ISSN: 0973-7510
E-ISSN: 2581-690X
WHO estimates show that 296 million people were living with chronic hepatitis B infection in 2019 with 1.5 million new infections occurring every year and approximately 290 000 people died from hepatitis C, mostly from cirrhosis and hepatocellular carcinoma. The prevalence and trends of Hepatitis B and Hepatitis C infections were affected during the pandemic, hence this study aimed to compare the difference in prevalence rates, trends, demographic data, and outcomes of Hepatitis B and Hepatitis C cases in pre-pandemic and pandemic era. The study was carried out in a 1060 bedded tertiary care teaching hospital located 90 kilometers away from Hyderabad, Telangana catering to a majorly rural population from around 200 villages. This study was a retrospective observational study where data of 4 years (March 2018 to Feb 2022) of patients whose samples were sent to Microbiology laboratory and were found to be positive for Hepatitis B surface antigen (HBsAg) or antibodies to Hepatitis C (Anti-HCV) were included. The medical records of Hepatitis B and Hepatitis C positive cases were analysed for demographic data like age, sex, address, requesting department, and present status retrieved from the hospital information system. The prevalence rates of Hepatitis B and Hepatitis C infections and trends every year were calculated and compared. Out of the total 39,578 samples tested for Hepatitis B surface antigen, 413 were positive with a seroprevalence of 1.04%. Among the 20,394 samples tested for anti-Hepatitis C antibodies, 53 samples were found to be positive showing a seroprevalence of 0.25%. There was a 23.63% decrease in the number of samples received during the pandemic period demonstrating the impact of COVID-19 on various laboratory testing. Male predominance was observed for both Hepatitis B (65.37%) and Hepatitis C (56.60%) positivity in this study. Hepatitis B was highest in the 61-80 years age group before the pandemic but during the pandemic, Hepatitis B positivity was equally distributed in the 41 to 60 years and 61-80 years age groups. Hepatitis C positive cases were equally distributed in the 41 to 60 years and 61-80 years age groups before the pandemic whereas during the pandemic Hepatitis C positivity was highest among the 41 to 60 years age group. Among the 413 positive cases of Hepatitis B, 315 (76.27%) cases belonged to the rural population and among the 53 Hepatitis C positive cases, 37 (69.81%) cases were from rural areas. The seroprevalence for Hepatitis B surface antigen displayed a decreasing trend in the pandemic era when compared to the pre-pandemic era. Seroprevalence for anti-HCV antibodies showed a small increase in the pandemic era when compared to the pre-pandemic era. Male predominance was observed for both Hepatitis B and Hepatitis C positivity in this study. Hepatitis B was highest in the 61-80 years age group before the pandemic but during the pandemic, Hepatitis B positivity was equally distributed in the 41 to 60 years and 61-80 years age groups. Hepatitis C positive cases were equally distributed in the 41 to 60 years and 61-80 years age groups before the pandemic whereas during the pandemic Hepatitis C positivity was highest among the 41 to 60 years age group. Detailed analysis of these variations in the trends during the pandemic will aid in guiding tertiary care hospitals on the way forward in the retrieval of medical services after the pandemic.
