, D.S.; Carrera, J.P.; Li, S.; Zarebski, A.E.; Pereira, R.H.M.; Prete, C.A.; de Souza-Santos, A.A.; Parag, K.V.; et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020, 4, 856–865. [Google Scholar] [CrossRef]
- Ribeiro, H.V.; Sunahara, A.S.; Sutton, J.; Perc, M.; Hanley, Q.S. City size and the spreading of COVID-19 in Brazil. PLoS ONE 2020, 15, e0239699. [Google Scholar] [CrossRef]
- National Center for Immunization and Respiratory Diseases (NCIRD). New Variants of the Virus That Causes COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/transmission/variant.html (accessed on 9 February 2021).
- Zheng, J. SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int. J. Biol. Sci. 2020, 2020, 1678–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, C.S.; Sahjwani, D.; Brown, A.W.; Feroz, S.; Cameron, P.; Osborn, E.; Desai, M.; Djurkovic, S.; Kasarabada, A.; Hinerman, R.; et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLoS ONE 2020, 15, e0242651. [Google Scholar] [CrossRef]
- Dhama, K.; Khan, S.; Tiwari, R.; Sircar, S.; Bhat, S.; Malik, Y.S.; Singh, K.P.; Chaicumpa, W.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Coronavirus Disease 2019–COVID-19. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef]
- Carrillo Hernandez-Rubio, J.; Sanchez-Carpintero Abad, M.; Yordi Leon, A.; Doblare Higuera, G.; Garcia Rodriguez, L.; Garcia Torrejon, C.; Mayor Cacho, A.; Jimenez Rodriguez, A.; Garcia-Salmones Martin, M. Outcomes of an intermediate respiratory care unit in the COVID-19 pandemic. PLoS ONE 2020, 15, e0243968. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Jin Yan Ang, M.; Yin Chan, S.; Yi, Z.; Yiing Goh, Y.; Yan, S.; Tao, J.; Liu, K.; Li, X.; Zhang, H.; et al. Combating the Coronavirus Pandemic: Early Detection, Medical Treatment, and a Concerted Effort by the Global Community. Research 2020, 2020, 6925296. [Google Scholar] [CrossRef]
- Summ, O.; Schute, J.; Byhahn, C.; Kahle, T.; Herrmann, M.; Schulte, C.; Bergold, M.N.; Groß, M. COVID-19 pandemic: Structured expansion of ventilation capacities using home respirators. Anaesthesist 2020, 69, 323–330. [Google Scholar] [CrossRef]
- Group, B.C.-A. Early indicators of intensive care unit bed requirement during the COVID-19 epidemic: A retrospective study in Ile-de-France region, France. PLoS ONE 2020, 15, e0241406. [Google Scholar] [CrossRef]
- Notz, Q.; Herrmann, J.; Stumpner, J.; Schmid, B.; Schlesinger, T.; Kredel, M.; Kranke, P.; Meybohm, P.; Lotz, C. Anesthesia and intensive care ventilators: Differences and usability in COVID-19 patients. Anaesthesist 2020, 69, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Dhanani, J.; Pang, G.; Pincus, J.; Ahern, B.; Goodwin, W.; Cowling, N.; Whitten, G.; Abdul-Aziz, M.H.; Martin, S.; Corke, P.; et al. Increasing ventilator surge capacity in COVID 19 pandemic: Design, manufacture and in vitro-in vivo testing in anaesthetized healthy pigs of a rapid prototyped mechanical ventilator. BMC Res. Notes 2020, 13, 421. [Google Scholar] [CrossRef]
- Talley, N.J. SARS-CoV-2, the medical profession, ventilator beds, and mortality predictions: Personal reflections of an Australian clinician. Med. J. Aust. 2020, 212, 302–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical supply shortages—The need for ventilators and personal protective equipment during the Covid-19 pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef]
- Douglas, B. White; Bernard Lo A Framework for Rationing Ventilators and Critical Care Beds During the COVID-19 Pandemic. JAMA 2020, 323, 1773–1774. [Google Scholar] [CrossRef] [Green Version]
- Severo, E.A.; De Guimarães, J.C.F.; Dellarmelin, M.L. Impact of the COVID-19 pandemic on environmental awareness, sustainable consumption and social responsibility: Evidence from generations in Brazil and Portugal. J. Clean. Prod. 2020, 226, 124947. [Google Scholar] [CrossRef] [PubMed]
- Baqui, P.; Bica, I.; Marra, V.; Ercole, A.; van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: A cross-sectional observational study. Lancet Glob. Health 2020, 8, e1018–e1026. [Google Scholar] [CrossRef]
- Velasco, C. Especialistas Apontam Falta de Coordenação Federal na Compra de Respiradores Por Parte dos Estados. Available online: https://g1.globo.com/bemestar/coronavirus/noticia/2020/06/29/especialistas-apontam-falta-de-coordenacao-federal-na-compra-de-respiradores-por-parte-dos-estados.ghtml (accessed on 29 June 2020).
