Association of Viral Infections With Oral Cavity Lesions: Role of SARS-CoV-2 Infection
- 1Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania, Italy
- 2Department of Biomedical and Biotechnological Sciences, Oncologic, Clinic and General Pathology Section, University of Catania, Catania, Italy
Different viral agents, such as herpesviruses, human papillomavirus, and Coxsackie virus, are responsible for primary oral lesions, while other viruses, such as human immunodeficiency virus, affect the oral cavity due to immune system weakness. Interestingly, it has been reported that coronavirus disease 2019 (COVID-19) patients can show cutaneous manifestations, including the oral cavity. However, the association between oral injuries and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is still unclear. This narrative review aimed to summarize the available literature and provide an overview of oral lesions associated with COVID-19. An online literature search was conducted to select relevant studies published up to November 2020. The results of 17 studies showed variability in oral lesions associated with COVID-19, including ulcerations, aphthous-like lesions, and macules. The tongue, lips, and palate were the most frequent anatomical locations. According to current knowledge, the etiopathogenesis of multiple COVID-19-associated lesions seems to be multifactorial. The appearance of such lesions could be related to the direct or indirect action of SARS-CoV-2 over the oral mucosa cells, coinfections, immunity impairment, and adverse drug reactions. Nevertheless, COVID-19-associated oral lesions may be underreported, mainly due to lockdown periods and the lack of mandatory dispositive protection. Consequently, further research is necessary to determine the diagnostic and pathological significance of oral manifestations of COVID-19. All medical doctors, dentists, and dermatologists are encouraged to perform an accurate and thorough oral examination of all suspected and confirmed COVID-19 cases to recognize the disease's possible early manifestations.
Introduction
The oral cavity is particularly susceptible to viral infections because of its conformation, particularly its soft tissue and salivary glands. Several viruses, including herpes simplex virus (HSV) and human papillomavirus (HPV), are associated with oral disease-causing primary lesions. Furthermore, oral mucosa can be affected by the secondary pathological processes of a bacterial or fungal nature due to viral immunosuppression, such as the human immunodeficiency virus (HIV). Consequently, the oral cavity could be considered a “biological barometer” of the viral immunosuppression advancement. Moreover, an implication of certain viral agents has been seen in dysplastic and neoplastic transformations of squamous epithelium (i.e., HPV) (1, 2). General and specialist dentists play a crucial role in evaluating, diagnosing, and managing such lesions, particularly considering the impacts of oral diseases on overall health and quality of life.
A new coronavirus, known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), responsible for coronavirus disease 2019 (COVID-19), was identified in Wuhan, Hubei Province, China, at the end of 2019. It then spread worldwide, becoming a global pandemic (3).
The main transmission route is via large respiratory droplets, even though the virus has also been identified in the stool and urine of affected individuals. It presents great variability in the severity of clinical manifestations, such as dry cough, shortness of breath, and fever (4, 5), passing from a mild flu-like illness to severe respiratory syndrome. Mortality rates vary according to region and change as the number of affected individuals is updated (6). Angiotensin-converting enzyme 2 (ACE2) receptor is considered the main functional receptor through which SARS-CoV-2 infects cells. The wide expression of ACE2 receptors in different anatomical sites, including the respiratory and gastrointestinal tracts, could explain the variability of reported clinical manifestations (7).
Interestingly, dermatological manifestations have also been observed in some patients affected by COVID-19. The most common skin lesions in these subjects include erythematous rash, urticaria, and vesicle formation, especially localized on the trunk, which seems to be the anatomic region most involved (5, 8). Oral lesions, such as unspecific ulceration, desquamative gingivitis, petechiae, and coinfections, such as candidiasis (9–13), have also been reported. Moreover, Xu et al. (14) reported a high level of expression of ACE2 receptors in epithelial cells of the oral mucosa, particularly tongue epithelial cells. These results suggest that oral mucosa could be a target of SARS-CoV-2 infection. Nevertheless, it is still unclear whether these manifestations are a specific clinical pattern derived from direct SARS-CoV-2 infection or a consequence of systemic involvement because of the possibility of coinfections, a compromised immune system, and adverse reactions to medical treatment (13, 15, 16). Because the prevalence of clinical oral manifestations is still unknown, the range of COVID-19 manifestations in the oral mucosa is of broad and current interest.
Therefore, after a brief excursus into the main viral agents associated with oral mucosa lesions, this review aims to summarize the updated literature on oral lesions in patients with COVID-19 and emphasize their clinical implications.