Hepatitis B, Hepatitis C, Seroprevalence, Viral Hepatitis, Impact of COVID-19 Pandemic
Hepatitis B and Hepatitis C are global diseases that are endemic in many countries, especially in the South East Asian Region.1 Hepatitis B is a vaccine-preventable liver infection caused by the Hepatitis B virus (HBV). The WHO estimates that 296 million people were living with chronic Hepatitis B infection in 2019, and 1.5 million new infections occur every year.2 Hepatitis B is spread through infected blood, semen, or other body fluids. Transmission occurs through sexual contact; sharing needles, syringes, or from mother to baby at birth. Symptoms can include fatigue, poor appetite, stomach pain, nausea, and jaundice. Hepatitis B is a short-term illness for many but few patients, it can become a long-term, chronic infection that can lead to serious, even life-threatening health issues like cirrhosis or liver cancer.3
Hepatitis C is a liver infection caused by the Hepatitis C virus (HCV) which spreads through contact with blood from an infected person. More than 50 percent of patients infected with the Hepatitis C virus, develop a long-term, chronic infection leading to cirrhosis and liver cancer.4 WHO estimated that in 2019, approximately 290 000 people died from Hepatitis C, mostly from cirrhosis and hepatocellular carcinoma.5
The COVID-19 pandemic had an impact on medical services globally. There was a reduction in patient check-ups and travel restrictions which caused a delay in diagnosis and interventions of other medical illnesses.6 The prevalence and trends of Hepatitis B and Hepatitis C infections also were affected during this pandemic. This study aims to compare the difference in prevalence rates, trends, demographic data, and outcomes of Hepatitis B and Hepatitis C cases in our hospital in the pre-pandemic and pandemic era. This comparison was intended to assess the impact of the pandemic on viral hepatitis services and any major differences in the trends will aid in guiding tertiary care hospitals on the way forward in the retrieval of medical services after the pandemic.
The study was carried out in a 1060 bedded tertiary care teaching hospital located 90 kilometers away from Hyderabad, Telangana catering to a majorly rural population from around 200 villages. This study was a retrospective observational study where data of 4 years (March 2018 to Feb 2022) of patients whose samples were sent to Microbiology laboratory and were found to be positive for Hepatitis B surface antigen (HBsAg) or antibodies to Hepatitis C (Anti-HCV) were included. Blood samples received by the Microbiology laboratory for testing from various outpatient and inpatient departments in our hospital were first centrifuged to separate the serum and screening for Hepatitis B surface antigen (HBsAg) and antibodies to Hepatitis C (Anti-HCV) were performed using ELISA kits according to manufacturer’s instructions. The medical records of Hepatitis B and Hepatitis C positive cases from March 2018 to Feb 2020 were included under the pre-pandemic period and records from March 2020 to Feb 2022 were included under the pandemic period. Demographic data like age, sex, address, requesting department, and present status was retrieved from the hospital information system. The prevalence rates of Hepatitis B and Hepatitis C infections and trends every year were calculated. A comparison of collected data in pre-pandemic and pandemic times was further analyzed, and results were calculated using statistical analysis by SPSS software version 23. The institutional ethical committee clearance was taken before starting the study.
The total number of blood samples received by the Microbiology laboratory for Hepatitis B and Hepatitis C testing from March 2018 to Feb 2022 was 59,972 samples. Out of the 59,972 blood samples received, 39,578 samples were received for HBsAg testing and 20,394 samples were received for anti-Hepatitis C antibodies testing. The numbers of samples received in the pre-pandemic era i.e. from March 2018 to Feb 2020 were 37,070 (61.81%) samples and the number of samples in the pandemic era i.e. from March 2020 to Feb 2022 was 22,902 (38.18%) samples. There was a substantial decrease, almost 23.63% in the number of samples received during the pandemic period demonstrating the impact of COVID-19 on various laboratory testing.
Out of the total 39,578 samples tested for Hepatitis B surface antigen, 413 were positive with a seroprevalence of 1.04%. Among the 20,394 samples tested for anti-Hepatitis C antibodies, 53 samples were found to be positive showing a seroprevalence of 0.25%. The seroprevalence for Hepatitis B surface antigen in the pre-pandemic era was 1.11% and in the pandemic era, it was found to be 0.91% displaying a decreasing trend. Seroprevalence for anti-HCV antibodies in the pre-pandemic was found to be 0.24% whereas in the pandemic the seroprevalence for Hepatitis C infection showed a small increase to 0.27%.