- Klein, A. Who will get ventilators in a covid-19 crisis? New Sci. 2020, 245, 12. [Google Scholar] [CrossRef]
- Requia, W.J.; Kondo, E.K.; Adams, M.D.; Gold, D.R.; Struchiner, C.J. Risk of the Brazilian health care system over 5572 municipalities to exceed health care capacity due to the 2019 novel coronavirus (COVID-19). Sci. Total Environ. 2020, 730, 139144. [Google Scholar] [CrossRef]
- Da Lino, D.O.C.; Barreto, R.; de Souza, F.D.; de Lima, C.J.M.; da Silva Junior, G.B. Impact of lockdown on bed occupancy rate in a referral hospital during the COVID-19 pandemic in northeast Brazil. Braz. J. Infect. Dis. 2020, 24, 466–469. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Gallego, V.; Escalera-Antezana, J.P.; Méndez, C.A.; Zambrano, L.I.; Franco-Paredes, C.; Suárez, J.A.; Rodriguez-Enciso, H.D.; Balbin-Ramon, G.J.; Savio-Larriera, E.; et al. COVID-19 in Latin America: The implications of the first confirmed case in Brazil. Travel Med. Infect. Dis. 2020, 35, 101613. [Google Scholar] [CrossRef]
- Carvalho, T.A.; Boschiero, M.N.; Marson, F.A.L. COVID-19 in Brazil: 150,000 deaths and the Brazilian underreporting. Diagn. Microbiol. Infect. Dis. 2020, 99, 115258. [Google Scholar] [CrossRef]
- SENAI. SENAI e Indústrias Fazem Manutenção de Respiradores Mecânicos—Portal da Indústria. Available online: http://www.portaldaindustria.com.br/canais/industria-contra-covid-19/iniciativas/senai-e-industrias-fazem-manutencao-de-respiradores-mecanicos/ (accessed on 21 February 2021).
- Boaventura, H. Rede Voluntária Coordenada Pelo SENAI Conserta Mais de 2 Mil Respiradores. Available online: https://noticias.portaldaindustria.com.br/noticias/saude-e-qualidade-de-vida/rede-voluntaria-coordenada-pelo-senai-conserta-mais-de-2-mil-respiradores/ (accessed on 11 August 2020).
- Jones, F. Governos e Hospitais Correm Contra o Tempo em Busca de Respiradores. Available online: https://www.uol.com.br/vivabem/noticias/redacao/2020/04/06/governos-e-hospitais-correm-contra-o-tempo-em-busca-de-respiradores.htm?next=0001H846U22N (accessed on 5 June 2021).
- Universidade Johns Hopkins. Coronavírus (COVID-19). Available online: https://news.google.com/covid19/map?hl=pt-BR&mid=%2Fm%2F015fr&gl=BR&ceid=BR%3Apt-419 (accessed on 5 June 2021).
- Ministério da Saúde do Brasil. DATASUS. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?cnes/cnv/leiintbr.def (accessed on 5 June 2021).
- Ministério da Saúde do Brasil. Resolução No 7, de 24 de Fevereiro de 2010. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2010/res0007_24_02_2010.html (accessed on 5 June 2021).