The Host Defense of Mouth and Viral Diseases
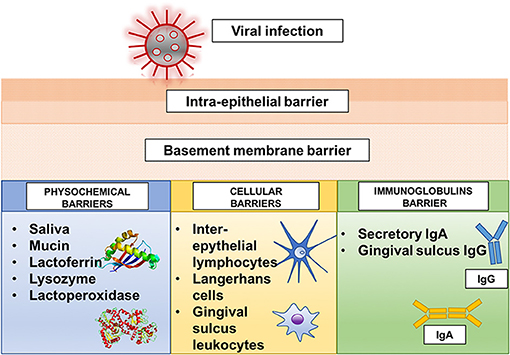
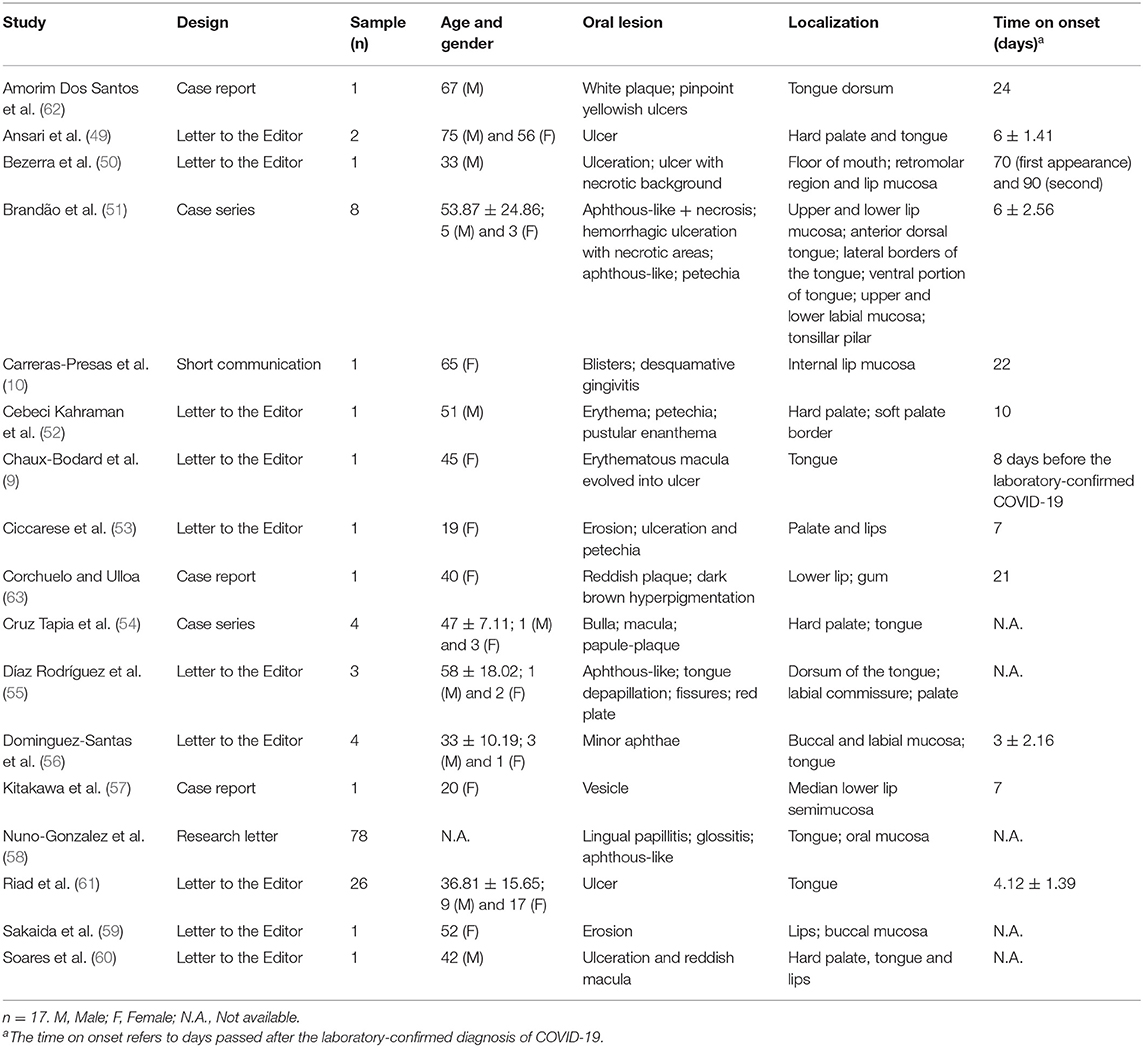
The oral cavity possesses a series of physicochemical, cellular, and immunoglobulin barriers that prevent the entrance of harmful substances and microorganisms (2) (Figure 1). However, physicochemical barriers within the oral mucosa, including saliva and oral epithelium, are not absolute. The saliva secreted by the major and minor salivary glands contains many non-specifically protective agents, such as mucin, lysozyme, lactoperoxidase, and lactoferrin. In particular, lactoferrin, an iron-binding glycoprotein of the transferrin family, can inactivate many deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) viruses, including cytomegalovirus, HSV, and rotavirus (17, 18). Cellular barriers involve the cells of the gingival sulcus, inter-epithelial lymphocytes, and Langerhans cells. In particular, Langerhans cells, which are dendritic inter-epithelial cells and act as mucosa “sentinels,” are localized in the mouth inverse to the degree of oral mucosa keratinization and are primarily implicated in immune reactions (2, 19, 20). Despite these defense mechanisms, the oral mucosa is particularly subjected to viral infections (Figure 2). A virus is a sub-microscopic entity formed by a protein shell (known as a capsid) surrounding a single nucleic acid, DNA or RNA, only able to replicate in bacterial, animal, and vegetal cells (21). Viral genetic material is distinguishable from human genetic material due to its unique chemical and/or physical features. Also, a lipid envelope derived from the host cell membrane can be identified in some viruses (22, 23). Even though viral infection can involve any human cell, the oral cavity offers an ideal entry into a new host (24). Some of the most well-known viral agents associated with oral lesions are HSVs and HPVs. HSVs contain a double-stranded linear DNA molecule enveloped by an icosahedral capsid and a lipid casing (25). Initially involved in primary infections, they then remain dormant but can later cause secondary or recurrent infections. Eight types of HSV have been identified as human pathogens, and most are responsible for oral diseases (1, 23, 25–35). HPVs are non-enveloped viruses containing double-stranded DNA (36). Over 100 subtypes of HPV have been identified, with at least 13 correlated to an insurgence of oral lesions (37–42). The oral wart is a generic term used to identify all papillary and verrucal proliferations. Squamous papilloma is one of the most represented papillary lesions in the oral cavity (1, 36). Oral lesions can also be secondary due to an immunosuppression state, such as occurs in HIV infection (43, 44). HIV is part of the Lentivirus genus, part of the Orthoretroviridae subfamily of the Retroviridae family. Two identical single-stranded RNA molecules form the HIV genome. The most frequent oral manifestations of HIV infection are opportunistic infections, such as candidiasis (45, 46), and malignancies, such as Kaposi's sarcoma (47, 48). The other most important viral agents associated with oral lesions are reported in Table 1. The association between oral lesions and SARS-CoV-2 remains controversial.