(Table 1)
Table (1):
Seroprevalence rates of Hepatitis B and Hepatitis C in study.
| Total samples tested | Hepatitis B positives | Prevalence rate | Total samples tested | Hepatitis C positives | Prevalence rate | |
|---|---|---|---|---|---|---|
| Pre-pandemic | 24,945 | 279 (67.55%) | 1.11% | 12,125 | 30 (56.60%) | 0.24% |
| Pandemic | 14,633 | 134 (32.44%) | 0.91% | 8,269 | 23 (43.39%) | 0.27% |
| Total | 39,578 | 413 | 1.04% | 20,394 | 53 | 0.25% |
Out of 413 Hepatitis B positive samples, 140 samples were outpatient samples and 273 samples were from inpatients. Out of the 53 Hepatitis C positive samples, 18 were outpatient samples and 35 were inpatient samples. During the pre-pandemic and pandemic periods, Hepatitis B and Hepatitis C positivity was found to be more prevalent among the inpatient samples. (Table 2).
Table (2):
Hepatitis B and Hepatitis C positivity during pre-pandemic and pandemic periods in IP and OP.
| Positive samples | Hepatitis B positive (n=413) | Total | Hepatitis C positive (n=53) | Total | ||
|---|---|---|---|---|---|---|
| Inpatient | Outpatient | Inpatient | Outpatient | |||
| Pre-pandemic | 182 | 97 | 279 | 19 | 11 | 30 |
| Pandemic | 91 | 43 | 134 | 16 | 7 | 23 |
| Total | 273 | 140 | 413 | 35 | 18 | 53 |
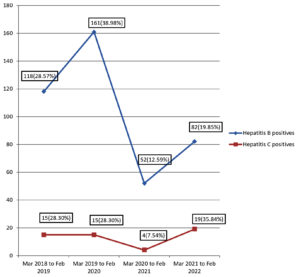
There was a decrease in the number of HBsAg and anti-HCV antibody positive samples during the pandemic era which could be attributed to the reduction of samples received during the pandemic. The prevalence of Hepatitis B has decreased during the pandemic time when compared to the pre-pandemic era whereas the prevalence rate of Hepatitis C showed a small increase during the pandemic. The trends of Hepatitis B and Hepatitis C positivity from March 2018 to March 2022 in Figure.
Male predominance was observed for both Hepatitis B (270, 65.37%) and Hepatitis C (30, 56.60%) positivity in this study. Similar findings were observed for Hepatitis B positivity in both pre-pandemic and pandemic times. Hepatitis C positivity was also seen majorly in males in pre-pandemic and pandemic periods. (Table 3).
Table (3):
Sex distribution of Hepatitis B and Hepatitis C positives during Pre-pandemic and Pandemic times.
Sex distribution |
Hepatitis B positives Pre-pandemic (n=279) |
Hepatitis B positives Pandemic (n=134) |
Hepatitis C positives Pre-pandemic (n=30) |
Hepatitis C positives Pandemic (n=23) |
|---|---|---|---|---|
Male |
189 (67.74%) |
81 (60.44%) |
17 (56.66%) |
13 (56.52%) |
Female |
90 (32.25%) |
53 (39.55%) |
13 (43.33%) |
10 (43.47%) |
The age of patients in years was divided into 5 groups, with ages less than 20 years in the first group, 21 to 40 years in the second group, 41 to 60 years in the third group, and 61 to 80 and above 80 years in the fourth and fifth groups. The positivity rate of Hepatitis B was highest in the fourth (61-80) age group and the positivity rate of Hepatitis C was highest in the third (41-60) age group as represented in Table 4.
Table (4):
Age distribution of Hepatitis B and Hepatitis C positives in 4 years.
Age group |
Hepatitis B positives |
Mean Age + SD (years) |
Percentage |
Hepatitis C positives |
Mean Age + SD (years) |
Percentage |
|---|---|---|---|---|---|---|
Less than 20 years |
2 |
14.5±4.49 |
0.48% |
0 |
0 |
0 |
21 to 40 years |
92 |
32.3±5.83 |
22.27% |
2 |
29±2.44 |
3.77% |
41 to 60 years |
151 |
52.0±5.68 |
36.56% |
25 |
52.2±5.12 |
47.16% |
61 to 80 years |
158 |
67.6±4.90 |
38.25% |
23 |
67.7±4.59 |
43.39% |
Above 80 years |
10 |
82.6±1.22 |
2.42% |
3 |
82.6±0.81 |
5.66% |
Total |
413 |
53 |
Hepatitis B was highest in the fourth (61-80) age group with a mean age of 67.3±4.62 before the pandemic but during the pandemic, Hepatitis B positivity was equally distributed in the third (41 to 60) and fourth (61-80) age groups with a mean age of 52.2±5.34 and 68.3±5.32 respectively. (Table 5).