- Branson, R.; Dichter, J.R.; Feldman, H.; Devereaux, A.; Dries, D.; Benditt, J.; Hossain, T.; Ghazipura, M.; King, M.; Baldisseri, M.; et al. The US Strategic National Stockpile Ventilators in Coronavirus Disease 2019: A Comparison of Functionality and Analysis Regarding the Emergency Purchase of 200,000 Devices. Chest 2020, 159, 634–652. [Google Scholar] [CrossRef] [PubMed]
- Lentz, S.; Roginski, M.A.; Montrief, T.; Ramzy, M.; Gottlieb, M.; Long, B. Initial emergency department mechanical ventilation strategies for COVID-19 hypoxemic respiratory failure and ARDS. Am. J. Emerg. Med. 2020, 38, 2194–2202. [Google Scholar] [CrossRef]
- Berlin, D.A.; Gulick, R.M.; Martinez, F.J. Severe Covid-19. N. Engl. J. Med. 2020, 383, 2451–2460. [Google Scholar] [CrossRef]
- Bhandary, R. Practical ventilator management. Surgery 2018, 36, 699–704. [Google Scholar] [CrossRef]
- Lei, Y. Chapter 5—Ventilator System Composition. In Medical Ventilator System Basics: A Clinical Guide; Oxford University Press: Oxford, UK, 2017; pp. 1–30. [Google Scholar]
- Arar, S. An Engineer’s Introduction to Mechanical Ventilation. Available online: https://www.allaboutcircuits.com/technical-articles/engineer-introduction-to-mechanical-ventilation/ (accessed on 15 February 2021).
- World Health Organization. Country & Technical Guidance—Coronavirus Disease (COVID-19). Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications (accessed on 19 February 2021).
- Brazilian National Standards Organization. NBR ISO 17025—General Requirements for the Competence of Testing and Calibration Laboratories; Brazilian Association of Technical Standards: São Paulo, Brazil, 2017; pp. 1–32. [Google Scholar]
- Brazilian National Standards Organization. NBR IEC 60601-1—Medical Electrical Equipment Part 1: General Requirements for Basic Safety and Essential Performance; Brazilian Association of Technical Standards: São Paulo, Brazil, 2014; pp. 1–30. [Google Scholar]
- Brazilian National Standards Organization. NBR IEC 62353—Medical Electrical Equipment—Recurrent Test and Test after Repair of Medical Electrical Equipment; Brazilian Association of Technical Standards: São Paulo, Brazil, 2019; pp. 1–64. [Google Scholar]
- Agência Nacional de Vigilância Sanitária (ANVISA). Produtos Para Saúde. Available online: https://www.gov.br/anvisa/pt-br (accessed on 4 June 2021).
- Kacmarek, R. The mechanical ventilator: Past, present, and future. Respir. Care 2011, 56, 1170–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tallo, F.S.; de Abib, S.C.V.; de Negri, A.J.A.; Filho, P.C.; Lopes, R.D.; Lopes, A.C. Evaluation of self-perception of mechanical ventilation knowledge among brazilian final-year medical students, residents and emergency physicians. Clinics 2017, 72, 65–70. [Google Scholar] [CrossRef]
- Holanda, M.A.; Pinheiro, B.V. COVID-19 pandemic and mechanical ventilation: Facing the present, designing the future. J. Bras. Pneumol. 2020, 46, e20200282. [Google Scholar] [CrossRef] [PubMed]
- Vilela, P.R. Preço Médio Pago por Respiradores foi de R$ 87 mil, diz CGU. Available online: https://agenciabrasil.ebc.com.br/politica/noticia/2020-05/preco-medio-pago-por-respiradores-foi-de-r-87-mil-diz-cgu#:~:text=O (accessed on 11 January 2021).