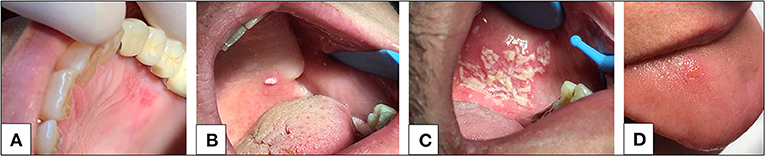
Figure 2. (A) Herpetic-like oral lesion. (B) Oral mucosal verruca lesion. (C) Opportunistic infection (candidiasis) in patient affected by HIV. (D) Unspecific ulcerous lesion in a SARS-CoV-2 infected patient. Image 2D is a case courtesy of Chaux-Bodard et al. (9).
Materials and Methods
An electronic search was conducted in PubMed, Scopus, and Web of Science for literature updated to November 20, 2020. A combination of the following keywords was used: “oral mucosal lesions” OR “oral lesions” AND “COVID-19” OR “SARS-CoV-2” OR “novel coronavirus disease.” The full-text articles of all potential studies were evaluated, and the references cited by the relevant studies were manually searched for further studies. Given the lack of available data, all types of studies reporting oral mucosal lesions in patients with laboratory-confirmed COVID-19 were included; only literature reviews were excluded. However, we decided to include only those with laboratory-confirmed COVID-19 when evaluating the reported cases; suspected COVID-19 were excluded. Other exclusion criteria were articles for which the full text was not accessible or not available in English. Duplicate articles were removed, and a first screening was performed by reading only the titles and abstracts of the studies.
Results
From the 86 studies retrieved, only 17 satisfied the inclusion criteria, of which 11 were letters to the editor, 3 were case reports, 2 were case series, and 1 was a short communication. Table 2 provides a detailed description of the cases included. Given that the studies were published between April and November 2020, the results were listed in the table in alphabetical order of the first author's surname. Excluding the only study in which the gender and age of the participants were not reported (58), 33 cases were female and 24 cases were male. The mean age of reported cases was 42.92 ± 18.05.
The documented manifestations of oral mucosa were quite heterogeneous, varying in the kind of lesion and the location. The most frequent findings were ulcerations (9, 50, 60–62), sometimes associated with necrotic areas (50, 51), aphthous-like lesions (51, 55, 56, 58), and petechiae (51, 52, 54). Maculae (53, 60), blisters (10, 57), lingual papillitis or depapillation (58), and erythema or red plaques (52, 63) were also among the described oral lesions. Besides, a case of dark brown hyperpigmentation was documented by Corchuelo and Ulloa (63).
Tongue (9, 51, 54, 55, 58–62), lips (10, 50, 51, 57, 59, 60, 63) and palate (52, 54, 60) were the most frequently described anatomical locations.