Table (5):
Comparison of Hepatitis B among the different age groups in pre-pandemic and pandemic times.
Age group |
Hep B positives Pre-pandemic (n=279) |
Mean Age + SD (years) |
Hep B positives Pandemic (n=134) |
Mean Age + SD (years) |
|---|---|---|---|---|
less than 20 years |
1(0.35%) |
9 |
1(0.74%) |
20 |
21 to 40 years |
61(21.86%) |
31.7±6.36 |
31(23.13%) |
33.5±4.27 |
41 to 60 years |
100 (35.84%) |
50.7±5.76 |
51(38.05%) |
52.2±5.34 |
61 to 80 years |
107 (38.35%) |
67.3±4.62 |
51 (38.05%) |
68.3±5.32 |
above 80 years |
10 (3.58%) |
82.6±1.22 |
0 (0%) |
0 |
Hepatitis C positive cases were equally distributed in the third (41 to 60) and fourth (61-80) age groups with a mean age of 52.5±5.86 and 67.6±3.75 years, respectively, before the pandemic, whereas during the pandemic Hepatitis C positivity was highest among the third (41 to 60) age group with a mean age of 51.9±3.91years. (Table 6).
Table (6):
Comparison of Hepatitis C among the different age groups in pre-pandemic and pandemic times.
Age group |
Hep C positives Pre-pandemic (n=30) |
Mean Age + SD (years) |
Hep C positives Pandemic (n=23) |
Mean Age + SD (years) |
|---|---|---|---|---|
less than 20 years |
0 (0%) |
0 |
0 (0%) |
0 |
21 to 40 years |
1 (3.33%) |
26 |
1 (4.34%) |
32 |
41 to 60 years |
13 (43.33%) |
52.5±5.86 |
12 (52.17%) |
51.9±3.91 |
61 to 80 years |
13 (43.33%) |
67.6±3.75 |
10 (43.47%) |
67.9±5.41 |
above 80 years |
3 (10%) |
82.6±0.81 |
0 (0%) |
0 |
Out of the 413 Hepatitis B positive cases, 3 cases expired (0.72%) and out of the 53 Hepatitis C positive cases, 1 case expired (0.18%) while being admitted to our hospital. This percentage does not represent the case fatality of viral hepatitis as there were other comorbid conditions in these cases. Among the 413 positive samples of Hepatitis B infection, the majority of the samples were sent from the Ophthalmology (27.6%) department and among the 53 positive samples of Hepatitis C infection, 39.6% samples were from the General Medicine department.
Based on the addresses submitted by Hepatitis B and Hepatitis C cases during registration with our tertiary center, they were divided into urban and rural populations. Among the 413 positive cases of Hepatitis B, 315 cases belonged to the rural population and among the 53 Hepatitis C positive cases, 37 cases were from rural areas. The rural and urban population distribution among Hepatitis B and Hepatitis C cases before the pandemic and during the pandemic are displayed in Table 7.
Table (7):
Rural and urban population distribution among Hepatitis B and Hepatitis C cases before the pandemic and during the pandemic.