- Rainisch, G.; Undurraga, E.A.; Chowell, G. A dynamic modeling tool for estimating healthcare demand from the COVID19 epidemic and evaluating population-wide interventions. Int. J. Infect. Dis. 2020, 96, 376–383. [Google Scholar] [CrossRef]
- Rubin, R.; Abbasi, J.; Voelker, R. Latin America and Its Global Partners Toil to Procure Medical Supplies as COVID-19 Pushes the Region to Its Limit. JAMA J. Am. Med. Assoc. 2020, 324, 217–219. [Google Scholar] [CrossRef]
- Stein, F.; Perry, M.; Banda, G.; Woolhouse, M.; Mutapi, F. Oxygen provision to fight COVID-19 in sub-Saharan Africa. BMJ Glob. Health 2020, 5, e002786. [Google Scholar] [CrossRef]
- U.S. Food & Drug Administration. Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency FDA; Food and Drug Administration (FDA): Silver Spring, MD, USA, 2020; pp. 1–14.
- Bernardo, T.M.; Sobkowich, K.E.; Forrest, R.O.; Stewart, L.S.; D’Agostino, M.; Perez, E.; Gillis, D. Collaborating in the time of COVID-19: The scope and scale of innovative responses to a global pandemic. JMIR Public Health Surveill. 2020, 7, e25935. [Google Scholar] [CrossRef]
- JMP Solutions. JMP Solutions Plays a Key Role in Canadian Ventilator Manufacturing Team to Aid in the Ongoing Fight against COVID-19. Available online: https://www.jmpsolutions.com/jmp-solutions-plays-a-key-role-in-canadian-ventilator-manufacturing-team-to-aid-in-the-ongoing-fight-against-covid-19-jmp-solutions/ (accessed on 9 February 2021).
- Jet Propulsion Laboratory. Brazilian Partnership to Begin Producing NASA-Designed COVID-19 Ventilator. Available online: https://www.jpl.nasa.gov/news/brazilian-partnership-to-begin-producing-nasa-designed-covid-19-ventilator (accessed on 9 February 2021).
- King, W.P.; Amos, J.; Azer, M.; Baker, D.; Bashir, R.; Best, C.; Bethke, E.; Boppart, S.A.; Bralts, E.; Corey, R.M.; et al. Emergency ventilator for COVID-19. PLoS ONE 2020, 15, e0244963. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, A.I.; Wang, H.; Zhang, R.; Mai, X.; Pan, T. AmbuBox: A Fast-Deployable Low-Cost Ventilator for COVID-19 Emergent Care. SLAS Technol. 2020, 25, 573–584. [Google Scholar] [CrossRef]
- Cole, J.H.; Hughey, S.B.; Rector, C.H.; Booth, G.J. A Novel Low-Cost Ventilator for Use in a Worldwide Pandemic: The Portsmouth Ventilator. Crit. Care Explor. 2020, 2, e0292. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Ntagios, M.; Hart, A.; Dahiya, R. GlasVent—The Rapidly Deployable Emergency Ventilator. Glob. Chall. 2020, 4, e2000046. [Google Scholar] [CrossRef] [PubMed]
- Ohnsman, A. Medtronic Gives away Ventilator Design Specs in Coronavirus Fight, Ahead of Tesla Alliance. Available online: https://www.forbes.com/sites/alanohnsman/2020/03/30/medtronic-gives-away-ventilator-design-specs-in-coronavirus-fight-ahead-of-tesla-alliance/?sh=16e1daed4591 (accessed on 9 February 2021).
- Pearce, J.M. A review of open source ventilators for COVID-19 and future pandemics. F1000 Res. 2020, 9, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Licempre Como é Feito o Registro de Respiradores na Anvisa? Available online: https://www.licempre.com.br/como-e-feito-o-registro-de-respiradores-na-anvisa/ (accessed on 5 June 2021).
- Jones, D.; Womack, J. A Mentalidade Enxuta Nas Empresas: Elimine o Desperdício e Crie Riqueza, 6th ed.; Elsevier: Rio de Janeiro, Brasil, 2004. [Google Scholar]
- Pearce, J.M. Authors from all over the world share their tech in HardwareX to battle COVID-19. HardwareX 2021, 9, e00190. [Google Scholar] [CrossRef]
- SENAI CIMATEC Ventilador Pulmonar Projetado Pela NASA Está em Produção no Brasil. Available online: http://www.senaicimatec.com.br/noticias/ventilador-pulmonar-projetado-pela-nasa-esta-em-producao-no-brasil/ (accessed on 5 June 2021).