Discussion
An increasing number of atypical clinical presentations have been reported during SARS-CoV-2 infection, including dermatological and oral manifestations (16, 64–67). The pathogenesis of skin damage during COVID-19 is not well known, but some hypotheses have been formulated. For example, the presence of viral particles in cutaneous blood vessels could induce lymphocytic vasculitis through cytokine production, i.e., interleukin-1 (IL-1), interferon gamma (IFN-γ), and tumor necrosis factor alfa (TNF-α) by CD4+ T helper lymphocytes and the migration of eosinophils, CD8+ cytotoxic T cells, B cells, and natural killer (NK) cells (68, 69). Another possible explanation of cutaneous disturbances correlated to SARS-CoV-2 is the formation and accumulation of microthromboses, which could reduce the blood flow to the cutaneous microvasculature (70), and the presence of deoxygenated blood in venous plexi could further contribute to these cutaneous lesions. Moreover, the deposition of complement components C5b-9 and C4d in pauci-inflammatory thrombogenic vasculopathy and their co-localization with COVID-19 spike glycoproteins were shown by Magro et al. (71). It is reasonable to hypothesize that skin involvement is due to a combination of these mechanisms rather than a single one (5). Taste disorders were the most common oral symptom in patients with COVID-19, probably due to a local inflammatory response resulting from rhinitis triggers, which can hamper taste buds' normal function (15, 72). Additionally, oral mucosa involvement was described during SARS-CoV-2 infection. Since the first description of oral lesions in SARS-CoV-2 positive patients, reported by Martín Carreras-Presas et al. (10), several more recent studies have also reported oral mucosa lesions in COVID-19, such as ulcers (50, 60–62), aphthae (51, 55), and maculae (53, 60). The clinical significance of oral mucosa involvement during SARS-CoV-2 infection remains controversial.
As previously reported (14), the high expression of ACE2 on oral epithelial cells, especially on the tongue, suggests that the oral cavity might be an anatomical site particularly susceptible to SARS-CoV-2 infection. Consequently, as suggested by Brandão et al. (51), the interaction between SARS-CoV-2 and ACE2 might disrupt the oral keratinocytes' function, resulting in painful oral ulcers. Furthermore, oral mucosa lesions during COVID-19 could be justified by the variable inflammatory reaction, which can induce vascular inflammation, as observed for cutaneous manifestations (5, 73). The most recent publications on oral mucosa lesions in patients affected by COVID-19 support an association with organic damage and/or complications for thrombocytopenia, anticoagulant therapy, disseminated intravascular coagulation, and systemic inflammation (60, 62, 63). According to Cruz Tapia et al. (54), clinical manifestations and histological findings suggest the possibility that the oral cavity presents the primary or secondary alterations of vascular-hematologic damage associated with COVID-19. Nevertheless, as reported by Martín Carreras-Presas et al. (10) and Hedou et al. (74), ulcers or vesiculobullous lesions can occur as in other viral infections. It is largely documented that high levels of fatigue and stress can increase the risk of the reactivation of HSV (75).
Moreover, oral damage could also be a manifestation of an immunosuppression state and microbiome dysbiosis caused by a viral infection (76). According to Bezerra et al. (50), it is reasonable to think that COVID-19 systemic immune deregulation may cause a more prolonged immune imbalance, which could predispose these late, secondary oral lesions. In addition, as stated by de Sousa et al. (13), most patients developed oral mucosal injury during the hospitalization period, which supports the hypothesis of coinfections, immunity impairment, and adverse reactions to COVID-19 treatment medications.
Interestingly, Martín Carreras-Presas et al. (10) suggested that oral lesions, such as ulcers, could be an inaugural symptom of COVID-19. According to Amorim Dos Santos et al. (15), in mild cases, oral mucosal lesions occurred before or at the same time as the initial respiratory symptoms; however, in those who required medication and hospitalization, the lesions developed approximately 7–24 days after the onset of symptoms. Limited to the cases reported, the time to onset was variable, ranging from 4 to 90 days; however, the time on onset was unavailable in several reported studies, confirming the necessity to verify this data in larger patients' cohorts.
Regarding the age of patients presenting with oral injuries, Brandão et al. (51) reported two distinct oral lesions patterns. One was represented by aphthous-like ulcers in young patients with mild cases of COVID-19, and the other resembled HSV-1 necrotic ulcers in more severe cases of immunosuppressed older patients. Again, the lack of data available from a large sample of participants makes further investigations necessary to support these hypotheses.
Another interesting fact emerging from the reported cases is how online professional consultation using photography (telemedicine) could be a very useful, additional tool to support clinicians in the early diagnosis of oral lesions, especially when direct observation is not possible (57, 63). Indeed, an early clinical diagnosis and the development of sensitive diagnostic tools are essential for a correct management of the disease (77, 78).
There are some limitations to underline in this study. First, almost all the reports were published as letters to the editor, thus imposing editorial limitations that reduce reporting comprehensibility (12). Moreover, the oral manifestations' real incidence could have been underestimated due to exposure and contamination risk while conducting photographic imaging (15) and the limited data available on oral lesion injuries in asymptomatic patients. Another important aspect to consider is the limited availability of microscopic and histological data of oral mucosa lesions in COVID-19. The only accessible data referred to histological characterizations performed by Soares et al. (60) and Ansari et al. (49), which confirmed the presence of an inflammatory infiltrate, suggesting that the patients' lesions could be associated with COVID-19 disease. It is auspicious that the characterization of the oral lesions of COVID-19 infected patients should include incisional biopsies, followed by direct viral testing for SARS-CoV-2, as suggested by Brandão et al. (51).