Total samples tested positive |
Hepatitis B positives |
Urban |
Rural |
Hepatitis C positives |
Urban |
Rural |
|---|---|---|---|---|---|---|
Pre-pandemic |
279 |
72 (25.80%) |
207 (74.19%) |
30 |
8 (26.66%) |
22 (73.33%) |
Pandemic |
134 |
26 (19.40%) |
108 (80.59%) |
23 |
8 (34.78%) |
15 (65.21%) |
Total |
413 |
98 (23.72%) |
315 (76.27%) |
53 |
16 (30.18%) |
37 (69.81%) |
The assessment for co-infection with HIV and COVID was done and the Hepatitis B positive with HIV reactive cases were 6 (1.45%) cases out of the total of 413 Hepatitis B positives. There were no Hepatitis C and HIV co-infected cases. The COVID status of all the Hepatitis B positive cases during the pandemic was negative. Among the 23 Hepatitis C positive cases during the pandemic, one case (4.3%) was found to be COVID positive.
The total number of blood samples received for Hepatitis B and Hepatitis C testing from March 2018 to Feb 2022 was 59,972 samples, with 37,070 samples in pre-pandemic and 22,902 samples in pandemic times. There was a substantial decrease of 23.63% in the number of samples received during the pandemic period demonstrating the impact of COVID-19 on various laboratory testing. The occurrence of late medical diagnosis among individuals with viral hepatitis could be interrelated with travel restriction guidelines during the COVID-19 pandemic. There was also channeling of equipment, infrastructure, and manpower for the sole purpose of the pandemic which led to a lack of attention to other laboratory tests. Similar findings are demonstrated by studies done by Gupta N et al. and Ismail Z et al. where they have discussed the impact of COVID-19 pandemic on viral hepatitis diagnosis and treatment in Africa.6,7
Out of the total 39,578 samples tested for Hepatitis B surface antigen, 413 were positive with a seroprevalence of 1.04%. Among the 20,394 samples tested for anti-Hepatitis C antibodies, 53 samples were found to be positive showing a seroprevalence of 0.25%. These results are concordant with the results of a similar study done by Baitha B et al. where seroprevalence rates for HIV, HBsAg, and Hepatitis C were calculated in blood donors.8
The seroprevalence for Hepatitis B surface antigen in the pre-pandemic era was 1.11% and in the pandemic era, it was found to be 0.91% displaying a decreasing trend. Seroprevalence for anti-HCV antibodies in the pre-pandemic was found to be 0.24% whereas in the pandemic the seroprevalence for Hepatitis C infection showed a small increase to 0.27%.
The overall prevalence of Hepatitis B has decreased during the pandemic time when compared to the pre-pandemic era. The decreased trend in Hepatitis B positive cases was also reported by Baitha B et al., Padmalatha et al. and Ray K et al.8-10 The prevalence rate of Hepatitis C showed a small increase in the later part of the pandemic in our study. The increased trend in Hepatitis C positive cases has been reported by Arora I et al. and Masood Z et al. during the pre-pandemic times but no studies have reported Hepatitis C trends in the late pandemic era.11, 12
Male predominance was observed for both Hepatitis B (65.37%) and Hepatitis C (56.60%) positivity in this study which is identical to studies done by Padmalatha et al. and Khan et al.9, 13 The reason for male predominance could be more chances of exposure to risk factors for Hepatitis B and Hepatitis C infections, like promiscuous sexual practices and needle sharing in drug abuse. The population catered by our tertiary care hospital has a sex ratio of 988 (females per thousand males) according to the Telangana state statistics which might have contributed to the male predominance in this study.