- Corsini, L.; Dammicco, V.; Moultrie, J. Critical Factors for Implementing Open Source Hardware in a Crisis: Lessons Learned from the COVID-19 Pandemic. J. Open Hardw. 2020, 4, 1–11. [Google Scholar] [CrossRef]
- Vaughan, T.J.; Kirrane, F.; Moerman, K.M.; Cahill, T.; O’Regan, A.; O’Keeffe, D.T. A Novel Dual Non-Invasive Ventilator Continuous Positive Airway Pressure Non-Aerosolization Circuit for Emergency Use in the COVID-19 Pandemic. J. Open Hardw. 2020, 4, 1–8. [Google Scholar] [CrossRef]












| Procedures | Description | Documents |
|---|---|---|
| Reception | Receipt and registration of ventilators. Protocols and procedures were defined in terms of necessary personal protective equipment (PPE) as well as the need for a photographic registry and registration in the warehouse management system (WMS). This step was important to ensure that ventilators and accessories were properly returned to healthcare units and to establish first in, first out (FIFO) process in maintenance line. | Receiving protocol |
| Hygiene/Disinfection | In this step, specific rules for maintenance lines were documented, validated, and distributed to personnel involved. Some rules defined that: a. External cleaning and disinfection of equipment should be carried out with 70% isopropyl alcohol. b. Breathing circuits (ventilator endotracheal tubes) should be disassembled and sanitized with 70% ethyl alcohol and sent in sealed plastic bags to a biotechnology laboratory for autoclave decontamination. c. After external cleaning, ventilator should undergo 48 h quarantine to ensure decontamination of internal parts. d. Equipment should be opened in a room with an exhaust fan fitted with a bag filter (F9) and an absolute filter (F772 or F782). | Basic cleaning protocol |
| Screening | At this step the objective was to identify equipment issues by evaluating and comparing with operational end service manuals. Many ventilators needed consumables such as batteries and oxygen cells replaced, but in order to evaluate their parameters, tests should include assembly of endotracheal tube circuits and initial calibration test should be run. Performance at initial trial generally demonstrated, through alarms, actions to be taken. Qualified technical teams, including clinical engineers and biomedical equipment technicians, and infrastructure, including oxygen and compressed air cylinders, artificial lungs, and gas flow ventilator analyzers, were crucial at this point forward. | Operation and service manuals |
| Electronic maintenance | Once the need for electronic repair is identified, the ventilator should be examined at this stage to repair defects, such as damaged electric key and sensors. Importantly, all ventilators were in use by healthcare units; consequently, their use was approved by the National Health Surveillance Agency (ANVISA). Thus maintenance did not change any aspect related to ventilator design, such as printed circuit board design or component specifications. | Basic requirements for ventilator maintenance |
| Mechanical maintenance | Most malfunctions were related to electronics and consumables, but in some cases, ventilator models were primarily based on a mechanical operating mechanism. It was usual to find punctured and dry hoses in such ventilators. After electronic and/or mechanical repair, calibration pre-trial was performed to ensure maintenance was done well. | Basic requirements for ventilator maintenance |
| Calibration and safety tests | A fundamental step in the maintenance process was final calibration and electrical safety tests. Calibration followed guidelines and procedures based on ABNT NBR ISO/IEC 17025:2017 standard [45]. Electrical safety followed ABNT NBR IEC 60601-1 [46] and ABNT NBR IEC 62353 [47] standards. This step ensured that every ventilator, once approved, met expected performance and posed no risk when used by hospitals. It was very important that healthcare units used the equipment according to operation manuals. | Operation and service manuals |