Further research is necessary to determine the diagnostic and pathological significance of oral manifestations during COVID-19. Indeed, oral mucosa involvement during viral infection may assume differing clinical significance: it could represent either the first sign of viral disease or coexist as a co-symptom or represent a unique sign of the viral infection (26). In this context, the importance of the clinical oral examination of patients with confirmed or suspicious COVID-19 infection should be emphasized, given the need for support, pain control, and quality of life. In addition, dental operators are potentially exposed to a high degree of contamination with SARS-CoV-2 because of dental procedures that produce aerosols (79, 80). Consequently, an accurate inspection of the oral cavity, always with the mandatory dispositive protection (79), could be crucial in the dental setting to perform a more accurate triage of patients and improve safety operator, avoiding underestimation and misdiagnoses of oral signs and symptoms.
Conclusions
The new SARS-CoV-2 responsible for the global COVID-19 pandemic has become a sanitary emergency of primary importance. Although the typical symptoms include fever, shortness of breath, and a dry cough, cutaneous manifestations have also been reported, including some oral lesions. An association between oral diseases and SARS-CoV-2 infection is still unclear and currently poorly investigated. The appearance of such lesions could be related to the direct or indirect action of SARS-CoV-2 over the oral mucosa cells, coinfections, immunity impairment, and adverse drug reactions. Nevertheless, oral manifestations of this disease seem to be underreported, especially due to lockdown periods and the lack of mandatory dispositive protection. Consequently, based on these outcomes, we can conclude that (1) further studies are necessary to establish the diagnostic and pathological significance of oral manifestations during COVID-19; (2) the oral examination in patients with COVID-19 should not be unattended but rather promote a specialist multidisciplinary approach, including especially dental practitioners; (3) early recognition of oral lesions associated to COVID-19 could be crucial in the dental setting to perform a more accurate triage of patients and improve operator safety, avoiding underestimation and misdiagnosis of oral manifestations.
Author Contributions
GL, ML, and EP contributed to all steps of manuscript preparation. RD and SF participated in editing and critical revision of article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Dr. Chaux-Bodard for authorizing to use the Figure 2D.
References
1. Lynch DP. Oral manifestations of viral diseases. In: Tyring S, editor. Mucosal Immunology and Virology. Cham: Springer (2006). p. 99–156.
2. Dolby AE. The host defence system of the mouth. In: Ivanyi L, editor. Immunological Aspects of Oral Diseases. London: MTP Press Limited (1986). p. 1–11.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. (2020) 323:1239–42. doi: 10.1001/jama.2020.2648
4. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
5. Sachdeva M, Gianotti R, Shah M, Lucia B, Tosi D, Veraldi S, et al. Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J Dermatol Sci. (2020) 98:75–81. doi: 10.1016/j.jdermsci.2020.04.011
6. Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. (2020) 14:211–2. doi: 10.1016/j.dsx.2020.03.002
7. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. (2020) 9:45. doi: 10.1186/s40249-020-00662-x
8. Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J Eur Acad Dermatol Venereol. (2020) 34:e212–3. doi: 10.1111/jdv.16387
9. Chaux-Bodard AG, Deneuve S, Desoutter A. Oral manifestation of Covid-19 as an inaugural symptom? J Oral Med Oral Surg. (2020) 26:18. doi: 10.1051/mbcb/2020011
10. Martín Carreras-Presas C, Amaro Sánchez J, López-Sánchez AF, Jané-Salas E, Somacarrera Pérez ML. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. (2020). doi: 10.1111/odi.13382. [Epub ahead of print].
11. Capocasale G, Nocini R, Faccioni P, Donadello D, Bertossi D, Albanese M, et al. How to deal with coronavirus disease 2019: a comprehensive narrative review about oral involvement of the disease. Clin Exp Dent Res. (2020). doi: 10.1002/cre2.332. [Epub ahead of print].
12. Riad A, Klugar M, Krsek M. COVID-19-related oral manifestations: early disease features? Oral Dis. (2020). doi: 10.1111/odi.13516. [Epub ahead of print].
13. de Sousa FACG, Paradella TC. Considerations on oral manifestations of COVID-19. J Med Virol. (2020). doi: 10.1002/jmv.26451
14. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. (2020) 12:8. doi: 10.1038/s41368-020-0074-x
15. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, Acevedo AC, De Luca Canto G, Sugaya N, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res. (2020). doi: 10.1177/0022034520957289. [Epub ahead of print].