The positivity rate of Hepatitis B was highest in the 61 to 80 years age group (38.25%) with the mean age being 67.6 years±4.90. The reason for the older age group showing Hepatitis B positivity in our study could be that the population seeking services in our hospital belongs to the older age group and surrounding rural areas. Moreover, the majority of samples sent to the Microbiology laboratory for screening of Hepatitis B were from patients who were seeking free services during the Ophthalmology cataract camps held by our tertiary care hospital. The majority of the population requesting such services are from the older age group. Hepatitis B was highest in the fourth (61-80) age group with a mean age of 67.3±4.62 before the pandemic but during the pandemic, Hepatitis B positivity was equally distributed in the third (41 to 60) and fourth (61-80) age groups with a mean age of 52.2±5.34 and 68.3±5.32 respectively. Padmalatha et al. and Khan et al. have reported high Hepatitis B positivity rates in the 41 to 59 years age group and 21 to 40 years age group respectively.9, 13
Hepatitis C positive cases were equally distributed in the third (41 to 60) and fourth (61-80) age groups with a mean age of 52.5±5.86 and 67.6±3.75 years, respectively, whereas during the pandemic, Hepatitis C positivity was highest among the third (41 to 60) age group with a mean age of 51.9±3.91years. The overall positivity rate of Hepatitis C was highest in the 41 to 60 years age group (47.16%) with a mean age of 52.2 years ±5.12 which is concordant with studies done by Padmalatha et al. and Tripathi PC et al.9,14
Based on the addresses submitted by Hepatitis B and Hepatitis C cases during registration with our tertiary center, they were divided into urban and rural populations. Among the 413 positive cases of Hepatitis B, 315 (76.27%) cases belonged to the rural population and among the 53 Hepatitis C positive cases, 37 (69.81%) cases were from rural areas. Similar findings have been reported by Shanmugam RP et al. and Bhate et al. Both these studies have confirmed that Hepatitis B and Hepatitis C positivity was high among the rural and low socioeconomic status populations.15,16 Bhate et al. also claim that the practice of tattooing in rural populations has a high risk of Hepatitis B positivity.16
The Hepatitis B positive with HIV reactive cases were 6 (1.45%) cases out of the total of 413 Hepatitis B positives. Shrestha LB et al. reported 2.95% of HIV and HBV coinfected cases in their study whereas J Sarkar et al. have shown a higher percentage of 8.33% HIV and HBV coinfected cases in their study.17,18 The COVID statuses of all the Hepatitis B positive cases during the pandemic were negative. Among the 23 Hepatitis C positive cases during the pandemic, one case (4.3%) was found to be COVID positive.
There was a decrease in the number of HBsAg and anti HCV antibodies’ positive samples in the pandemic era which could be attributed to the reduction of samples received during the pandemic, especially in the early pandemic times. Similar findings have been reported by Gupta N et al., Ismail Z et al. and Kaufman HW et al., where they describe the decrease in Hepatitis testing and treatment during the COVID-19 pandemic.6,7,19
The seroprevalence for Hepatitis B surface antigen displayed a decreasing trend in the pandemic era when compared to the pre-pandemic era. Seroprevalence for anti-HCV antibodies showed a small increase in the pandemic era when compared to the pre-pandemic era. Male predominance was observed for both Hepatitis B and Hepatitis C positivity in this study. Hepatitis B was highest in the 61-80 years age group before the pandemic but during the pandemic, Hepatitis B positivity was equally distributed in the 41 to 60 years and 61-80 years age groups. Hepatitis C positive cases were equally distributed in the 41 to 60 years and 61-80 years age groups before the pandemic whereas during the pandemic Hepatitis C positivity was highest among the 41 to 60 years age group. Detailed analysis of these variations in the trends during the pandemic will aid in guiding tertiary care hospitals on the way forward in the retrieval of medical services after the pandemic.
Limitations of the Study
Risk factors and outcomes of patients are lacking in this study. The present results represent the patient population seeking services in our hospital and may not reflect the entire community.
ACKNOWLEDGMENTS
The authors would like to thank Kamineni Institute of Medical Sciences, Narketpally for supporting this project. The authors would also like to thank the technical workers of the Department of Microbiology, Records section, Front office and Hospital Administration staff and Dr. Shruti Mohanty, Principal of Kamineni Institute of Medical Sciences, Narketpally for her support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
CR conceived the study. CR and BRR designed the study. CR, SK, PNS collected and contributed the relevant data. CR, SK, PNS and BRR performed the data analysis. CR and PNS wrote the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, Kamineni Institute of Medical Sciences, Narketpally, Telangana, India with reference number KIMS/NKP/2022/06.