| Expedition and delivery | Final step, including cleaning to ensure that oldest tags were removed and review of all accessories according to ventilator’s entrance registration and packing. Upon completion, the logistics team was triggered to return ventilators to healthcare units as soon as possible. | Devolution protocol |
| Type | Goal | Target Audience | Coverage | Workload (Hours) | Quantity | Number of Participants/ Viewers |
|---|---|---|---|---|---|---|
| Initial training | Demonstrate knowledge, standards, safety procedures, and basic infrastructure to perform ventilator maintenance | SENAI institutes and partner companies | National | 4 | 8 | 803 |
| International live | Companies in Latin America, Africa, and USA | International | 2 | 3 | 617 | |
| Conversation with a specialist | Transfer specialized knowledge of ventilator maintenance focusing on most common brands/models | Maintenance sites (I+M) | National | 1 | 3 | 187 |
| Clinical engineering live | Discuss strategy and future developments in clinical engineering in Brazil | All partners and interested public | International | 2 | 1 | 605 |
| Question sections | Specific sections to answer questions regarding maintenance process and equipment | SENAI institutes and partner companies | National | 1 | 3 | 08 |
| State of Brazil | Number of Ventilators Sent | % of Ventilators Sent | Number of Ventilators Repaired | % of Ventilators Repaired |
|---|---|---|---|---|
| Acre | 8 | 0.20 | 6 | 75.00 |
| Alagoas | 10 | 0.25 | 3 | 30.00 |
| Amapá | 17 | 0.42 | 13 | 76.47 |
| Amazonas | 40 | 0.99 | 26 | 65.00 |
| Bahia | 439 | 10.85 | 279 | 63.55 |
| Ceará | 127 | 3.14 | 110 | 86.61 |
| Distrito Federal | 104 | 2.57 | 71 | 68.27 |
| Espírito Santo | 88 | 2.17 | 65 | 73.86 |
| Goiás | 198 | 4.89 | 118 | 59.60 |
| Maranhão | 25 | 0.62 | 16 | 64.00 |
| Mato Grosso | 153 | 3.78 | 83 | 54.25 |
| Mato Grosso do Sul | 137 | 3.39 | 103 | 75.18 |
| Minas Gerais | 559 | 13.81 | 281 | 50.27 |
| Pará | 128 | 3.16 | 55 | 42.97 |
| Paraíba | 82 | 2.03 | 39 | 47.56 |
| Paraná | 146 | 3.61 | 52 | 35.62 |
| Pernambuco | 89 | 2.20 | 32 | 35.96 |
| Rio de Janeiro | 296 | 7.31 | 124 | 41.89 |
| Rio Grande do Norte | 44 | 1.09 | 40 | 90.91 |
| Rio Grande do Sul | 168 | 4.15 | 120 | 71.43 |
| Rondônia | 9 | 0.22 | 6 | 66.67 |
| Roraima | 28 | 0.69 | 20 | 71.43 |
| Santa Catarina | 79 | 1.95 | 54 | 68.35 |
| São Paulo | 1018 | 25.15 | 753 | 73.97 |
| Tocantins | 55 | 1.36 | 47 | 85.45 |
| Total | 4047 | 100 | 2516 | 62.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, D.; Amaral, L.F.T.G.; Silva, B.C.d.S.; Gomes, L.d.F.; Barbosa, W.T.; Coelho, R.S.; Machado, B.A.S. Collaborative and Structured Network for Maintenance of Mechanical Ventilators during the SARS-CoV-2 Pandemic. Healthcare 2021, 9, 754. https://doi.org/10.3390/healthcare9060754
Motta D, Amaral LFTG, Silva BCdS, Gomes LdF, Barbosa WT, Coelho RS, Machado BAS. Collaborative and Structured Network for Maintenance of Mechanical Ventilators during the SARS-CoV-2 Pandemic. Healthcare. 2021; 9(6):754. https://doi.org/10.3390/healthcare9060754
Chicago/Turabian StyleMotta, Daniel, Luiz Fernando Taboada Gomes Amaral, Bruno Caetano dos Santos Silva, Lucas de Freitas Gomes, Willams Teles Barbosa, Rodrigo Santiago Coelho, and Bruna Aparecida Souza Machado. 2021. "Collaborative and Structured Network for Maintenance of Mechanical Ventilators during the SARS-CoV-2 Pandemic" Healthcare 9, no. 6: 754. https://doi.org/10.3390/healthcare9060754