16. Halboub E, Al-Maweri SA, Alanazi RH, Qaid NM, Abdulrab S. Orofacial manifestations of COVID-19: a brief review of the published literature. Braz Oral Res. (2020) 34:e124. doi: 10.1590/1807-3107bor-2020.vol34.0124
17. Ng TB, Cheung RCF, Wong JH, Wang Y, Ip DTM, Wan DCC, et al. Antiviral activities of whey proteins. Appl Microbiol Biotechnol. (2015) 17:6997–7008. doi: 10.1007/s00253-015-6818-4
18. Wakabayashi H, Oda H, Yamauchi K, Abe F. Lactoferrin for prevention of common viral infections. J Infect Chemother. (2014) 20:666–71. doi: 10.1016/j.jiac.2014.08.003
19. Upadhyay J, Upadhyay RB, Agrawal P, Jaitley S, Shekhar R. Langerhans cells and their role in oral mucosal diseases. N Am J Med Sci. (2013) 5:505–14. doi: 10.4103/1947-2714.118923
20. Sistig S, Vucićević-Boras V, Lukac J, Kusić Z. Salivary IgA and IgG subclasses in oral mucosal diseases. Oral Dis. (2002) 8:282–6. doi: 10.1034/j.1601-0825.2002.20844.x
21. Kiselev NA, Sherman MB, Tsuprun VL. Negative staining of proteins. Electron Microsc Rev. (1990) 3:43–72. doi: 10.1016/0892-0354(90)90013-I
22. Caspar DL, Klug A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. (1962) 27:1–24. doi: 10.1101/SQB.1962.027.001.005
24. Grinde B, Olsen I. The role of viruses in oral disease. J Oral Microbiol. (2010) 2:2127. doi: 10.3402/jom.v2i0.2127
25. Pellett PE, Roizman B. The family herpesviridae: a brief introduction. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia, PA: Lippincott, Williams and Wilkins (2007) p. 2479–99.
26. Santosh A, Muddana K. Viral infections of oral cavity. J Family Med Prim Care. (2020) 9:36–42. doi: 10.4103/jfmpc.jfmpc_807_19
27. Scully C. Ulcerative stomatitis, gingivitis, and skin lesions. An unusual case of primary herpes simplex infection. Oral Surg Oral Med Oral Pathol. (1985) 59:261–3. doi: 10.1016/0030-4220(85)90163-X
28. Nahmias AJ, Roizman B. Infection with herpes simplex viruses 1 and 2. III. N Engl J Med. (1973) 289:781–9. doi: 10.1056/NEJM197310112891505
29. Weller T. Varicella and herpes zoster. N Engl J Med. (1983) 309:1362–8. doi: 10.1056/NEJM198312013092205
30. Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. (2009) 84:274–80. doi: 10.4065/84.3.274
31. Lambore S, McSherry J, Kraus AS. Acute and chronic symptoms of mononucleosis. J Fam Pract. (1991) 33:33–7.
32. Mach M, Stamminger T, Jahn G. Human cytomegalovirus: recent aspects from molecular biology. J Gen Virol. (1989) 70:3117–46. doi: 10.1099/0022-1317-70-12-3117
33. Bagg J. Human herpesvirus-6: the latest human herpes virus. J Oral Pathol Med. (1991) 20:465–8. doi: 10.1111/j.1600-0714.1991.tb00404.x
34. De Araujo T, Berman B, Weinstein A. Human herpesviruses 6 and 7. Dermatol Clin. (2002) 20:301–6. doi: 10.1016/S0733-8635(01)00008-0
35. Cannon M, Cesarman E. Kaposi's sarcoma-associated herpes virus and acquired immunodeficiency syndrome-related malignancy. Semin Oncol. (2000) 27:409–19.