- MedlinePlus [Internet]. Bethesda (MD): National Library of Medicine (US). Hepatitis B. 2021. https://medlineplus.gov/hepatitisb.html
- WHO. World Health Organization: Factsheets: Hepatitis B. Accessed: 24 June 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. Accessed 24 June 2022.
- Centers for Disease Control and Prevention. Hepatitis B. https://www.cdc.gov/hepatitis/hbv/index.htm
- Centers for Disease Control and Prevention. Hepatitis C. https://www.cdc.gov/hepatitis/hcv/index.htm
- WHO. World Health Organization: Factsheets: Hepatitis C. Accessed: 24 June 2022. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 24 June 2022
- Gupta N, Desalegn H, Ocama P, et al. Converging pandemics: implications of COVID-19 for the viral hepatitis response in sub-Saharan Africa. Lancet Gastroenterol Hepatol. 2020;5(7):634-636.
Crossref - Ismail Z, Aborode AT, Oyeyemi AA, et al. Impact of COVID-19 pandemic on viral hepatitis in Africa: Challenges and way forward. Int J Health Plann Manage. 2022;37(1):547-552.
Crossref - Baitha B, Murmu S, Singh US. Prevalence and trends of markers of Hepatitis B Virus, Hepatitis C Virus and human immunodeficiency virus in Jamshedpur blood donors: A hospital based study. IOSR Journal of Dental and Medical Sciences. 2017;16(8):08-10x.
- Mahalakshmi PA, Gowtham RR, Mudhigeti N, et al. Seroprevalence and Trend of Hepatitis B and C Viral Infections in Patients at a Tertiary Care Hospital in Southern India- A Retrospective Study. J Clin Diagn Res. 2020;14(1):DC08-DC12.
Crossref - Ray K, Roy H, Das M. Trends of transfusion transmissible infections among blood donors in a rural medical college of West Bengal, India. Al Ameen J Med Sci. 2018;11(2):93-100.
- Arora I, Singh S, Singh S. Seroprevalence and trends of transfusion transmitted infections in blood donors of rural tertiary care hospital blood bank: A 3 year retrospective study in Chamba (HP). Int J Community Med Public Health. 2018;5(6):2453-57.
Crossref - Masood Z, Qureshi Z. Trends of Hepatitis B and C in general public living in the vicinity of M. Islam Medical College, Gujranwala. PJMHS. 2018;12(2):521-522.
- Khan AA. An update on the prevalence of viral hepatitis B and C among patients attending at tertiary hospital in South India. J Med Allied Sci. 2018;8(1):01-02.
Crossref - Tripathi PC, Chakraverti TK, Khant NR. Seroprevalence of hepatitis B surface antigen and antibody to hepatitis C virus at a tertiary care centre in Telangana. Int J Res Med Sci. 2015;3:297-300.
Crossref - Shanmugam RP, Balakrishnan S, Varadhan H, Shanmugam V. Prevalence of hepatitis B and hepatitis C infection from a population-based study in Southern India. Eur J Gastroenterol Hepatol. 2018;30(11):1344-1351.
Crossref - Bhate P, Saraf N, Parikh P, Ingle M, Phadke A, Sawant P. Cross sectional study of prevalence and risk factors of hepatitis B and hepatitis C infection in a rural village of India. Arquivos de Gastroenterologia. 2015;52(4):321-324.
Crossref - Shrestha LB, Yadav GK, Pradhan S, et al. Co-infection of Hepatitis B and Hepatitis C among HIV-infected patients: A cross-sectional study from tertiary care hospital of eastern Nepal. PLoS ONE. 2022;17(3):e0264791.
Crossref - Sarkar J, Bandyopadhyay B, Chakrabarty R, et al. HIV-HBV coinfection among individuals attending the ICTC of a tertiary care hospital in West Bengal, India. ISRN Virol. 2013;2013:180150.
Crossref - Kaufman HW, Bull-Otterson L, Meyer WA 3rd, et al. Decreases in Hepatitis C Testing and Treatment During the COVID-19 Pandemic. Am J Prev Med. 2021;61(3):369-376.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.