36. Handisurya A, Schellenbacher C, Kirnbauer R. Diseases caused by human papillomaviruses (HPV). J Dtsch Dermatol Ges. (2009) 7:453–66. doi: 10.1111/j.1610-0387.2009.06988.x
37. Garlick JA, Taichman LB. Human papillomavirus infection of the oral mucosa. Am J Dermatopathol. (1991) 13:386–95. doi: 10.1097/00000372-199108000-00010
38. Kellokoski J, Syrjanen S, Syrjanen K, Yliskoski M. Oral mucosal changes in women with genital HPV infection. J Oral Pathol Med. (1990) 19:142–8. doi: 10.1111/j.1600-0714.1990.tb00813.x
39. Ficarra G, Adler-Storthz K, Galeotti F, Shillitoe E. Focal epithelial hyperplasia (Heck's disease): the first reported case from Italy. Tumori. (1991) 77:83–5. doi: 10.1177/030089169107700119
40. Morrow DJ, Sandhu HS, Daley TD. Focal epithelial hyperplasia (Heck's disease) with generalized lesions of the gingiva. A case report. J Periodontol. (1993) 64:63–5. doi: 10.1902/jop.1993.64.1.63
41. Miller CS, Johnstone BM. Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982-1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. (2001) 91:622–35. doi: 10.1067/moe.2001.115392
42. Hennessey PT, Westra WH, Califano JA. Human papillomavirus and head and neck squamous cell carcinoma: recent evidence and clinical implications. J Dent Res. (2009) 88:300–6. doi: 10.1177/0022034509333371
43. German Advisory Committee Blood (Arbeitskreis Blut) Subgroup 'Assessment of Pathogens Transmissible by Blood'. Human Immunodeficiency Virus (HIV). Transfus Med Hemother. (2016) 43:203–22. doi: 10.1159/000445852
44. Mbopi-Keou FX, Belec L, Teo CG, Scully C, Porter SR. Synergism between HIV and other viruses in the mouth. Lancet Infect Dis. (2002) 2:416–24. doi: 10.1016/S1473-3099(02)00317-1
45. Warrier SA, Sathasivasubramanian S. Human immunodeficiency virus induced oral candidiasis. J Pharm Bioallied Sci. (2015) 7:S81214. doi: 10.4103/0975-7406.163577
46. Shetti A, Gupta I, Charantimath SM. Oral candidiasis: aiding in the diagnosis of HIV-a case report. Case Rep Dent. (2011) 2011:929616. doi: 10.1155/2011/929616
47. Curtiss P, Strazzulla LC, Friedman-Kien AE. An update on Kaposi's Sarcoma: epidemiology, pathogenesis and treatment. Dermatol Ther. (2016) 6:465–70. doi: 10.1007/s13555-016-0152-3
48. Cesarman E, Damania B, Krown SE, Martin J, Bower M, Whitby D. Kaposi sarcoma. Nat Rev Dis Primers. (2019) 5:9. doi: 10.1038/s41572-019-0060-9
49. Ansari R, Gheitani M, Heidari F, Heidari F. Oral cavity lesions as a manifestation of the novel virus (COVID-19). Oral Dis. (2020). doi: 10.1111/odi.13465. [Epub ahead of print].
50. Bezerra TMM, Feitosa SG, Carneiro DTO, Costa FWG, Pires FR, Pereira KMA. Oral lesions in COVID-19 infection: is long term follow-up important in the affected patients? Oral Dis. (2020). doi: 10.1111/odi.13705. [Epub ahead of print].
51. Brandão TB, Gueiros LA, Melo TS, Prado-Ribeiro AC, Nesrallah A, Prado G, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol. (2020). doi: 10.1016/j.oooo.2020.07.014. [Epub ahead of print].
52. Cebeci Kahraman F, Çaşkurlu H. Mucosal involvement in a COVID-19-positive patient: a case report. Dermatol Ther. (2020) 33:e13797. doi: 10.1111/dth.13797
53. Ciccarese G, Drago F, Boatti M, Porro A, Muzic SI, Parodi A. Oral erosions and petechiae during SARS-CoV-2 infection. J Med Virol. (2020). doi: 10.1002/jmv.26221. [Epub ahead of print].
54. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, Matos Valdez LH. Oral mucosal lesions in patients with SARS-CoV-2 infection. Report of four cases. Are they a true sign of COVID-19 disease? Spec Care Dentist. (2020) 40:555–60. doi: 10.1111/scd.12520
55. Díaz Rodríguez M, Jimenez Romera A, Villarroel M. Oral manifestations associated with COVID-19. Oral Dis. (2020). doi: 10.1111/odi.13555. [Epub ahead of print].
56. Dominguez-Santas M, Diaz-Guimaraens B, Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, Suarez-Valle A. Minor aphthae associated with SARS-CoV-2 infection. Int J Dermatol. (2020). doi: 10.31128/AJGP-COVID-32. [Epub ahead of print].
57. Kitakawa D, Oliveira FE, Neves de Castro P, Carvalho LFCS. Short report - Herpes simplex lesion in the lip semimucosa in a COVID-19 patient. Eur Rev Med Pharmacol Sci. (2020) 24:9151–3. doi: 10.26355/eurrev_202009_22863
58. Nuno-Gonzalez A, Martin-Carrillo P, Magaletsky K, Martin Rios MD, Herranz Mañas C, Artigas Almazan J, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol. (2020). doi: 10.1111/bjd.19564. [Epub ahead of print].
59. Sakaida T, Tanimoto I, Matsubara A, Nakamura M, Morita A. Unique skin manifestations of COVID-19: is drug eruption specific to COVID-19? J Dermatol Sci. (2020) 99:62–4. doi: 10.1016/j.jdermsci.2020.05.002
60. Soares CD, Carvalho RA, Carvalho KA, Carvalho MG, Almeida OP. Letter to editor: oral lesions in a patient with Covid-19. Med Oral Patol Oral Cir Bucal. (2020) 25:e563–4. doi: 10.4317/medoral.24044
61. Riad A, Kassem I, Hockova B, Badrah M, Klugar M. Tongue ulcers associated with SARS-CoV-2 infection: a case series. Oral Dis. (2020). doi: 10.1111/odi.13635. [Epub ahead of print].
62. Amorim Dos Santos J, Normando AGC, Carvalho da Silva RL, De Paula RM, Cembranel AC, Santos-Silva AR, et al. Oral mucosal lesions in a COVID-19 patient: New signs or secondary manifestations? Int J Infect Dis. (2020) 97:326–8. doi: 10.1016/j.ijid.2020.06.012
63. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis. (2020) 100:154–7. doi: 10.1016/j.ijid.2020.08.071
64. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in china. N Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
65. Aziz M, Perisetti A, Lee-Smith WM, Gajendran M, Bansal P, Goyal H. Taste changes (dysgeusia) in Covid-19: a systematic review and metaanalysis. Gastroenterology. (2020) 159:1132–3. doi: 10.1053/j.gastro.2020.05.003
66. Gianotti R, Zerbi P, Dodiuk-Gad RP. Clinical and histopathological study of skin dermatoses in patients affected by COVID-19 infection in the Northern part of Italy. J Dermatol Sci. (2020) 98:141–3. doi: 10.1016/j.jdermsci.2020.04.007
67. Xue X, Mi Z, Wang Z, Pang Z, Liu H, Zhang F. High expression of ACE2 on the keratinocytes reveals skin as a potential target for SARS-CoV-2. J Invest Dermatol. (2020) 141:206–9.e1. doi: 10.1016/j.jid.2020.05.087
68. Lee JS, Kossard S, McGrath MA. Lymphocytic thrombophilic arteritis: a newly described medium-sized vessel arteritis of the skin. Arch Dermatol. (2008) 144:1175–82. doi: 10.1001/archderm.144.9.1175
70. Manalo IF, Smith MK, Cheeley J, Jacobs R. A dermatologic manifestation of COVID-19: transient livedo reticularis. J Am Acad Dermatol. (2020) 83:700. doi: 10.1016/j.jaad.2020.04.018
71. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
72. Finsterer J, Stollberger C. Causes of hypogeusia/hyposmia in SARS- CoV2 infected patients. J Med Virol. (2020) 92:1793–4. doi: 10.1002/jmv.25903
73. Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. (2020) 5:831–40. doi: 10.1001/jamacardio.2020.1286
74. Hedou M, Carsuzaa F, Chary E, Hainaut E, Cazenave-Roblot F, Masson Regnault M. Comment on 'Cutaneous manifestations in COVID-19: a first perspective' by Recalcati S. J Eur Acad Dermatol Venereol. (2020) 34:e299–300. doi: 10.1111/jdv.16519
75. Forbes H, Warne B, Doelken L, Brenner N, Waterboe T, Luben R, et al. Risk factors for herpes simplex virus type-1 infection and reactivation: cross-sectional studies among EPIC- Norfolk participants. PLoS ONE. (2019) 14:e0215553. doi: 10.1371/journal.pone.0215553
76. Santacroce L, Charitos IA, Ballini A, Inchingolo F, Luperto P, De Nitto E, et al. The human respiratory system and its microbiome at a glimpse. Biology. (2020) 9:318. doi: 10.3390/biology9100318
77. Pham VH, Gargiulo Isacco C, Nguyen KCD, Le SH, Tran DK, Nguyen QV, et al. Rapid and sensitive diagnostic procedure for multiple detection of pandemic Coronaviridae family members SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV: a translational research and cooperation between the Phan Chau Trinh University in Vietnam and University of Bari “Aldo Moro” in Italy. Eur Rev Med Pharmacol Sci. (2020) 24:7173–91. doi: 10.26355/eurrev_202006_21713
78. Charitos IA, Ballini A, Bottalico L, Cantore S, Passarelli PC, Inchingolo F, et al. Special features of SARS-CoV-2 in daily practice. World J Clin Cases. (2020) 8:3920–33. doi: 10.12998/wjcc.v8.i18.3920
79. Bordea IR, Xhajanka E, Candrea S, Bran S, Oni?or F, Inchingolo AD, etal. Coronavirus (SARS-CoV-2) pandemic: future challenges for dental practitioners. Microorganisms. (2020) 8:1704. doi: 10.3390/microorganisms8111704
Keywords: COVID-19, novel coronavirus, oral health, oral lesions, oral mucosa, SARS-CoV-2, viral infection
Citation: La Rosa GRM, Libra M, De Pasquale R, Ferlito S and Pedullà E (2021) Association of Viral Infections With Oral Cavity Lesions: Role of SARS-CoV-2 Infection. Front. Med. 7:571214. doi: 10.3389/fmed.2020.571214
Received: 10 June 2020; Accepted: 09 December 2020;
Published: 14 January 2021.
Edited by:
Chaminda Jayampath Seneviratne, National Dental Centre of Singapore, SingaporeReviewed by:
Andrea Ballini, University of Bari Aldo Moro, ItalyStefania Cantore, City Unity College Athens, Greece
Copyright © 2021 La Rosa, Libra, De Pasquale, Ferlito and Pedullà. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giusy Rita Maria La Rosa, g_larosa92@live.it
 Giusy Rita Maria La Rosa
Giusy Rita Maria La Rosa Massimo Libra
Massimo Libra Rocco De Pasquale1
Rocco De Pasquale1